 Found 425 hits with Last Name = 'cecchetti' and Initial = 'o'
Found 425 hits with Last Name = 'cecchetti' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195108
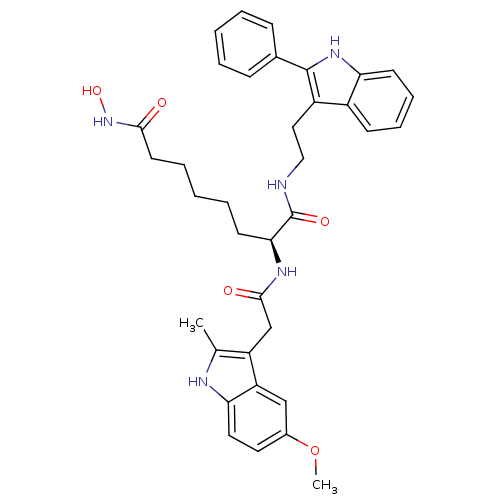
((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM25146
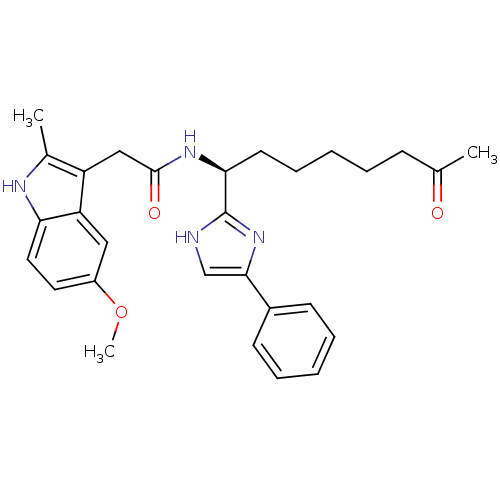
(2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-[(1S)-7-oxo...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)c3nc(c[nH]3)-c3ccccc3)c2c1 |r| Show InChI InChI=1S/C29H34N4O3/c1-19(34)10-6-4-9-13-26(29-30-18-27(33-29)21-11-7-5-8-12-21)32-28(35)17-23-20(2)31-25-15-14-22(36-3)16-24(23)25/h5,7-8,11-12,14-16,18,26,31H,4,6,9-10,13,17H2,1-3H3,(H,30,33)(H,32,35)/t26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50258539
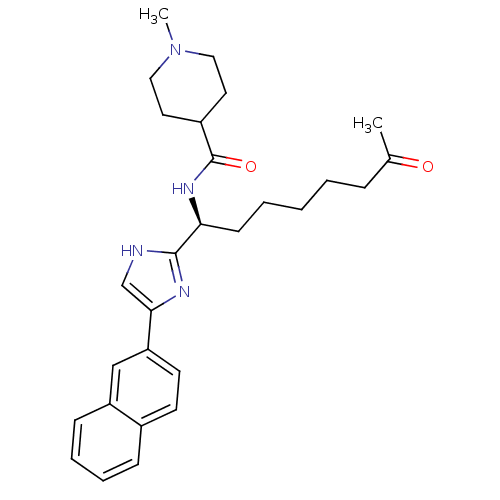
((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM25146

(2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-[(1S)-7-oxo...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)c3nc(c[nH]3)-c3ccccc3)c2c1 |r| Show InChI InChI=1S/C29H34N4O3/c1-19(34)10-6-4-9-13-26(29-30-18-27(33-29)21-11-7-5-8-12-21)32-28(35)17-23-20(2)31-25-15-14-22(36-3)16-24(23)25/h5,7-8,11-12,14-16,18,26,31H,4,6,9-10,13,17H2,1-3H3,(H,30,33)(H,32,35)/t26-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50258539

