 Found 159 hits with Last Name = 'bull' and Initial = 'hg'
Found 159 hits with Last Name = 'bull' and Initial = 'hg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Granzyme B
(Homo sapiens (Human)) | BDBM50116262
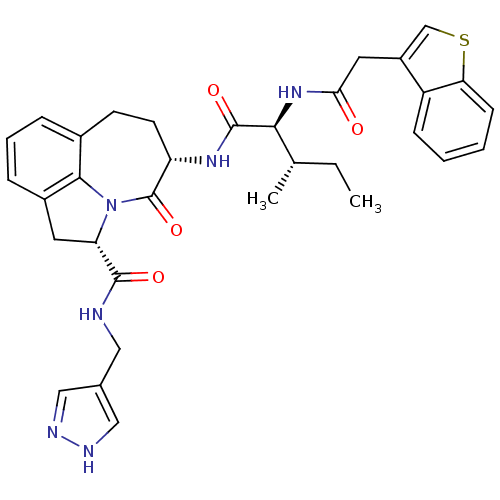
((2S,5S)-5-[(S)-2-(2-Benzo[b]thiophen-3-yl-acetylam...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1csc2ccccc12)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cn[nH]c1 Show InChI InChI=1S/C33H36N6O4S/c1-3-19(2)29(38-28(40)14-23-18-44-27-10-5-4-9-24(23)27)32(42)37-25-12-11-21-7-6-8-22-13-26(39(30(21)22)33(25)43)31(41)34-15-20-16-35-36-17-20/h4-10,16-19,25-26,29H,3,11-15H2,1-2H3,(H,34,41)(H,35,36)(H,37,42)(H,38,40)/t19-,25-,26-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116264

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H38N6O8/c1-5-18(2)27(39(4)19(3)41)31(46)35-23-15-14-20-12-9-13-22-16-25(40(28(20)22)34(23)47)30(45)36-24(17-26(42)43)29(44)33-38-37-32(48-33)21-10-7-6-8-11-21/h6-13,18,23-25,27H,5,14-17H2,1-4H3,(H,35,46)(H,36,45)(H,42,43)/t18-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116260
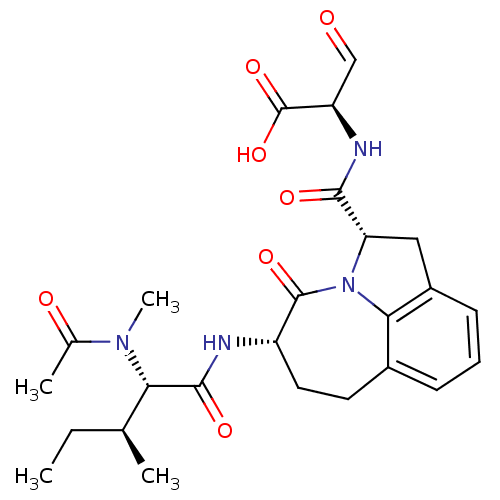
((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116255
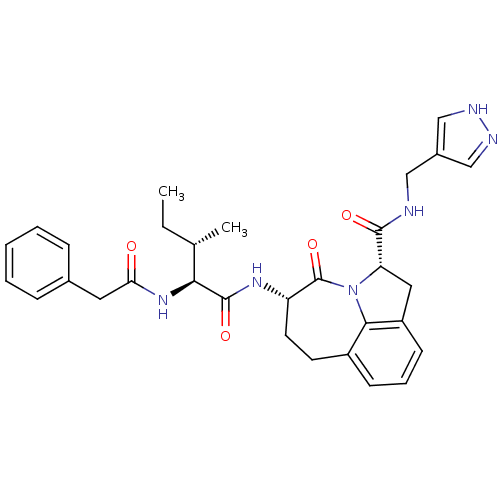
((2S,5S)-5-((S)-3-(S)-Methyl-2-phenylacetylamino-pe...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1ccccc1)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cn[nH]c1 Show InChI InChI=1S/C31H36N6O4/c1-3-19(2)27(36-26(38)14-20-8-5-4-6-9-20)30(40)35-24-13-12-22-10-7-11-23-15-25(37(28(22)23)31(24)41)29(39)32-16-21-17-33-34-18-21/h4-11,17-19,24-25,27H,3,12-16H2,1-2H3,(H,32,39)(H,33,34)(H,35,40)(H,36,38)/t19-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116256
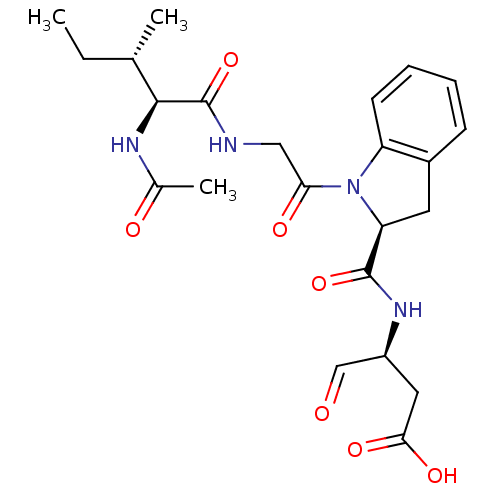
((S)-3-({1-[(S)-2-((3S,4S)-2-Acetylamino-3-methyl-p...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)NCC(=O)N1[C@@H](Cc2ccccc12)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C23H30N4O7/c1-4-13(2)21(25-14(3)29)23(34)24-11-19(30)27-17-8-6-5-7-15(17)9-18(27)22(33)26-16(12-28)10-20(31)32/h5-8,12-13,16,18,21H,4,9-11H2,1-3H3,(H,24,34)(H,25,29)(H,26,33)(H,31,32)/t13-,16-,18-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116267
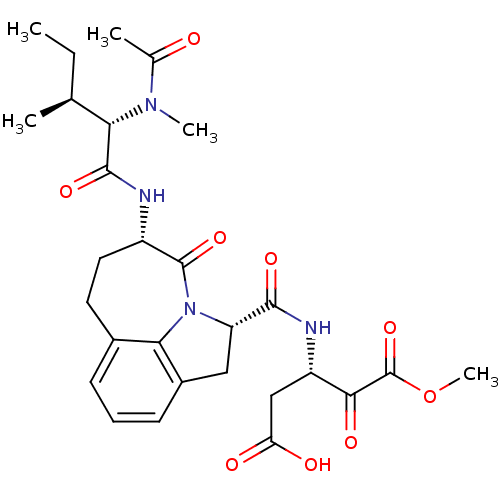
((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)C(=O)OC Show InChI InChI=1S/C28H36N4O9/c1-6-14(2)22(31(4)15(3)33)26(38)29-18-11-10-16-8-7-9-17-12-20(32(23(16)17)27(18)39)25(37)30-19(13-21(34)35)24(36)28(40)41-5/h7-9,14,18-20,22H,6,10-13H2,1-5H3,(H,29,38)(H,30,37)(H,34,35)/t14-,18-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116264

