

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412656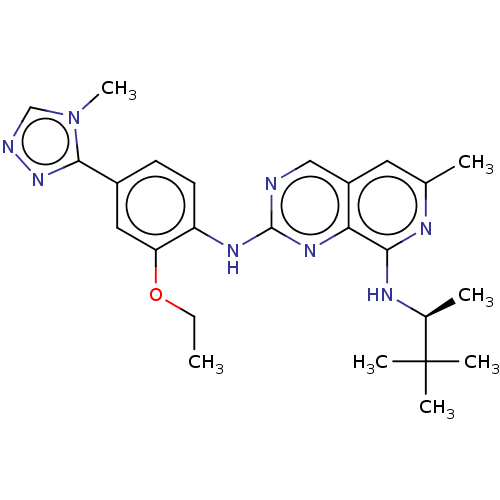 (US10399974, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241208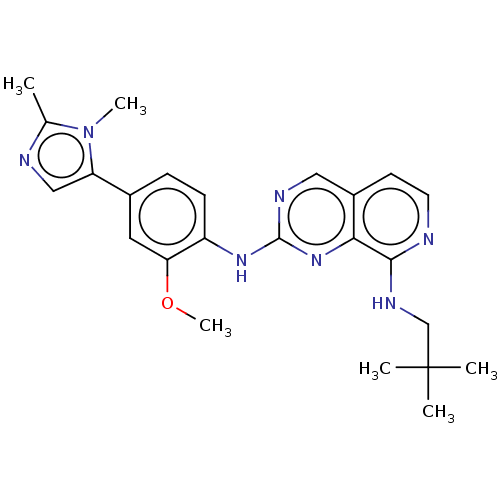 (US11046688, Example 50 | US9409907, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464039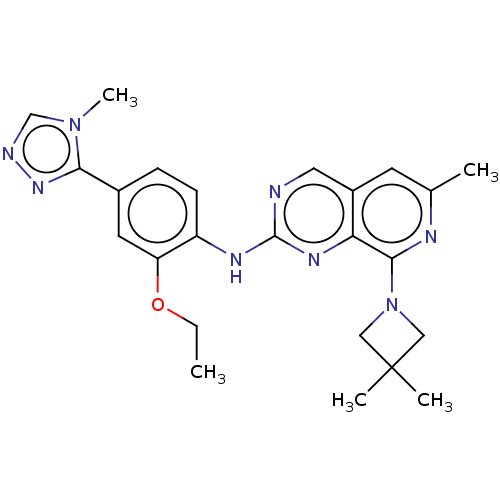 (CHEMBL4245639) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241333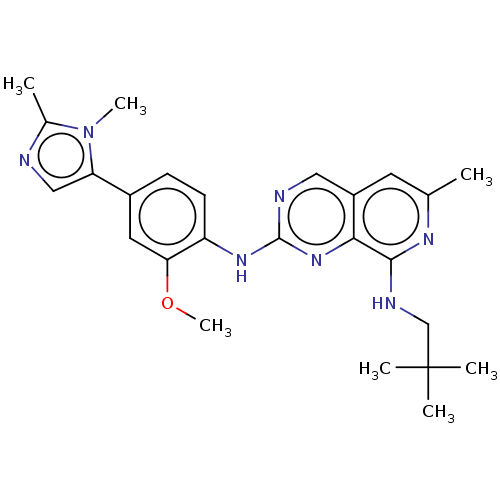 (US10479788, Example 177 | US11046688, Example 177 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241338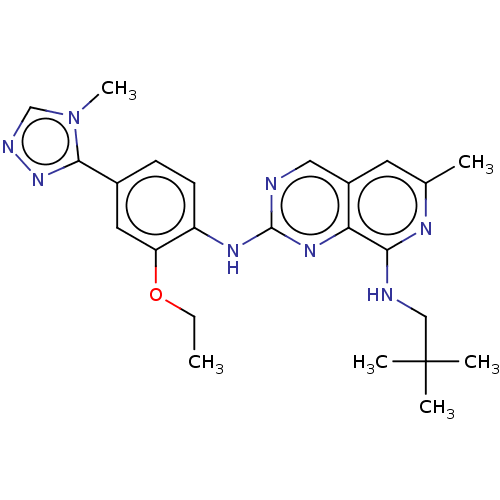 (US10479788, Example 182 | US11046688, Example 182 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611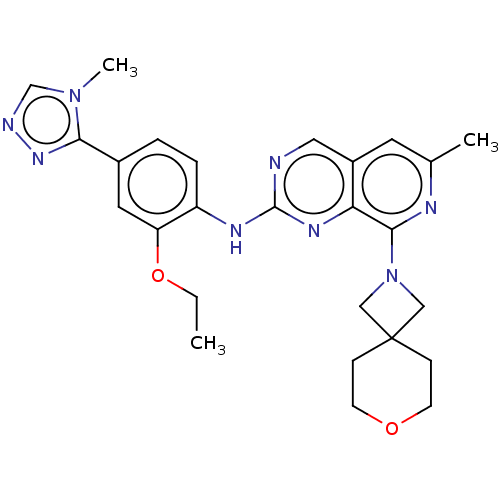 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464037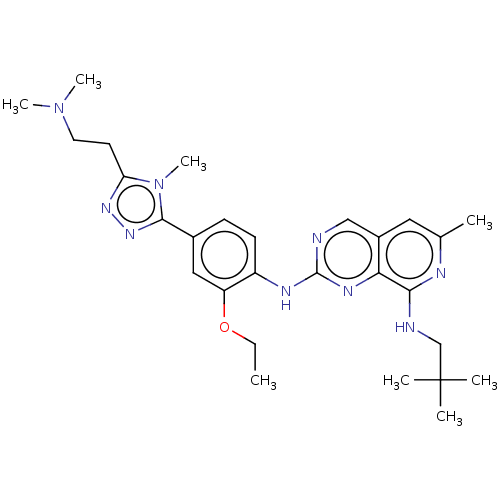 (CHEMBL4240502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412614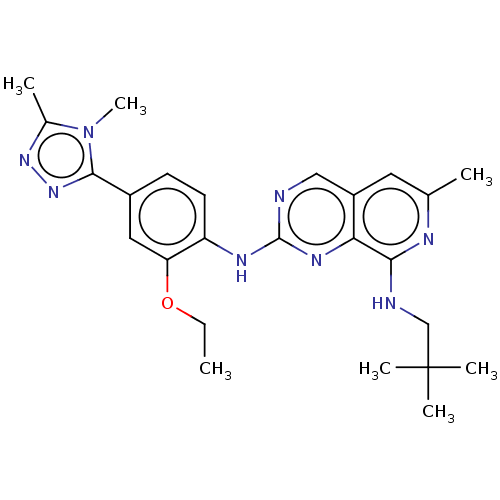 (N2-(4-(4,5-dimethyl-4H-1,2,4-triazol-3-yl)-2-ethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1D receptor beta | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241335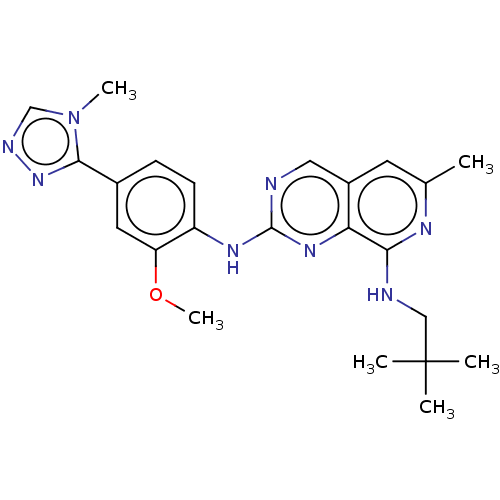 (US9409907, 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464040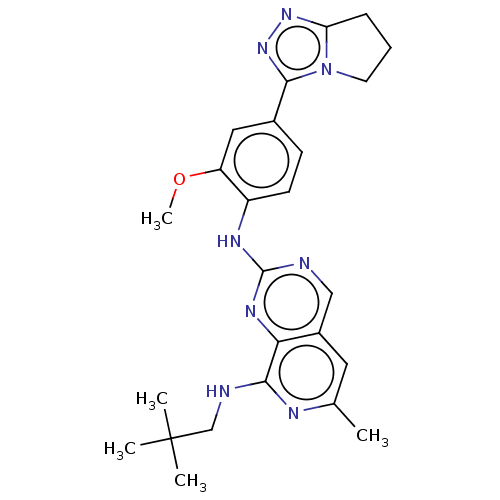 (CHEMBL4251352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412610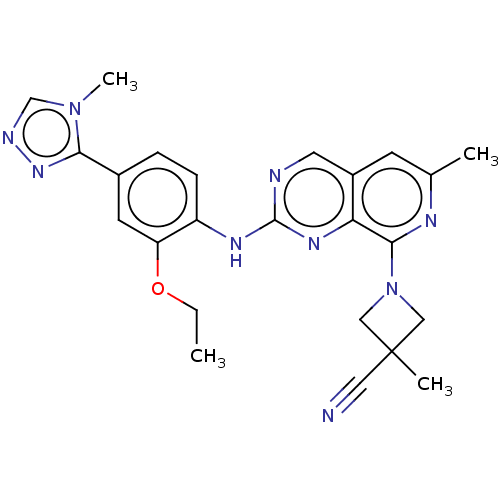 (1-(2-((2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464038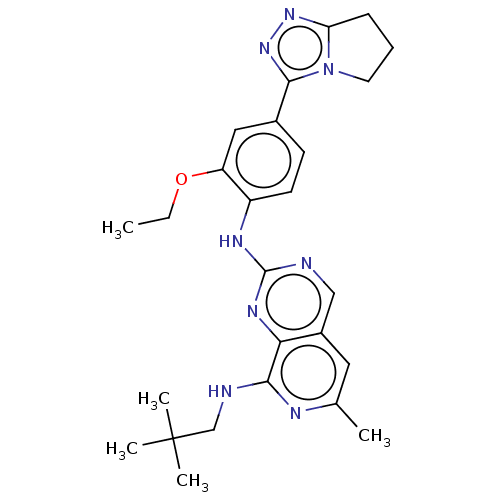 (CHEMBL4250961) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226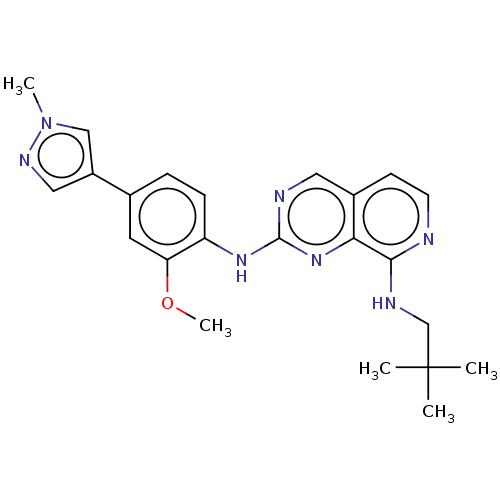 