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM25146

(2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-[(1S)-7-oxo...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)c3nc(c[nH]3)-c3ccccc3)c2c1 |r| Show InChI InChI=1S/C29H34N4O3/c1-19(34)10-6-4-9-13-26(29-30-18-27(33-29)21-11-7-5-8-12-21)32-28(35)17-23-20(2)31-25-15-14-22(36-3)16-24(23)25/h5,7-8,11-12,14-16,18,26,31H,4,6,9-10,13,17H2,1-3H3,(H,30,33)(H,32,35)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC7 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50258539

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC7 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC7 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM25146

(2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-[(1S)-7-oxo...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)c3nc(c[nH]3)-c3ccccc3)c2c1 |r| Show InChI InChI=1S/C29H34N4O3/c1-19(34)10-6-4-9-13-26(29-30-18-27(33-29)21-11-7-5-8-12-21)32-28(35)17-23-20(2)31-25-15-14-22(36-3)16-24(23)25/h5,7-8,11-12,14-16,18,26,31H,4,6,9-10,13,17H2,1-3H3,(H,30,33)(H,32,35)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50258539

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50175036
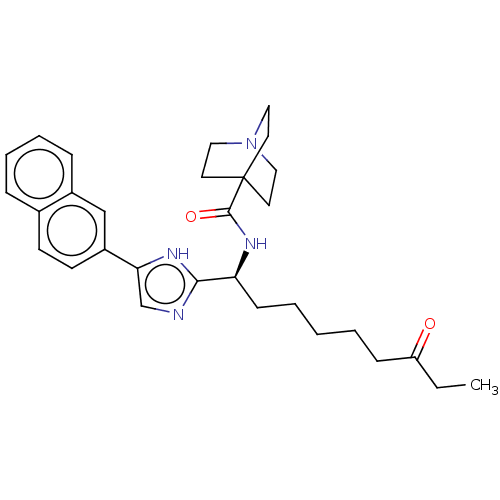
(CHEMBL3809599)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H11Cl2F3N2OS/c19-13-2-1-3-14(20)12(13)8-16-24-9-15(27-16)17(26)25-11-6-4-10(5-7-11)18(21,22)23/h1-7,9H,8H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged HDAC1 expressed in insect cells preincubated for 10 mins followed by addition of FLUOR DE LYS as fluoresce... |
ACS Med Chem Lett 7: 454-9 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00468
BindingDB Entry DOI: 10.7270/Q2S184FM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM25168
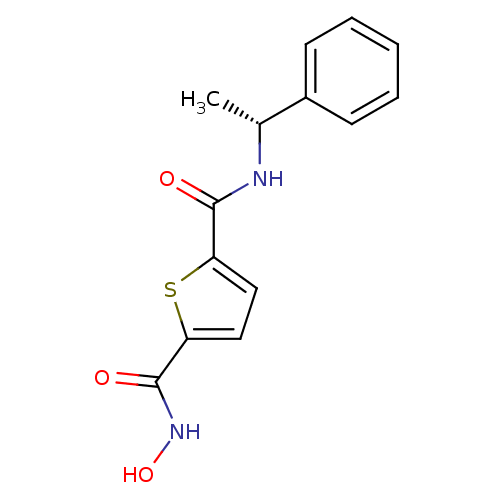
(2-N-hydroxy-5-N-[(1R)-1-phenylethyl]thiophene-2,5-...)Show SMILES C[C@@H](NC(=O)c1ccc(s1)C(=O)NO)c1ccccc1 |r| Show InChI InChI=1S/C14H14N2O3S/c1-9(10-5-3-2-4-6-10)15-13(17)11-7-8-12(20-11)14(18)16-19/h2-9,19H,1H3,(H,15,17)(H,16,18)/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | 37 |
IRBM/Merck
| Assay Description
The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... |
Bioorg Med Chem Lett 18: 3456-61 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.026
BindingDB Entry DOI: 10.7270/Q2MS3R28 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50245989
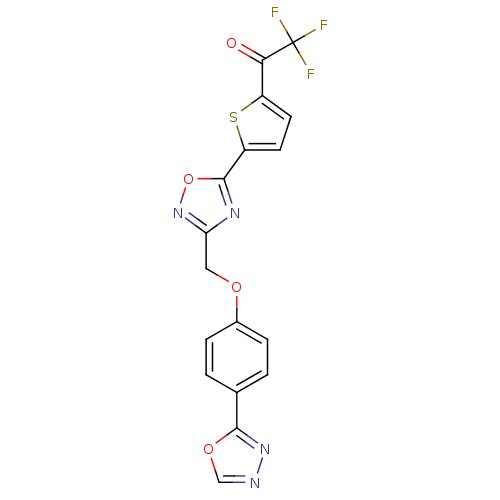
(1-(5-(3-((4-(1,3,4-oxadiazol-2-yl)phenoxy)methyl)-...)Show SMILES FC(F)(F)C(=O)c1ccc(s1)-c1nc(COc2ccc(cc2)-c2nnco2)no1 Show InChI InChI=1S/C17H9F3N4O4S/c18-17(19,20)14(25)11-5-6-12(29-11)16-22-13(24-28-16)7-26-10-3-1-9(2-4-10)15-23-21-8-27-15/h1-6,8H,7H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged HDAC4 catalytic domain (unknown origin) expressed in Escherichia coli |
Bioorg Med Chem Lett 18: 6083-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.076
BindingDB Entry DOI: 10.7270/Q2TD9X6G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM25168