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H38N6O8/c1-5-18(2)27(39(4)19(3)41)31(46)35-23-15-14-20-12-9-13-22-16-25(40(28(20)22)34(23)47)30(45)36-24(17-26(42)43)29(44)33-38-37-32(48-33)21-10-7-6-8-11-21/h6-13,18,23-25,27H,5,14-17H2,1-4H3,(H,35,46)(H,36,45)(H,42,43)/t18-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116268
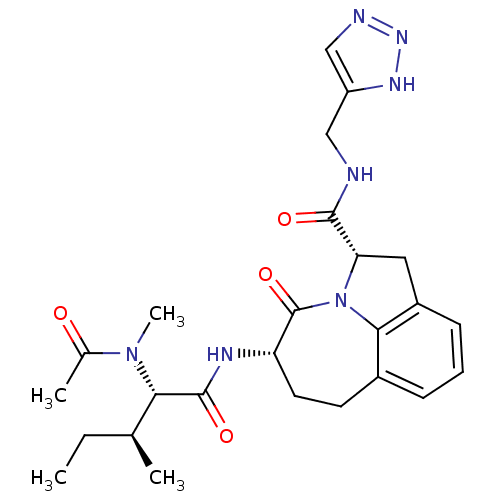
((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cnn[nH]1 Show InChI InChI=1S/C25H33N7O4/c1-5-14(2)21(31(4)15(3)33)24(35)28-19-10-9-16-7-6-8-17-11-20(32(22(16)17)25(19)36)23(34)26-12-18-13-27-30-29-18/h6-8,13-14,19-21H,5,9-12H2,1-4H3,(H,26,34)(H,28,35)(H,27,29,30)/t14-,19-,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116260

