

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061306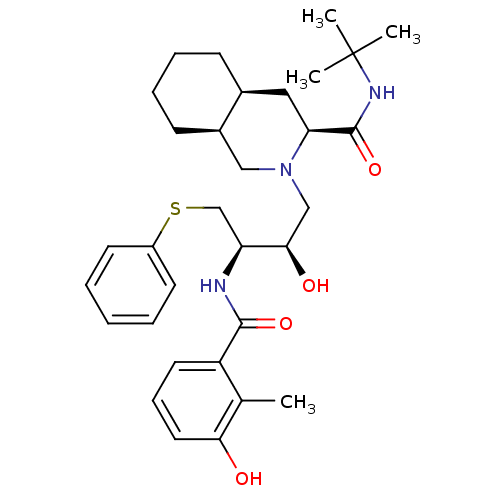 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061307 (AG-1254 | CHEMBL128696 | N-[(1R,2R)-3-(2-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061308 ((3S,4aS,8aS)-2-[(2R,3S)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061305 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50028854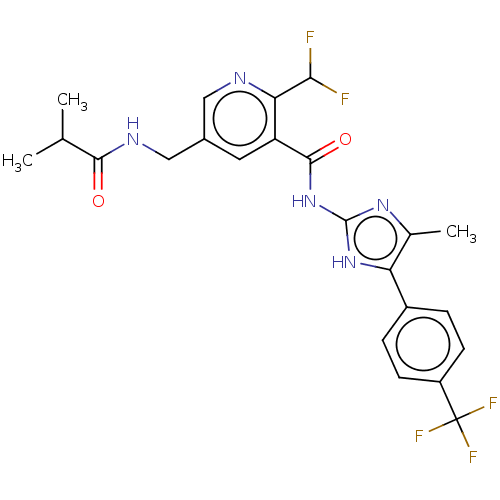 (CHEMBL3342693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 59: 194-205 (2016) Article DOI: 10.1021/acs.jmedchem.5b01249 BindingDB Entry DOI: 10.7270/Q2474CQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM823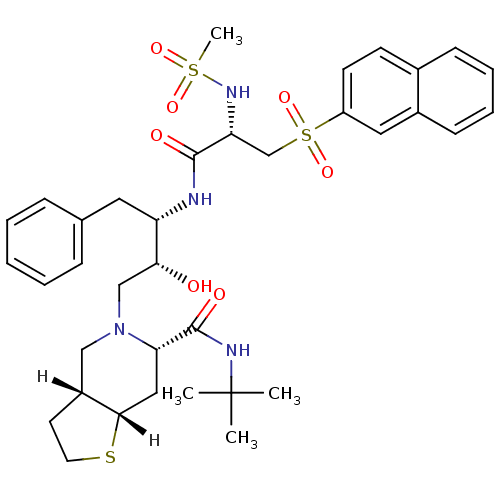 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM817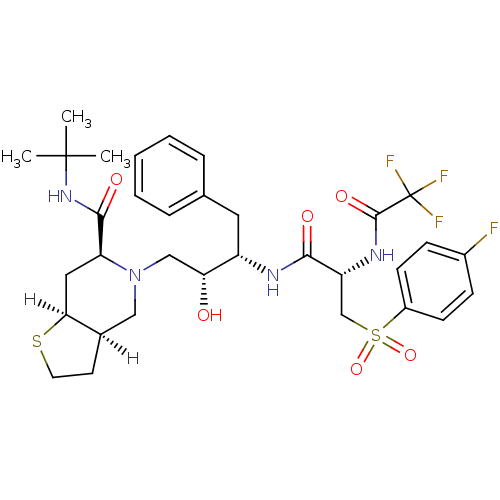 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-3-[(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM817 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-3-[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM821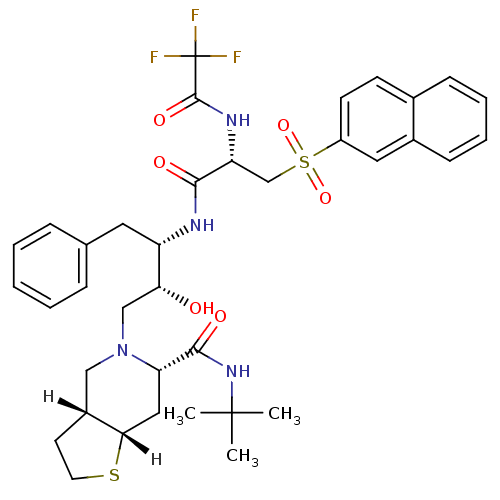 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM821 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM823 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM825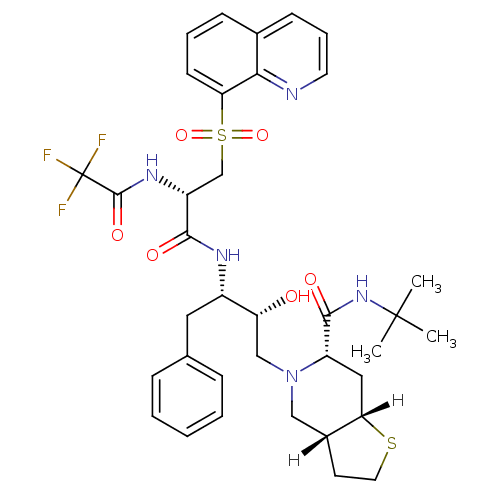 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-4-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM825 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-4-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM819 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-3-[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM819 