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241207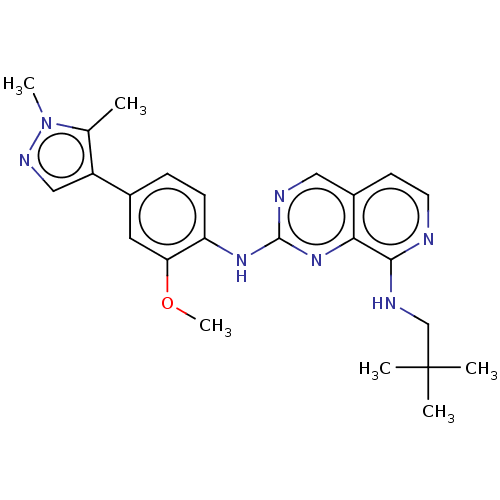 (US11046688, Example 49 | US9409907, 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412658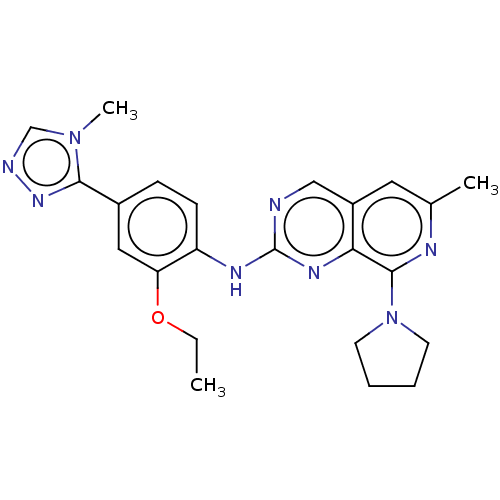 (US10399974, Example 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1D receptor alpha | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469882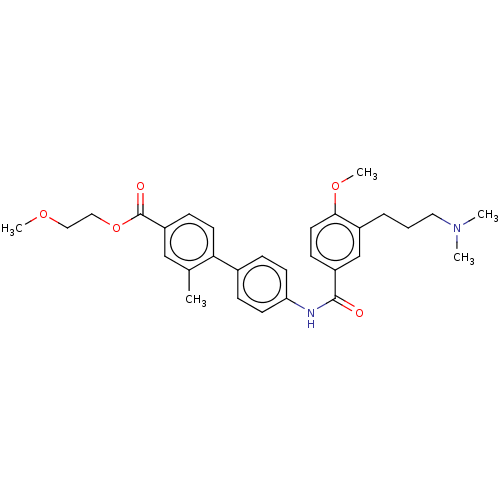 (CHEMBL73446) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469879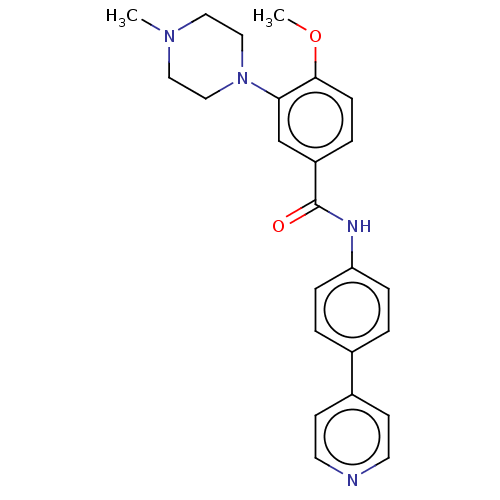 (CHEMBL72088) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412644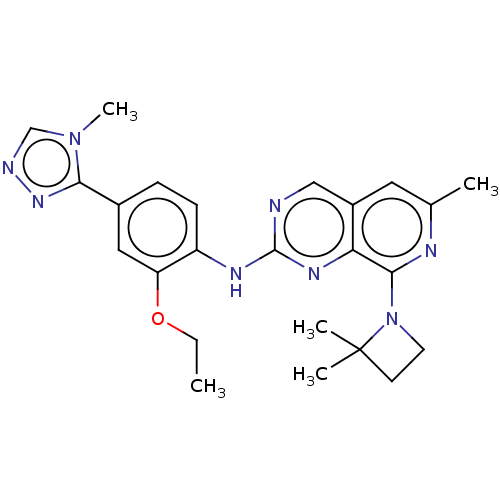 (US10399974, Example 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469881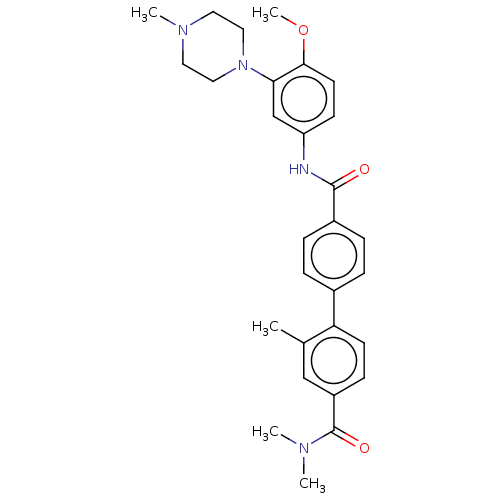 (CHEMBL311150) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304836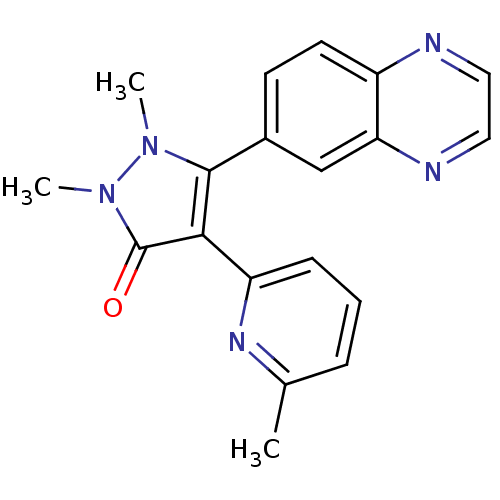 (1,2-dimethyl-4-(6-methylpyridin-2-yl)-5-(quinoxali...