((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116269
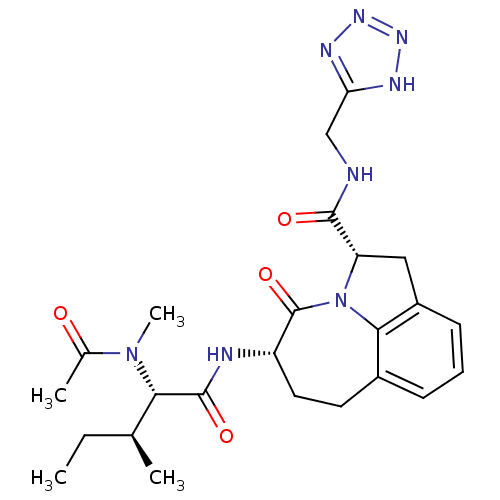
((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C24H32N8O4/c1-5-13(2)20(31(4)14(3)33)23(35)26-17-10-9-15-7-6-8-16-11-18(32(21(15)16)24(17)36)22(34)25-12-19-27-29-30-28-19/h6-8,13,17-18,20H,5,9-12H2,1-4H3,(H,25,34)(H,26,35)(H,27,28,29,30)/t13-,17-,18-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116261
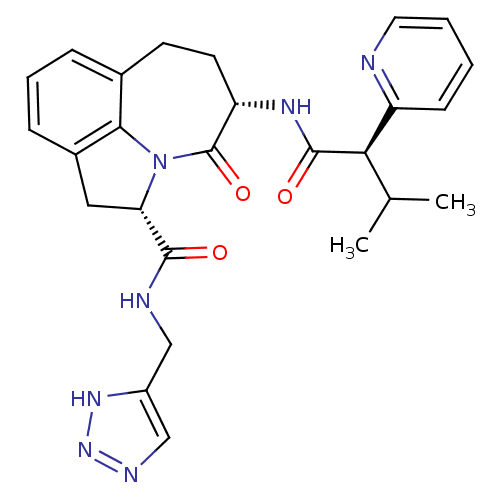
((2S,5S)-5-((R)-3-Methyl-2-pyridin-2-yl-butyrylamin...)Show SMILES CC(C)[C@@H](C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cnn[nH]1)c1ccccn1 Show InChI InChI=1S/C26H29N7O3/c1-15(2)22(19-8-3-4-11-27-19)25(35)30-20-10-9-16-6-5-7-17-12-21(33(23(16)17)26(20)36)24(34)28-13-18-14-29-32-31-18/h3-8,11,14-15,20-22H,9-10,12-13H2,1-2H3,(H,28,34)(H,30,35)(H,29,31,32)/t20-,21-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116259
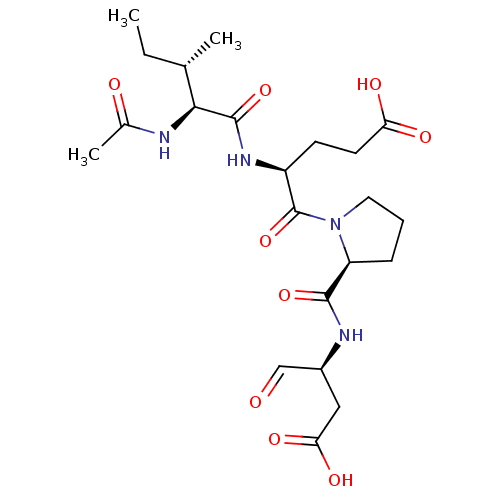
((S)-4-((3S,4S)-2-Acetylamino-3-methyl-pentanoylami...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C22H34N4O9/c1-4-12(2)19(23-13(3)28)21(34)25-15(7-8-17(29)30)22(35)26-9-5-6-16(26)20(33)24-14(11-27)10-18(31)32/h11-12,14-16,19H,4-10H2,1-3H3,(H,23,28)(H,24,33)(H,25,34)(H,29,30)(H,31,32)/t12-,14-,15-,16-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116265

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C33H37N5O7S/c1-5-17(2)27(37(4)18(3)39)31(44)34-22-14-13-19-9-8-10-20-15-24(38(28(19)20)33(22)45)30(43)35-23(16-26(40)41)29(42)32-36-21-11-6-7-12-25(21)46-32/h6-12,17,22-24,27H,5,13-16H2,1-4H3,(H,34,44)(H,35,43)(H,40,41)/t17-,22-,23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116263

((S)-3-{[(S)-(5S,7S)-6-((3S,4S)-2-Acetylamino-3-met...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CC[C@H]2CC[C@H](N2C1=O)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C21H32N4O7/c1-4-11(2)18(22-12(3)27)20(31)24-15-7-5-14-6-8-16(25(14)21(15)32)19(30)23-13(10-26)9-17(28)29/h10-11,13-16,18H,4-9H2,1-3H3,(H,22,27)(H,23,30)(H,24,31)(H,28,29)/t11-,13-,14-,15-,16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116258
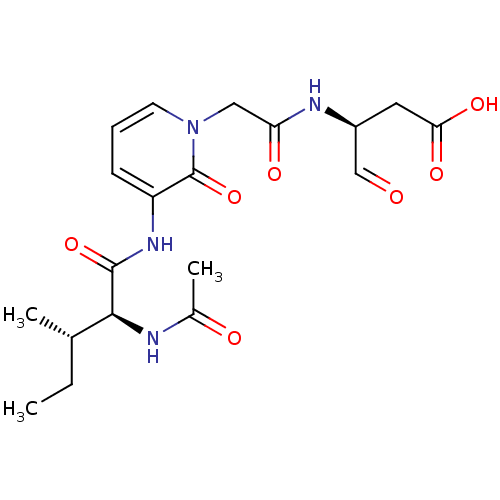
((S)-3-{2-[3-((2S,3S)-2-Acetylamino-3-methyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)Nc1cccn(CC(=O)N[C@@H](CC(O)=O)C=O)c1=O Show InChI InChI=1S/C19H26N4O7/c1-4-11(2)17(20-12(3)25)18(29)22-14-6-5-7-23(19(14)30)9-15(26)21-13(10-24)8-16(27)28/h5-7,10-11,13,17H,4,8-9H2,1-3H3,(H,20,25)(H,21,26)(H,22,29)(H,27,28)/t11-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116265