(2-N-hydroxy-5-N-[(1R)-1-phenylethyl]thiophene-2,5-...)Show SMILES C[C@@H](NC(=O)c1ccc(s1)C(=O)NO)c1ccccc1 |r| Show InChI InChI=1S/C14H14N2O3S/c1-9(10-5-3-2-4-6-10)15-13(17)11-7-8-12(20-11)14(18)16-19/h2-9,19H,1H3,(H,15,17)(H,16,18)/t9-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 25 |
IRBM/Merck
| Assay Description
The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... |
Bioorg Med Chem Lett 18: 3456-61 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.026
BindingDB Entry DOI: 10.7270/Q2MS3R28 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50258539

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258539

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminally flag tagged HDAC1 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50258574
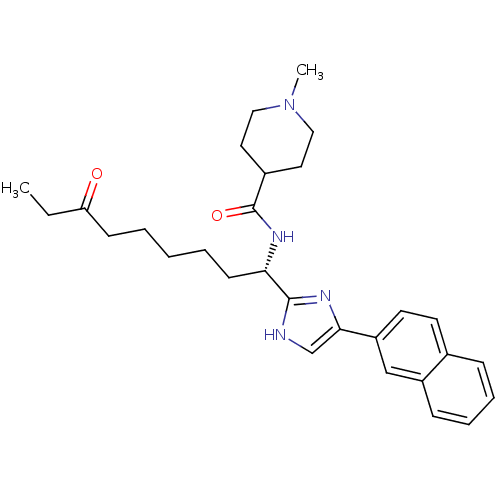
((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CCN(C)CC1)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C29H38N4O2/c1-3-25(34)11-5-4-6-12-26(32-29(35)22-15-17-33(2)18-16-22)28-30-20-27(31-28)24-14-13-21-9-7-8-10-23(21)19-24/h7-10,13-14,19-20,22,26H,3-6,11-12,15-18H2,1-2H3,(H,30,31)(H,32,35)/t26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258574

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CCN(C)CC1)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C29H38N4O2/c1-3-25(34)11-5-4-6-12-26(32-29(35)22-15-17-33(2)18-16-22)28-30-20-27(31-28)24-14-13-21-9-7-8-10-23(21)19-24/h7-10,13-14,19-20,22,26H,3-6,11-12,15-18H2,1-2H3,(H,30,31)(H,32,35)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminally flag tagged HDAC1 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM25144
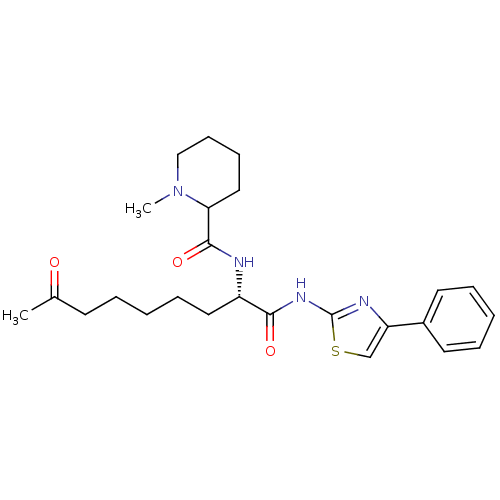
((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM25144