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469875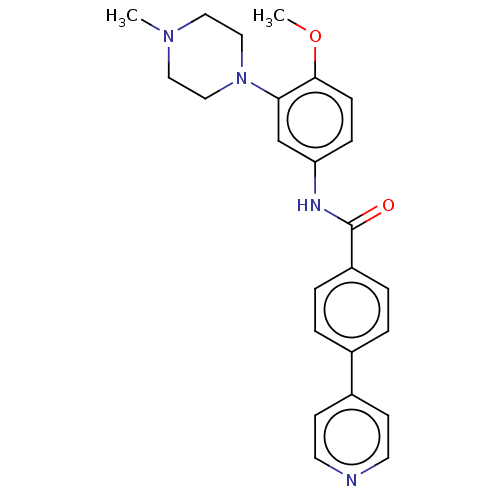 (CHEMBL72981) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304849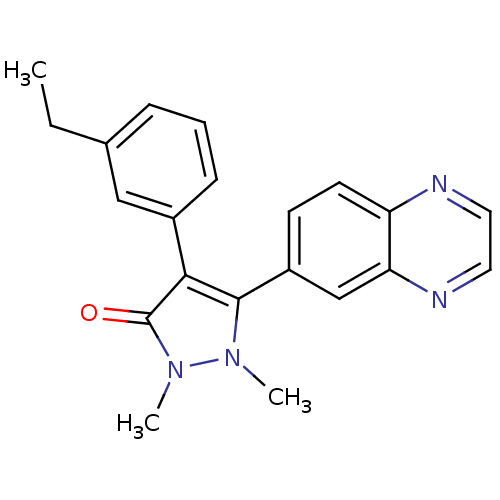 (4-(3-ethylphenyl)-1,2-dimethyl-5-(quinoxalin-6-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469878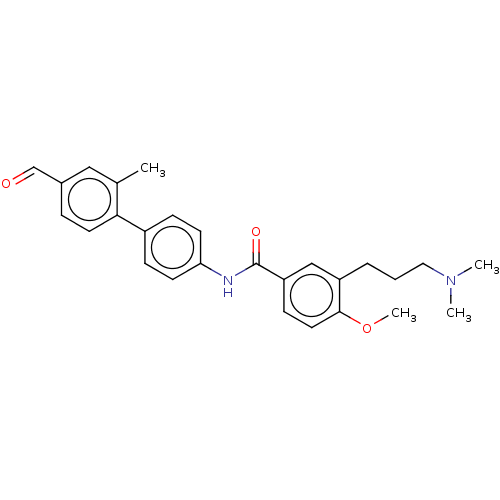 (CHEMBL72700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469876 (CHEMBL306384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50469881 (CHEMBL311150) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50469876 (CHEMBL306384) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM241331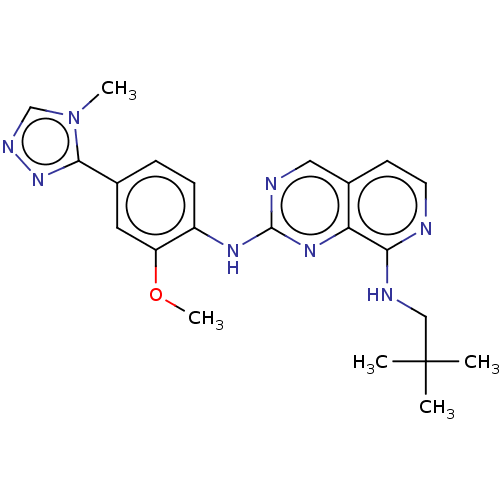 (US10479788, Example 175 | US11046688, Example 