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C33H37N5O7S/c1-5-17(2)27(37(4)18(3)39)31(44)34-22-14-13-19-9-8-10-20-15-24(38(28(19)20)33(22)45)30(43)35-23(16-26(40)41)29(42)32-36-21-11-6-7-12-25(21)46-32/h6-12,17,22-24,27H,5,13-16H2,1-4H3,(H,34,44)(H,35,43)(H,40,41)/t17-,22-,23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116267

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)C(=O)OC Show InChI InChI=1S/C28H36N4O9/c1-6-14(2)22(31(4)15(3)33)26(38)29-18-11-10-16-8-7-9-17-12-20(32(23(16)17)27(18)39)25(37)30-19(13-21(34)35)24(36)28(40)41-5/h7-9,14,18-20,22H,6,10-13H2,1-5H3,(H,29,38)(H,30,37)(H,34,35)/t14-,18-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116257
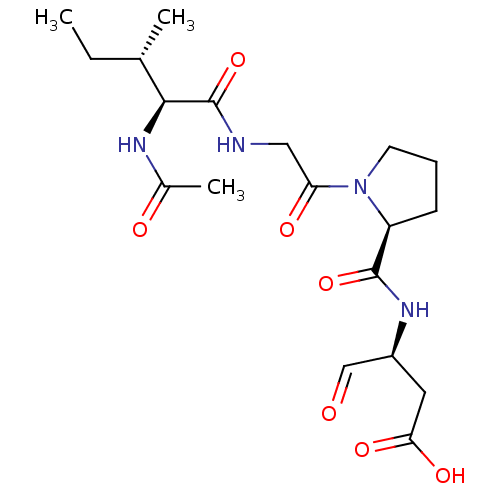
(3-[((S)-{1-[(S)-2-((3S,4S)-2-Acetylamino-3-methyl-...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C19H30N4O7/c1-4-11(2)17(21-12(3)25)19(30)20-9-15(26)23-7-5-6-14(23)18(29)22-13(10-24)8-16(27)28/h10-11,13-14,17H,4-9H2,1-3H3,(H,20,30)(H,21,25)(H,22,29)(H,27,28)/t11-,13-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-8
(Homo sapiens (Human)) | BDBM50116260

((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-8 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116270

((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cc(=O)[nH]o1 Show InChI InChI=1S/C26H33N5O6/c1-5-14(2)22(30(4)15(3)32)25(35)28-19-10-9-16-7-6-8-17-11-20(31(23(16)17)26(19)36)24(34)27-13-18-12-21(33)29-37-18/h6-8,12,14,19-20,22H,5,9-11,13H2,1-4H3,(H,27,34)(H,28,35)(H,29,33)/t14-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116254

(3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)-3-m...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H34N4O6/c1-5-14(2)21(28(4)15(3)30)24(34)27-18-10-9-16-7-6-8-17-13-19(29(22(16)17)25(18)35)23(33)26-12-11-20(31)32/h6-8,14,18-19,21H,5,9-13H2,1-4H3,(H,26,33)(H,27,34)(H,31,32)/t14-,18-,19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116266
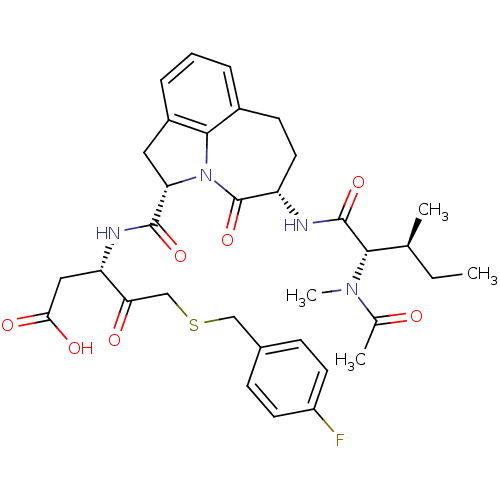
((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)CSCc1ccc(F)cc1 Show InChI InChI=1S/C34H41FN4O7S/c1-5-19(2)30(38(4)20(3)40)33(45)36-25-14-11-22-7-6-8-23-15-27(39(31(22)23)34(25)46)32(44)37-26(16-29(42)43)28(41)18-47-17-21-9-12-24(35)13-10-21/h6-10,12-13,19,25-27,30H,5,11,14-18H2,1-4H3,(H,36,45)(H,37,44)(H,42,43)/t19-,25-,26-,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Rattus norvegicus) | BDBM50300992
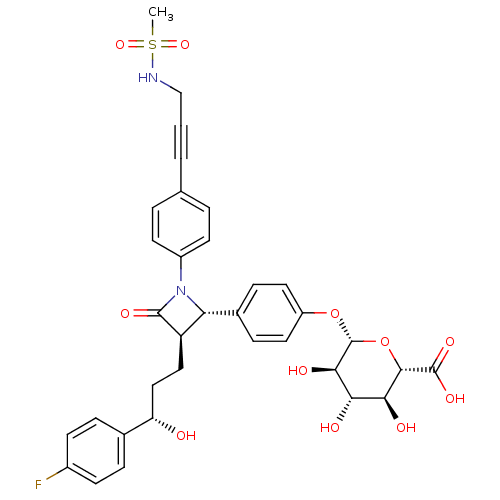
((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...)Show SMILES CS(=O)(=O)NCC#Cc1ccc(cc1)N1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C34H35FN2O11S/c1-49(45,46)36-18-2-3-19-4-12-23(13-5-19)37-27(25(32(37)42)16-17-26(38)20-6-10-22(35)11-7-20)21-8-14-24(15-9-21)47-34-30(41)28(39)29(40)31(48-34)33(43)44/h4-15,25-31,34,36,38-41H,16-18H2,1H3,(H,43,44)/t25-,26+,27-,28+,29+,30-,31+,34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NPC1-like intracellular cholesterol transporter 1
(Rattus norvegicus) | BDBM50300992