((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM25172
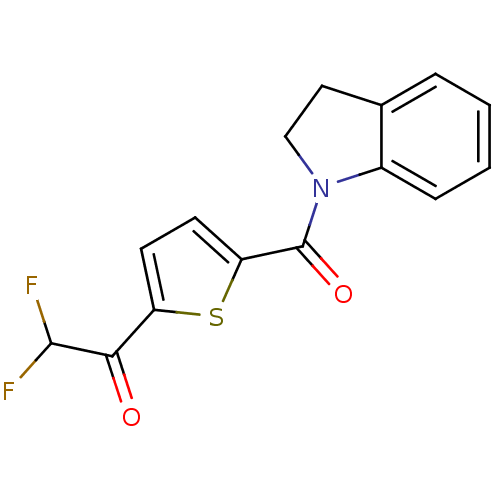
(1-[5-(2,3-dihydro-1H-indol-1-ylcarbonyl)thiophen-2...)Show InChI InChI=1S/C15H11F2NO2S/c16-14(17)13(19)11-5-6-12(21-11)15(20)18-8-7-9-3-1-2-4-10(9)18/h1-6,14H,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 25 |
IRBM/Merck
| Assay Description
The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... |
Bioorg Med Chem Lett 18: 3456-61 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.026
BindingDB Entry DOI: 10.7270/Q2MS3R28 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50245988
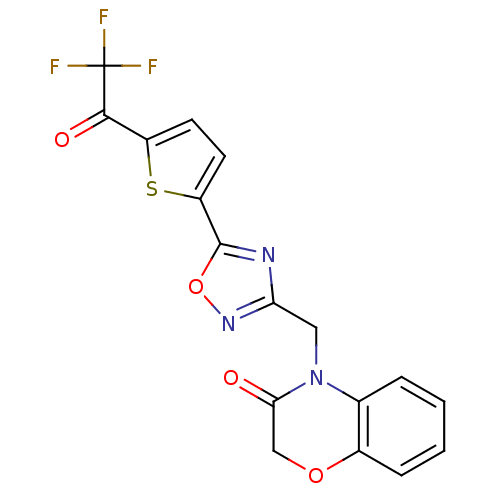
(4-((5-(5-(2,2,2-trifluoroacetyl)thiophen-2-yl)-1,2...)Show SMILES FC(F)(F)C(=O)c1ccc(s1)-c1nc(CN2C(=O)COc3ccccc23)no1 Show InChI InChI=1S/C17H10F3N3O4S/c18-17(19,20)15(25)11-5-6-12(28-11)16-21-13(22-27-16)7-23-9-3-1-2-4-10(9)26-8-14(23)24/h1-6H,7-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged HDAC4 catalytic domain (unknown origin) expressed in Escherichia coli |
Bioorg Med Chem Lett 18: 6083-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.076