175 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal His-tagged CDK2/cyclinA expressed in baculovirus expression system using 5FAM- peptide18 as substrate afte... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50060518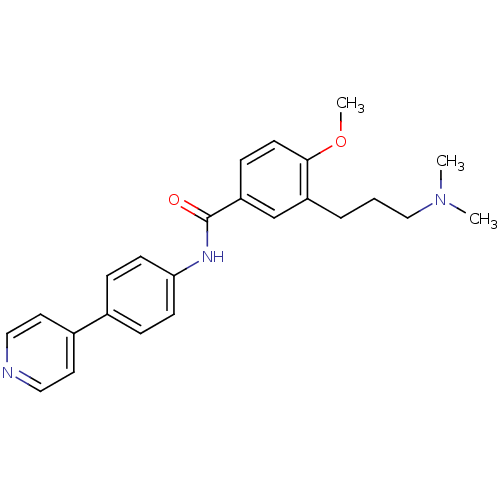 (3-(3-Dimethylamino-propyl)-4-methoxy-N-(4-pyridin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1/3 (Rattus norvegicus) | BDBM50496697 (CHEMBL3218460) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Antagonist activity at LPAR1/LPAR3 in rat glioma C62B cells assessed as inhibition of LPA-induced reduction in isoproterenol-stimulated [3H]cAMP accu... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304835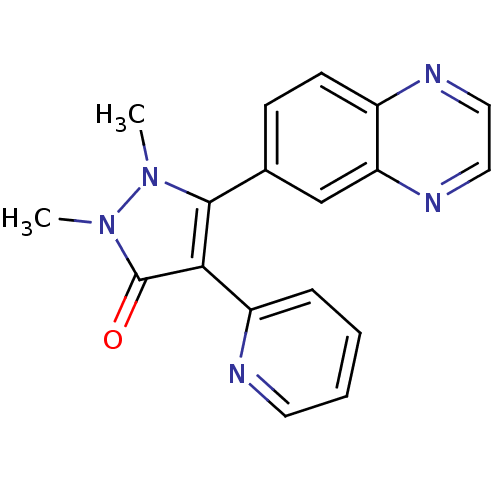 (1,2-dimethyl-4-(pyridin-2-yl)-5-(quinoxalin-6-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304864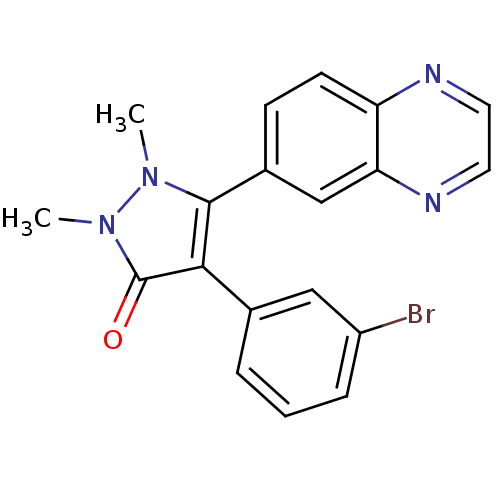 (4-(3-bromophenyl)-1,2-dimethyl-5-(quinoxalin-6-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304850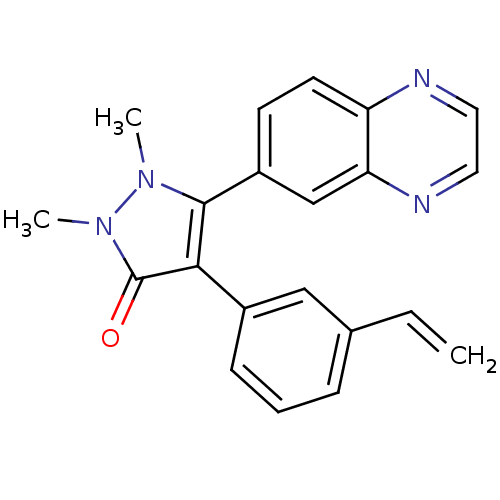 (1,2-dimethyl-5-(quinoxalin-6-yl)-4-(3-vinylphenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50110208 (4-(4-(benzo[d][1,3]dioxol-5-yl)-5-(pyridin-2-yl)-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50146232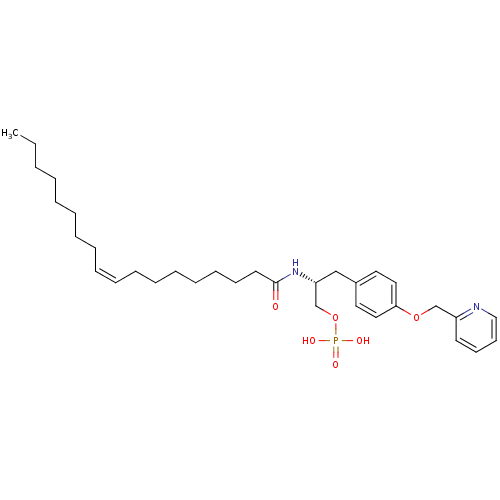 (CHEMBL440696 | Phosphoric acid mono-{(R)-2-((Z)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304863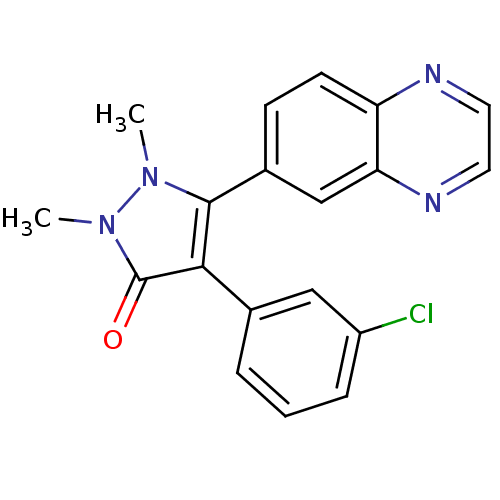 (4-(3-chlorophenyl)-1,2-dimethyl-5-(quinoxalin-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50150007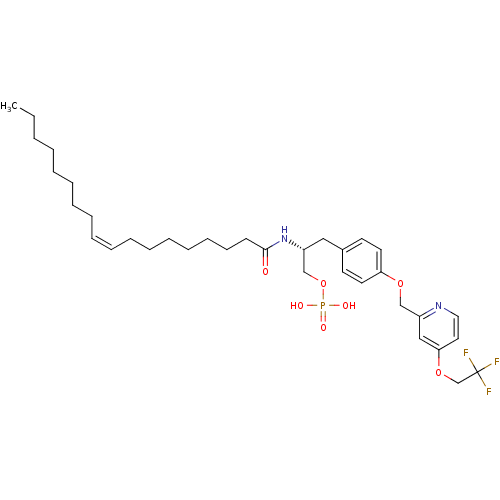 (CHEMBL183221 | Phosphoric acid