((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...)Show SMILES CS(=O)(=O)NCC#Cc1ccc(cc1)N1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C34H35FN2O11S/c1-49(45,46)36-18-2-3-19-4-12-23(13-5-19)37-27(25(32(37)42)16-17-26(38)20-6-10-22(35)11-7-20)21-8-14-24(15-9-21)47-34-30(41)28(39)29(40)31(48-34)33(43)44/h4-15,25-31,34,36,38-41H,16-18H2,1H3,(H,43,44)/t25-,26+,27-,28+,29+,30-,31+,34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50300992

((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...)Show SMILES CS(=O)(=O)NCC#Cc1ccc(cc1)N1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C34H35FN2O11S/c1-49(45,46)36-18-2-3-19-4-12-23(13-5-19)37-27(25(32(37)42)16-17-26(38)20-6-10-22(35)11-7-20)21-8-14-24(15-9-21)47-34-30(41)28(39)29(40)31(48-34)33(43)44/h4-15,25-31,34,36,38-41H,16-18H2,1H3,(H,43,44)/t25-,26+,27-,28+,29+,30-,31+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50300992

((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...)Show SMILES CS(=O)(=O)NCC#Cc1ccc(cc1)N1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C34H35FN2O11S/c1-49(45,46)36-18-2-3-19-4-12-23(13-5-19)37-27(25(32(37)42)16-17-26(38)20-6-10-22(35)11-7-20)21-8-14-24(15-9-21)47-34-30(41)28(39)29(40)31(48-34)33(43)44/h4-15,25-31,34,36,38-41H,16-18H2,1H3,(H,43,44)/t25-,26+,27-,28+,29+,30-,31+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50258479
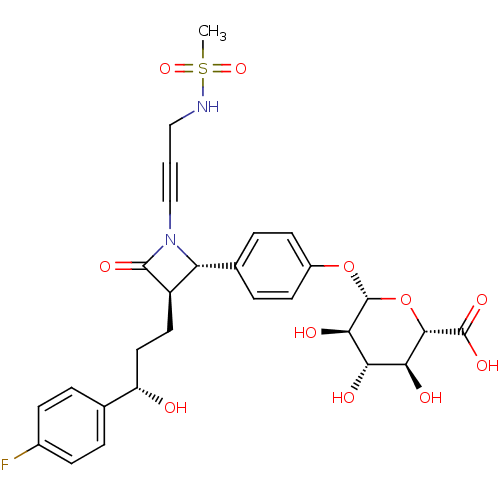
((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
Niemann-Pick C1-like 1 protein
(Canis familiaris) | BDBM50258479

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Rattus norvegicus) | BDBM50258479

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50258479

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
Niemann-Pick C1-like 1 protein
(Macaca mulatta) | BDBM50258479