BindingDB Entry DOI: 10.7270/Q2TD9X6G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50245986
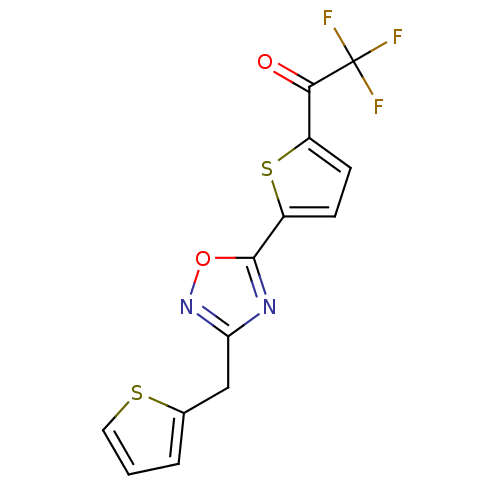
(2,2,2-trifluoro-1-(5-(3-(thiophen-2-ylmethyl)-1,2,...)Show InChI InChI=1S/C13H7F3N2O2S2/c14-13(15,16)11(19)8-3-4-9(22-8)12-17-10(18-20-12)6-7-2-1-5-21-7/h1-5H,6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged HDAC4 catalytic domain (unknown origin) expressed in Escherichia coli |
Bioorg Med Chem Lett 18: 6083-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.076
BindingDB Entry DOI: 10.7270/Q2TD9X6G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275647
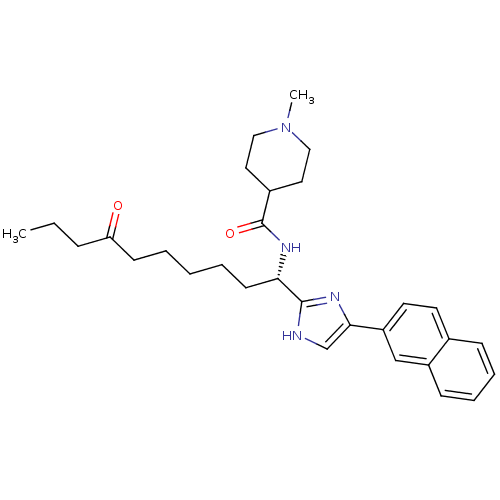
((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CCCC(=O)CCCCC[C@H](NC(=O)C1CCN(C)CC1)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C30H40N4O2/c1-3-9-26(35)12-5-4-6-13-27(33-30(36)23-16-18-34(2)19-17-23)29-31-21-28(32-29)25-15-14-22-10-7-8-11-24(22)20-25/h7-8,10-11,14-15,20-21,23,27H,3-6,9,12-13,16-19H2,1-2H3,(H,31,32)(H,33,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminally flag tagged HDAC1 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50258539