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-3-[(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121513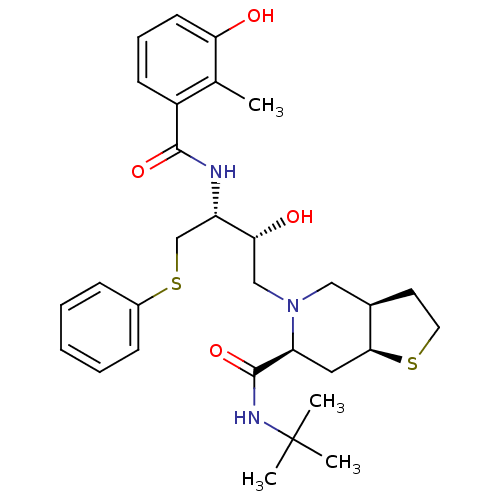 ((3aR,6S,7aS)-5-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration required against HIV-1 protease | Bioorg Med Chem Lett 5: 2885-2890 (1995) Article DOI: 10.1016/0960-894X(95)00506-O BindingDB Entry DOI: 10.7270/Q2930T45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM824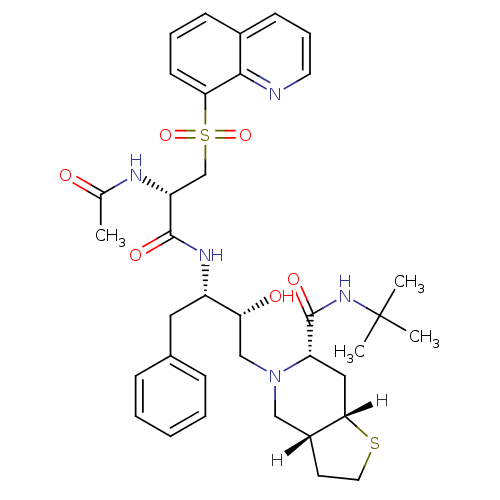 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM824 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM820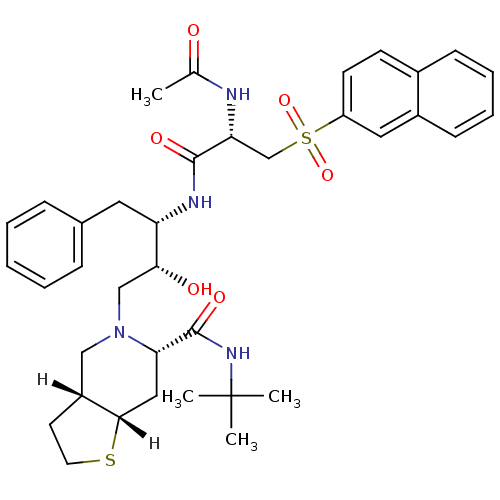 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM811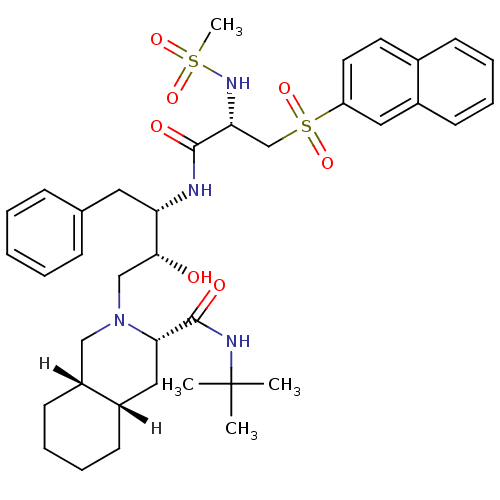 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM811 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM820 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285964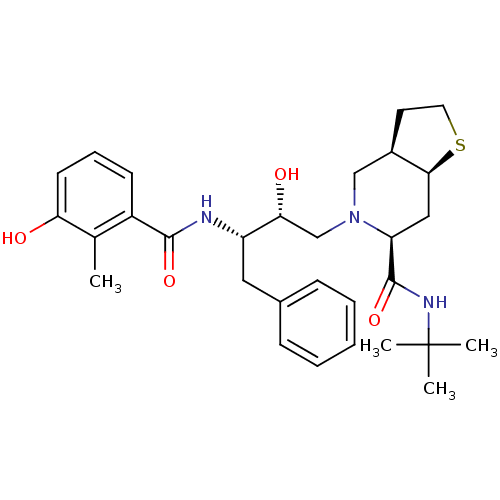 ((3aR,6S,7aS)-5-[(2R,3S)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration required against HIV-1 protease | Bioorg Med Chem Lett 5: 2885-2890 (1995) Article DOI: 10.1016/0960-894X(95)00506-O BindingDB Entry DOI: 10.7270/Q2930T45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM807 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-3-[(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM807 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-3-[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM826 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM826 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50028854 (CHEMBL3342693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysis | J Med Chem 59: 194-205 (2016) Article DOI: 10.1021/acs.jmedchem.5b01249 BindingDB Entry DOI: 10.7270/Q2474CQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration required against HIV-1 protease | Bioorg Med Chem Lett 5: 2885-2890 (1995) Article DOI: 10.1016/0960-894X(95)00506-O BindingDB Entry DOI: 10.7270/Q2930T45 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM815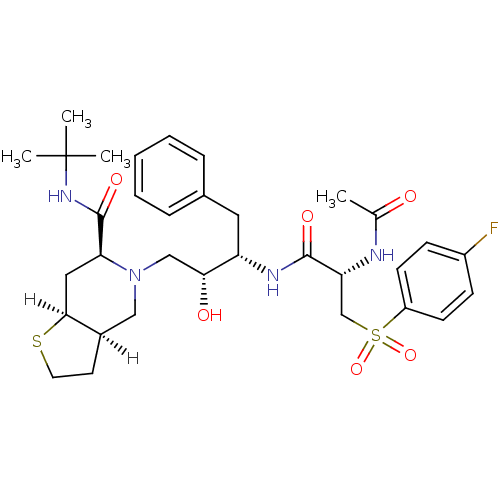 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM815 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM812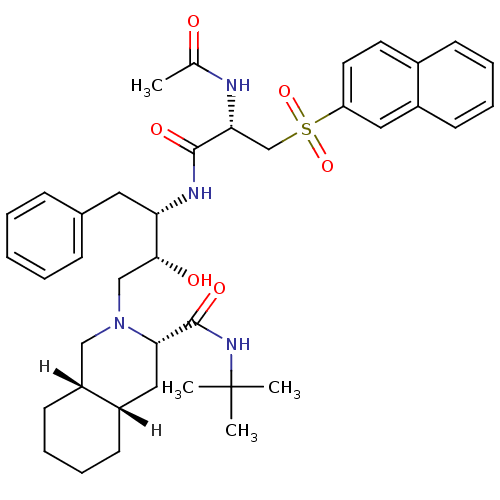 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-2-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM812 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-2-ace...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM813 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-2-ace...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM813 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-2-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM822 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM822 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3R)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM816 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3R)-3-[(2S)-2-ace...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM816 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3R)-3-[(2S)-2-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM808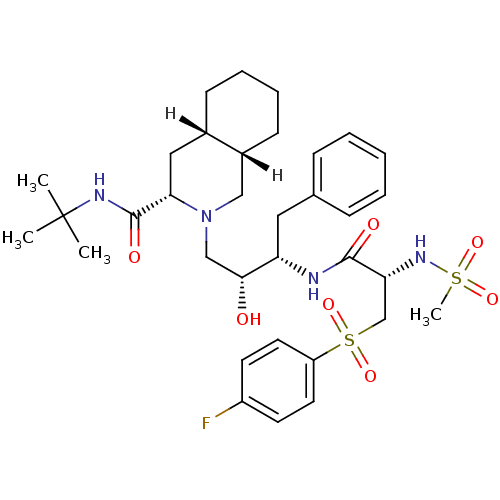 