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
Niemann-Pick C1-like 1 protein
(Macaca mulatta) | BDBM50240720
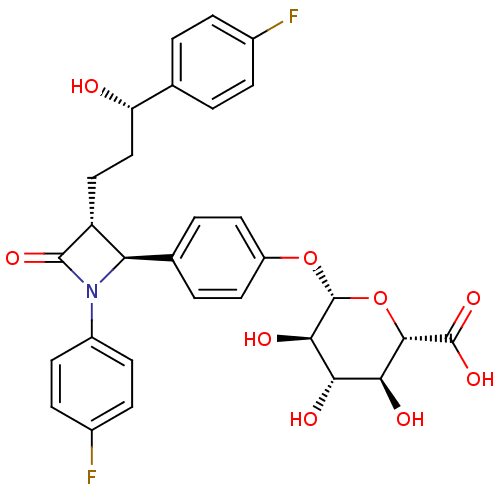
((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-1-(4-fluorophenyl)-...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccc(F)cc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H29F2NO9/c31-17-5-1-15(2-6-17)22(34)14-13-21-23(33(28(21)38)19-9-7-18(32)8-10-19)16-3-11-20(12-4-16)41-30-26(37)24(35)25(36)27(42-30)29(39)40/h1-12,21-27,30,34-37H,13-14H2,(H,39,40)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Mus musculus) | BDBM50300992

((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...)Show SMILES CS(=O)(=O)NCC#Cc1ccc(cc1)N1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C34H35FN2O11S/c1-49(45,46)36-18-2-3-19-4-12-23(13-5-19)37-27(25(32(37)42)16-17-26(38)20-6-10-22(35)11-7-20)21-8-14-24(15-9-21)47-34-30(41)28(39)29(40)31(48-34)33(43)44/h4-15,25-31,34,36,38-41H,16-18H2,1H3,(H,43,44)/t25-,26+,27-,28+,29+,30-,31+,34-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Niemann-Pick C1-like 1 protein
(Canis familiaris) | BDBM50240720

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-1-(4-fluorophenyl)-...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccc(F)cc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H29F2NO9/c31-17-5-1-15(2-6-17)22(34)14-13-21-23(33(28(21)38)19-9-7-18(32)8-10-19)16-3-11-20(12-4-16)41-30-26(37)24(35)25(36)27(42-30)29(39)40/h1-12,21-27,30,34-37H,13-14H2,(H,39,40)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Rattus norvegicus) | BDBM50300993

((2S,3S,4S,5R,6S)-6-{4-[(2S,3R)-3-[(3S)-3-(4-fluoro...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccccc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H30FNO9/c31-18-10-6-16(7-11-18)22(33)15-14-21-23(32(28(21)37)19-4-2-1-3-5-19)17-8-12-20(13-9-17)40-30-26(36)24(34)25(35)27(41-30)29(38)39/h1-13,21-27,30,33-36H,14-15H2,(H,38,39)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50300993

((2S,3S,4S,5R,6S)-6-{4-[(2S,3R)-3-[(3S)-3-(4-fluoro...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccccc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H30FNO9/c31-18-10-6-16(7-11-18)22(33)15-14-21-23(32(28(21)37)19-4-2-1-3-5-19)17-8-12-20(13-9-17)40-30-26(36)24(34)25(35)27(41-30)29(38)39/h1-13,21-27,30,33-36H,14-15H2,(H,38,39)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Rattus norvegicus) | BDBM50300993

((2S,3S,4S,5R,6S)-6-{4-[(2S,3R)-3-[(3S)-3-(4-fluoro...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccccc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H30FNO9/c31-18-10-6-16(7-11-18)22(33)15-14-21-23(32(28(21)37)19-4-2-1-3-5-19)17-8-12-20(13-9-17)40-30-26(36)24(34)25(35)27(41-30)29(38)39/h1-13,21-27,30,33-36H,14-15H2,(H,38,39)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50300993

((2S,3S,4S,5R,6S)-6-{4-[(2S,3R)-3-[(3S)-3-(4-fluoro...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccccc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H30FNO9/c31-18-10-6-16(7-11-18)22(33)15-14-21-23(32(28(21)37)19-4-2-1-3-5-19)17-8-12-20(13-9-17)40-30-26(36)24(34)25(35)27(41-30)29(38)39/h1-13,21-27,30,33-36H,14-15H2,(H,38,39)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50240720

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-1-(4-fluorophenyl)-...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccc(F)cc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H29F2NO9/c31-17-5-1-15(2-6-17)22(34)14-13-21-23(33(28(21)38)19-9-7-18(32)8-10-19)16-3-11-20(12-4-16)41-30-26(37)24(35)25(36)27(42-30)29(39)40/h1-12,21-27,30,34-37H,13-14H2,(H,39,40)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50240720

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-1-(4-fluorophenyl)-...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccc(F)cc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H29F2NO9/c31-17-5-1-15(2-6-17)22(34)14-13-21-23(33(28(21)38)19-9-7-18(32)8-10-19)16-3-11-20(12-4-16)41-30-26(37)24(35)25(36)27(42-30)29(39)40/h1-12,21-27,30,34-37H,13-14H2,(H,39,40)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50258548