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195119
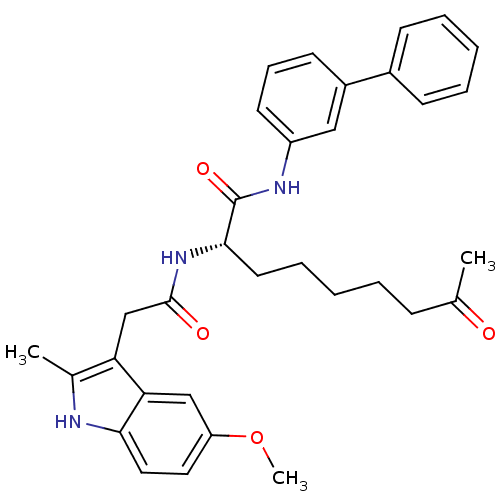
((S)-N-(biphenyl-3-yl)-2-(2-(5-methoxy-2-methyl-1H-...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3cccc(c3)-c3ccccc3)c2c1 Show InChI InChI=1S/C33H37N3O4/c1-22(37)11-6-4-9-16-31(33(39)35-26-15-10-14-25(19-26)24-12-7-5-8-13-24)36-32(38)21-28-23(2)34-30-18-17-27(40-3)20-29(28)30/h5,7-8,10,12-15,17-20,31,34H,4,6,9,11,16,21H2,1-3H3,(H,35,39)(H,36,38)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195129
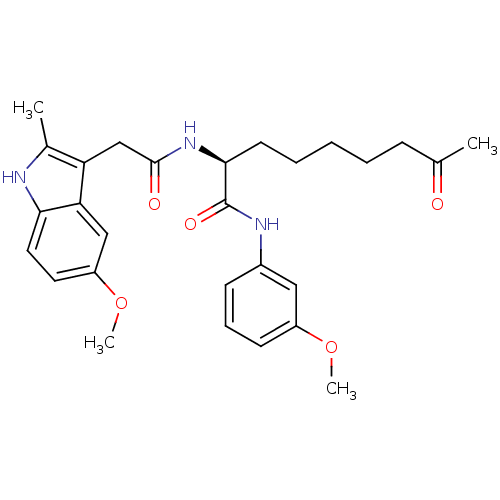
((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1cccc(NC(=O)[C@H](CCCCCC(C)=O)NC(=O)Cc2c(C)[nH]c3ccc(OC)cc23)c1 Show InChI InChI=1S/C28H35N3O5/c1-18(32)9-6-5-7-12-26(28(34)30-20-10-8-11-21(15-20)35-3)31-27(33)17-23-19(2)29-25-14-13-22(36-4)16-24(23)25/h8,10-11,13-16,26,29H,5-7,9,12,17H2,1-4H3,(H,30,34)(H,31,33)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195118