mono-((R)-2-((Z)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304865 (CHEMBL607429 | N-(3-(1,2-dimethyl-3-oxo-5-(quinoxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304840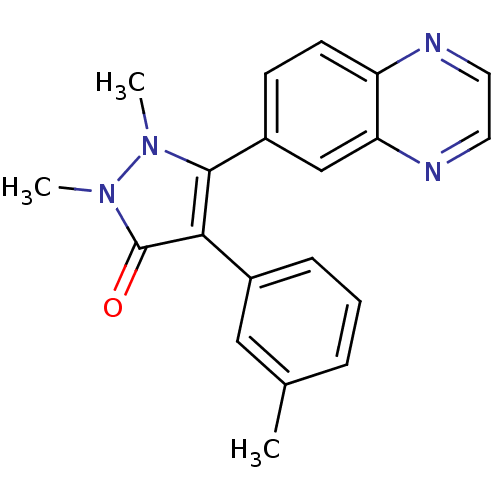 (1,2-dimethyl-5-(quinoxalin-6-yl)-4-m-tolyl-1H-pyra...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304853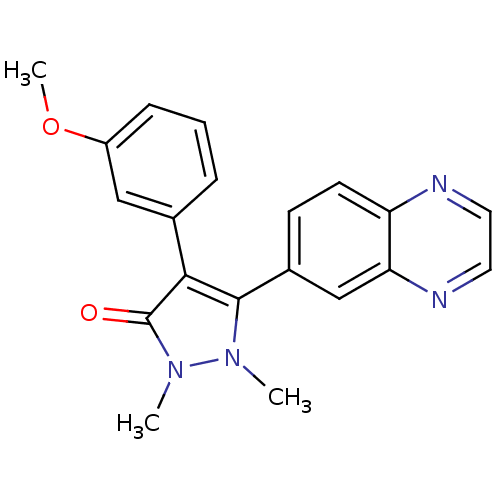 (4-(3-methoxyphenyl)-1,2-dimethyl-5-(quinoxalin-6-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241206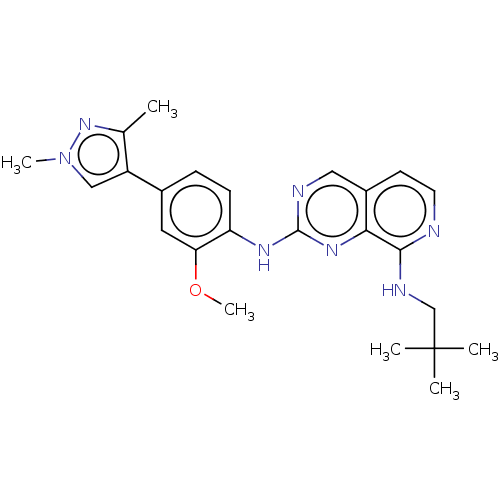 (US11046688, Example 48 | US9409907, 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304858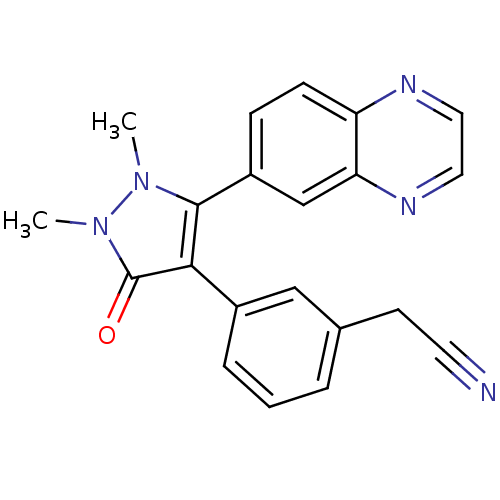 (2-(3-(1,2-dimethyl-3-oxo-5-(quinoxalin-6-yl)-2,3-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50149996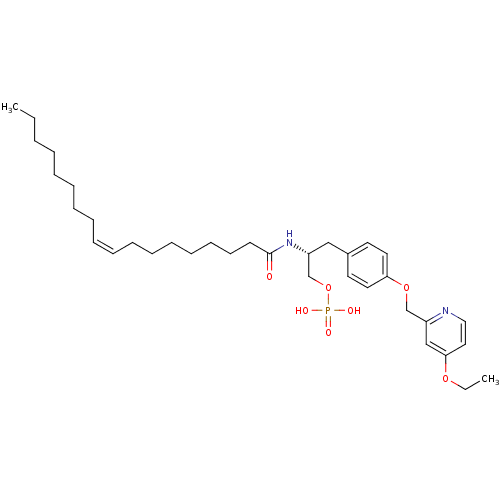 (CHEMBL183143 | Phosphoric acid