((1-(1-naphthoyl)-5-phenylpiperidin-3-yl)(spiro[ind...)Show SMILES O=C(C1CC(CN(C1)C(=O)c1cccc2ccccc12)c1ccccc1)N1CCC2(CC1)C=Cc1ccccc21 |c:37| Show InChI InChI=1S/C36H34N2O2/c39-34(37-21-19-36(20-22-37)18-17-28-12-5-7-16-33(28)36)30-23-29(26-9-2-1-3-10-26)24-38(25-30)35(40)32-15-8-13-27-11-4-6-14-31(27)32/h1-18,29-30H,19-25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Rattus norvegicus) | BDBM50240720

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-1-(4-fluorophenyl)-...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccc(F)cc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H29F2NO9/c31-17-5-1-15(2-6-17)22(34)14-13-21-23(33(28(21)38)19-9-7-18(32)8-10-19)16-3-11-20(12-4-16)41-30-26(37)24(35)25(36)27(42-30)29(39)40/h1-12,21-27,30,34-37H,13-14H2,(H,39,40)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50332397
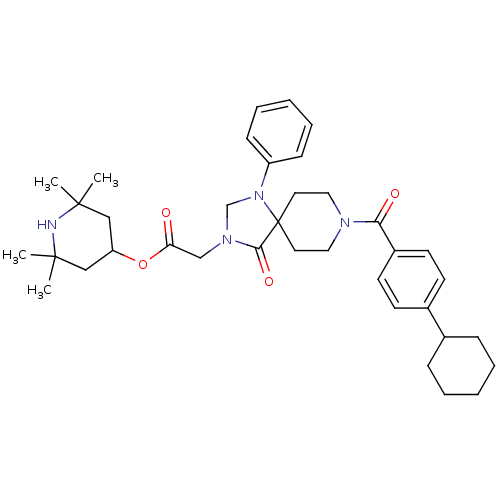
(2,2,6,6-tetramethylpiperidin-4-yl 2-(8-(4-cyclohex...)Show SMILES CC1(C)CC(CC(C)(C)N1)OC(=O)CN1CN(c2ccccc2)C2(CCN(CC2)C(=O)c2ccc(cc2)C2CCCCC2)C1=O Show InChI InChI=1S/C37H50N4O4/c1-35(2)23-31(24-36(3,4)38-35)45-32(42)25-40-26-41(30-13-9-6-10-14-30)37(34(40)44)19-21-39(22-20-37)33(43)29-17-15-28(16-18-29)27-11-7-5-8-12-27/h6,9-10,13-18,27,31,38H,5,7-8,11-12,19-26H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 20: 6929-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.138
BindingDB Entry DOI: 10.7270/Q29K4BG6 |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50258480
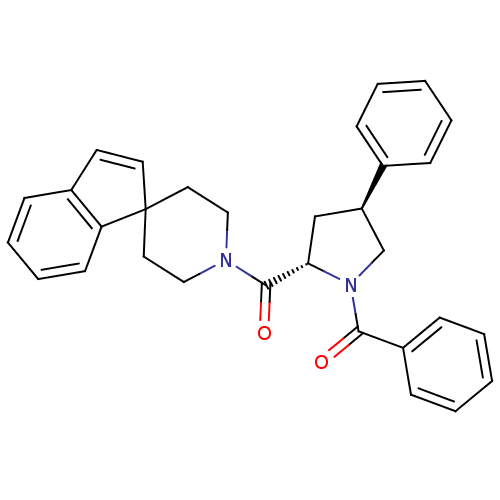
(((2S,4S)-1-benzoyl-4-phenylpyrrolidin-2-yl)(spiro[...)Show SMILES O=C([C@@H]1C[C@H](CN1C(=O)c1ccccc1)c1ccccc1)N1CCC2(CC1)C=Cc1ccccc21 |r,c:31| Show InChI InChI=1S/C31H30N2O2/c34-29(25-12-5-2-6-13-25)33-22-26(23-9-3-1-4-10-23)21-28(33)30(35)32-19-17-31(18-20-32)16-15-24-11-7-8-14-27(24)31/h1-16,26,28H,17-22H2/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50332389

(CHEMBL1632092 | ethyl 1-(2-(8-(4-cyclohexylbenzoyl...)Show SMILES CCOC(=O)C1CCN(CC1)C(=O)CN1CN(c2ccccc2)C2(CCN(CC2)C(=O)c2ccc(cc2)C2CCCCC2)C1=O Show InChI InChI=1S/C36H46N4O5/c1-2-45-34(43)30-17-21-37(22-18-30)32(41)25-39-26-40(31-11-7-4-8-12-31)36(35(39)44)19-23-38(24-20-36)33(42)29-15-13-28(14-16-29)27-9-5-3-6-10-27/h4,7-8,11-16,27,30H,2-3,5-6,9-10,17-26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 20: 6929-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.138
BindingDB Entry DOI: 10.7270/Q29K4BG6 |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50332404