((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3nc(cs3)-c3ccccc3)c2c1 Show InChI InChI=1S/C30H34N4O4S/c1-19(35)10-6-4-9-13-26(29(37)34-30-33-27(18-39-30)21-11-7-5-8-12-21)32-28(36)17-23-20(2)31-25-15-14-22(38-3)16-24(23)25/h5,7-8,11-12,14-16,18,26,31H,4,6,9-10,13,17H2,1-3H3,(H,32,36)(H,33,34,37)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50275647

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CCCC(=O)CCCCC[C@H](NC(=O)C1CCN(C)CC1)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C30H40N4O2/c1-3-9-26(35)12-5-4-6-13-27(33-30(36)23-16-18-34(2)19-17-23)29-31-21-28(32-29)25-15-14-22-10-7-8-11-24(22)20-25/h7-8,10-11,14-15,20-21,23,27H,3-6,9,12-13,16-19H2,1-2H3,(H,31,32)(H,33,36)/t27-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50258539

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminally flag tagged HDAC1 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50246033
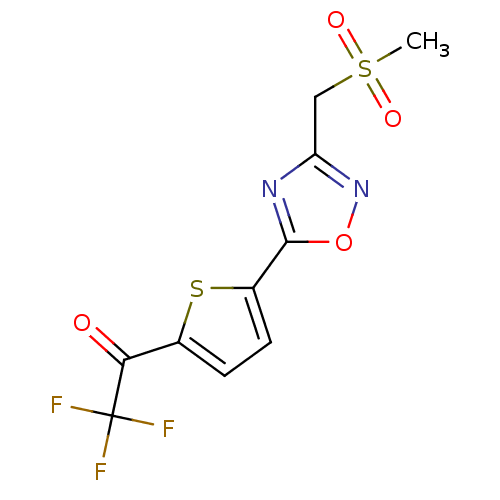
(2,2,2-Trifluoro-1-(5-{3-[(methylsulfonyl)methyl]-1...)Show SMILES CS(=O)(=O)Cc1noc(n1)-c1ccc(s1)C(=O)C(F)(F)F Show InChI InChI=1S/C10H7F3N2O4S2/c1-21(17,18)4-7-14-9(19-15-7)6-3-2-5(20-6)8(16)10(11,12)13/h2-3H,4H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged HDAC4 catalytic domain (unknown origin) expressed in Escherichia coli |
Bioorg Med Chem Lett 18: 6083-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.076
BindingDB Entry DOI: 10.7270/Q2TD9X6G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50246034
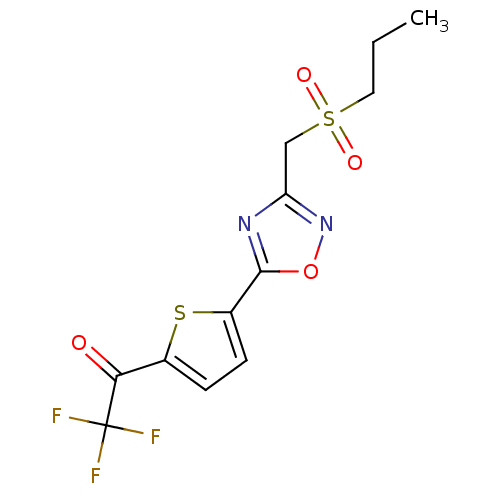
(2,2,2-Trifluoro-1-(5-{3-[(propylsulfonyl)methyl]-1...)Show SMILES CCCS(=O)(=O)Cc1noc(n1)-c1ccc(s1)C(=O)C(F)(F)F Show InChI InChI=1S/C12H11F3N2O4S2/c1-2-5-23(19,20)6-9-16-11(21-17-9)8-4-3-7(22-8)10(18)12(13,14)15/h3-4H,2,5-6H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged HDAC4 catalytic domain (unknown origin) expressed in Escherichia coli |
Bioorg Med Chem Lett 18: 6083-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.076
BindingDB Entry DOI: 10.7270/Q2TD9X6G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195111
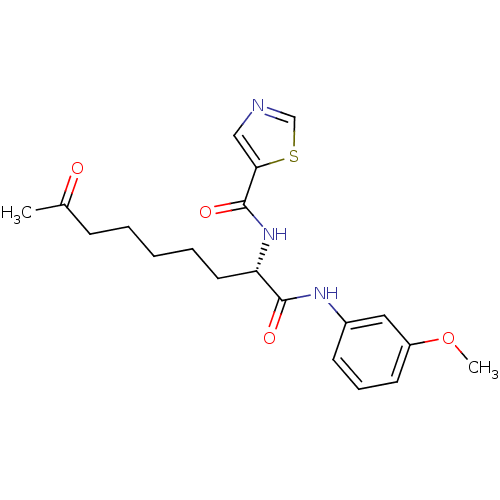
((S)-N-(1-(3-methoxyphenylamino)-1,8-dioxononan-2-y...)Show SMILES COc1cccc(NC(=O)[C@H](CCCCCC(C)=O)NC(=O)c2cncs2)c1 Show InChI InChI=1S/C20H25N3O4S/c1-14(24)7-4-3-5-10-17(23-20(26)18-12-21-13-28-18)19(25)22-15-8-6-9-16(11-15)27-2/h6,8-9,11-13,17H,3-5,7,10H2,1-2H3,(H,22,25)(H,23,26)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50246035