mono-[3-[4-(4-ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50149997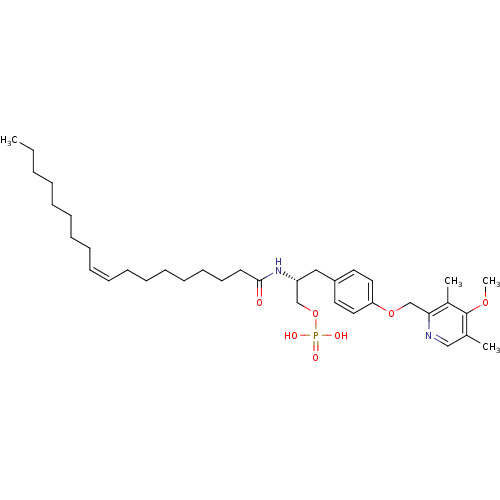 (CHEMBL362053 | Phosphoric acid mono-[3-[4-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469877 (CHEMBL72092) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50304855 (4-(3-(methoxymethyl)phenyl)-1,2-dimethyl-5-(quinox...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of [3H]HTS446284 from human recombinant His-tagged TGFbetaR1 after 1 hr by scintillation counting | Bioorg Med Chem Lett 20: 326-9 (2010) Article DOI: 10.1016/j.bmcl.2009.10.108 BindingDB Entry DOI: 10.7270/Q2RN37ZK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50150000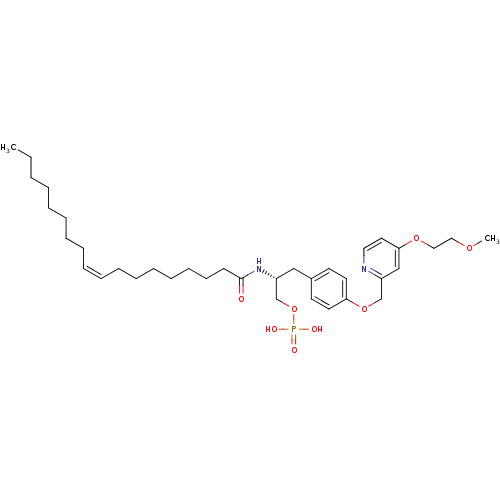 (CHEMBL182446 | Phosphoric acid mono-[(R)-3-{4-[4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 741 total ) | Next | Last >> |