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-3-[(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM808 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-3-[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM830 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(2-methylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM830 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(2-methylp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50142252 (CHEMBL3758953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysis | J Med Chem 59: 194-205 (2016) Article DOI: 10.1021/acs.jmedchem.5b01249 BindingDB Entry DOI: 10.7270/Q2474CQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50142254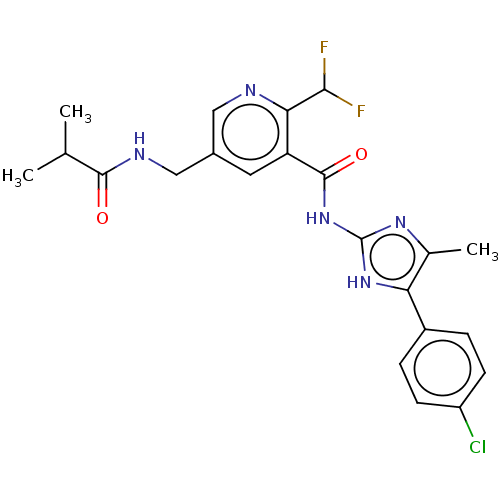 (CHEMBL3759288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysis | J Med Chem 59: 194-205 (2016) Article DOI: 10.1021/acs.jmedchem.5b01249 BindingDB Entry DOI: 10.7270/Q2474CQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50142253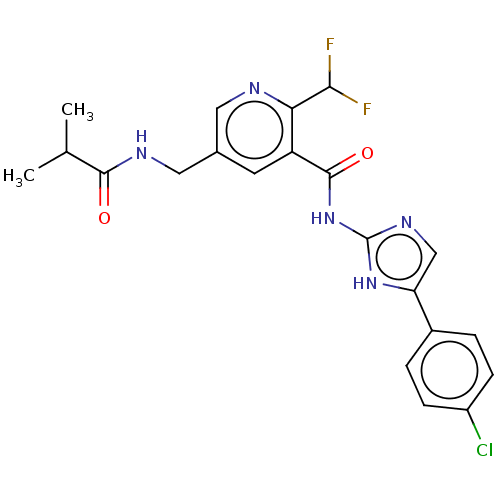 (CHEMBL3758924) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysis | J Med Chem 59: 194-205 (2016) Article DOI: 10.1021/acs.jmedchem.5b01249 BindingDB Entry DOI: 10.7270/Q2474CQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285962 (CHEMBL318217 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration required against HIV-1 protease | Bioorg Med Chem Lett 5: 2885-2890 (1995) Article DOI: 10.1016/0960-894X(95)00506-O BindingDB Entry DOI: 10.7270/Q2930T45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM809 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 122 total ) | Next | Last >> |