(2,2,2-Trifluoro-1-(5-{3-[(thiophen-2-ylsulfonyl)me...)Show SMILES FC(F)(F)C(=O)c1ccc(s1)-c1nc(CS(=O)(=O)c2cccs2)no1 Show InChI InChI=1S/C13H7F3N2O4S3/c14-13(15,16)11(19)7-3-4-8(24-7)12-17-9(18-22-12)6-25(20,21)10-2-1-5-23-10/h1-5H,6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged HDAC4 catalytic domain (unknown origin) expressed in Escherichia coli |
Bioorg Med Chem Lett 18: 6083-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.076
BindingDB Entry DOI: 10.7270/Q2TD9X6G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50238632
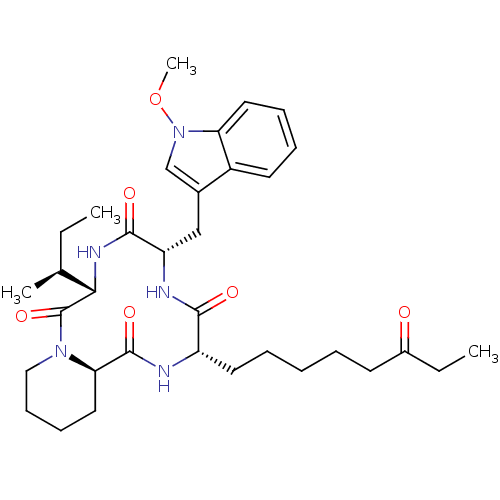
((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminally flag tagged HDAC1 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195135
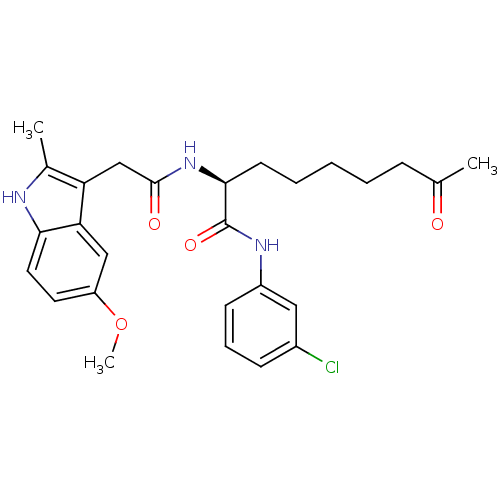
((S)-N-(3-chlorophenyl)-2-(2-(5-methoxy-2-methyl-1H...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H32ClN3O4/c1-17(32)8-5-4-6-11-25(27(34)30-20-10-7-9-19(28)14-20)31-26(33)16-22-18(2)29-24-13-12-21(35-3)15-23(22)24/h7,9-10,12-15,25,29H,4-6,8,11,16H2,1-3H3,(H,30,34)(H,31,33)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275542

((S)-N-(1-(5-(biphenyl-4-yl)-1H-imidazol-2-yl)-7-ox...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C30H38N4O2/c1-22(35)9-5-3-8-12-27(33-30(36)26-17-19-34(2)20-18-26)29-31-21-28(32-29)25-15-13-24(14-16-25)23-10-6-4-7-11-23/h4,6-7,10-11,13-16,21,26-27H,3,5,8-9,12,17-20H2,1-2H3,(H,31,32)(H,33,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminally flag tagged HDAC1 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195130
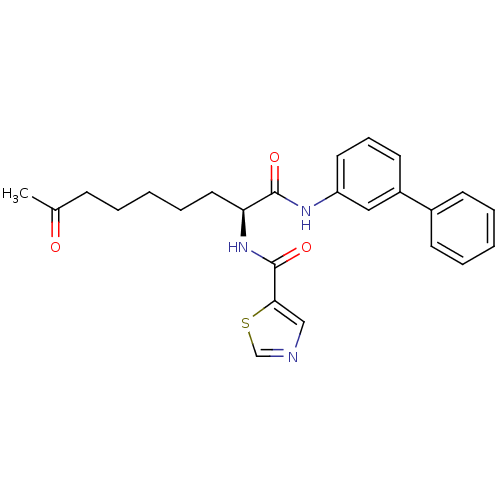
((S)-N-(1-(biphenyl-3-ylamino)-1,8-dioxononan-2-yl)...)Show SMILES CC(=O)CCCCC[C@H](NC(=O)c1cncs1)C(=O)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C25H27N3O3S/c1-18(29)9-4-2-7-14-22(28-25(31)23-16-26-17-32-23)24(30)27-21-13-8-12-20(15-21)19-10-5-3-6-11-19/h3,5-6,8,10-13,15-17,22H,2,4,7,9,14H2,1H3,(H,27,30)(H,28,31)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM25144

((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275995

((S)-3-nitro-N-(7-oxo-1-(5-phenyl-1H-imidazol-2-yl)...)Show SMILES CC(=O)CCCCC[C@H](NS(=O)(=O)c1cccc(c1)[N+]([O-])=O)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C23H26N4O5S/c1-17(28)9-4-2-7-14-21(23-24-16-22(25-23)18-10-5-3-6-11-18)26-33(31,32)20-13-8-12-19(15-20)27(29)30/h3,5-6,8,10-13,15-16,21,26H,2,4,7,9,14H2,1H3,(H,24,25)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminally flag tagged HDAC1 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data