(2-(8-(4-tert-butylbenzoyl)-4-oxo-1-phenyl-1,3,8-tr...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)N1CCC2(CC1)N(CN(CC(=O)NCCO)C2=O)c1ccccc1 Show InChI InChI=1S/C28H36N4O4/c1-27(2,3)22-11-9-21(10-12-22)25(35)30-16-13-28(14-17-30)26(36)31(19-24(34)29-15-18-33)20-32(28)23-7-5-4-6-8-23/h4-12,33H,13-20H2,1-3H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 20: 6929-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.138
BindingDB Entry DOI: 10.7270/Q29K4BG6 |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50332437

(CHEMBL1631064 | tert-butyl 4-(2-(8-(4-tert-butylbe...)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)C(=O)CN1CN(c2ccccc2)C2(CCN(CC2)C(=O)c2ccc(cc2)C(C)(C)C)C1=O Show InChI InChI=1S/C35H47N5O5/c1-33(2,3)27-14-12-26(13-15-27)30(42)37-18-16-35(17-19-37)31(43)39(25-40(35)28-10-8-7-9-11-28)24-29(41)36-20-22-38(23-21-36)32(44)45-34(4,5)6/h7-15H,16-25H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 20: 6929-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.138
BindingDB Entry DOI: 10.7270/Q29K4BG6 |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50332446
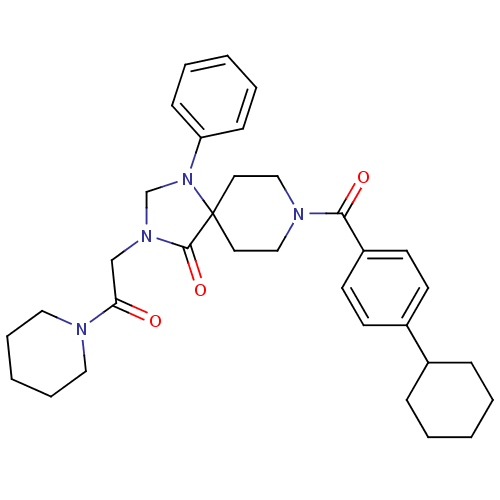
(8-(4-cyclohexylbenzoyl)-3-(2-oxo-2-(piperidin-1-yl...)Show SMILES O=C(CN1CN(c2ccccc2)C2(CCN(CC2)C(=O)c2ccc(cc2)C2CCCCC2)C1=O)N1CCCCC1 Show InChI InChI=1S/C33H42N4O3/c38-30(34-20-8-3-9-21-34)24-36-25-37(29-12-6-2-7-13-29)33(32(36)40)18-22-35(23-19-33)31(39)28-16-14-27(15-17-28)26-10-4-1-5-11-26/h2,6-7,12-17,26H,1,3-5,8-11,18-25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 20: 6929-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.138
BindingDB Entry DOI: 10.7270/Q29K4BG6 |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50332452
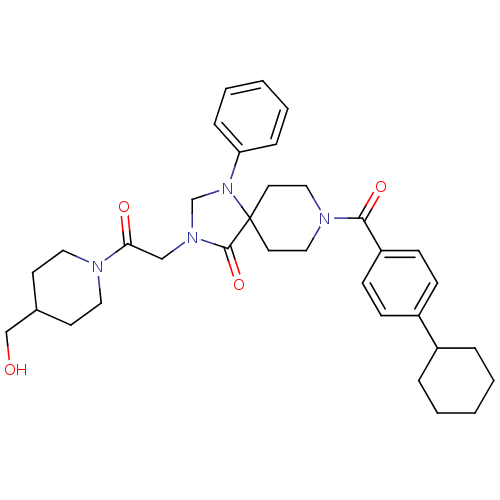
(8-(4-cyclohexylbenzoyl)-3-(2-(4-(hydroxymethyl)pip...)Show SMILES OCC1CCN(CC1)C(=O)CN1CN(c2ccccc2)C2(CCN(CC2)C(=O)c2ccc(cc2)C2CCCCC2)C1=O Show InChI InChI=1S/C34H44N4O4/c39-24-26-15-19-35(20-16-26)31(40)23-37-25-38(30-9-5-2-6-10-30)34(33(37)42)17-21-36(22-18-34)32(41)29-13-11-28(12-14-29)27-7-3-1-4-8-27/h2,5-6,9-14,26-27,39H,1,3-4,7-8,15-25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 20: 6929-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.138
BindingDB Entry DOI: 10.7270/Q29K4BG6 |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Mus musculus) | BDBM50300993

((2S,3S,4S,5R,6S)-6-{4-[(2S,3R)-3-[(3S)-3-(4-fluoro...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccccc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H30FNO9/c31-18-10-6-16(7-11-18)22(33)15-14-21-23(32(28(21)37)19-4-2-1-3-5-19)17-8-12-20(13-9-17)40-30-26(36)24(34)25(35)27(41-30)29(38)39/h1-13,21-27,30,33-36H,14-15H2,(H,38,39)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 5033-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.051
BindingDB Entry DOI: 10.7270/Q28P61GZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data