

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065118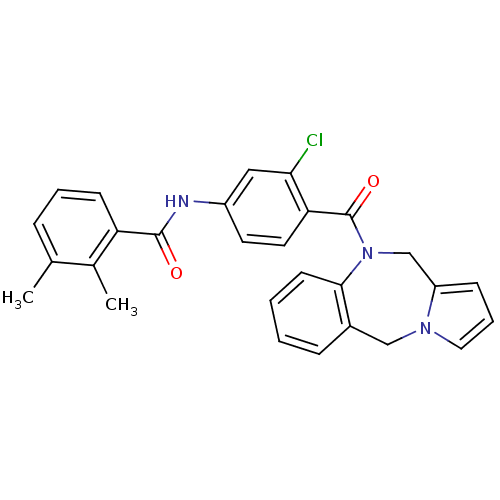 (CHEMBL311931 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50078646 (CHEMBL49322 | N-[4-(4,10-Dihydro-1-thia-9-aza-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065123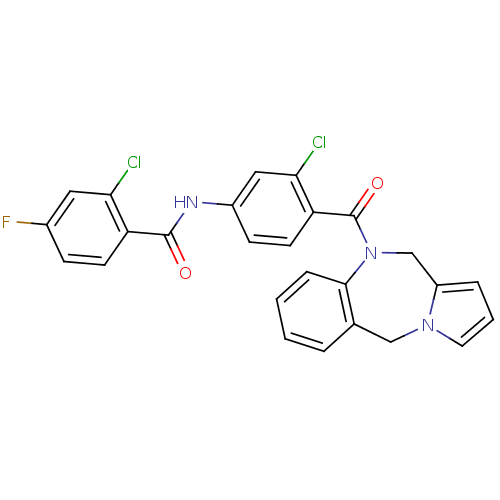 (CHEMBL420031 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA | J Med Chem 53: 8468-84 (2010) Article DOI: 10.1021/jm1004286 BindingDB Entry DOI: 10.7270/Q2154H9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA | J Med Chem 53: 8468-84 (2010) Article DOI: 10.1021/jm1004286 BindingDB Entry DOI: 10.7270/Q2154H9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065121 (CHEMBL310416 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078656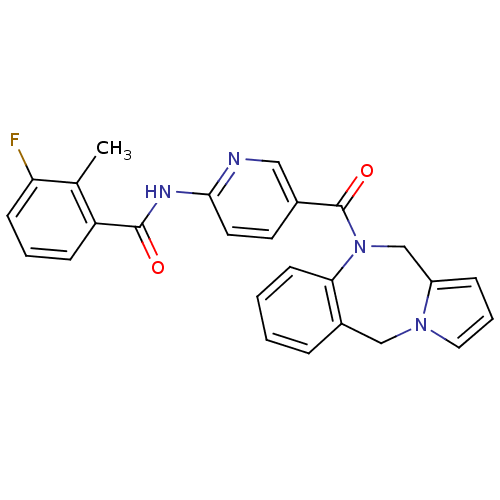 (CHEMBL46295 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human V2 receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078652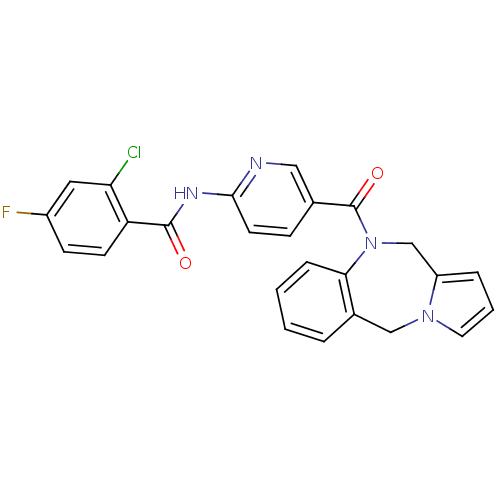 (CHEMBL301788 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50087550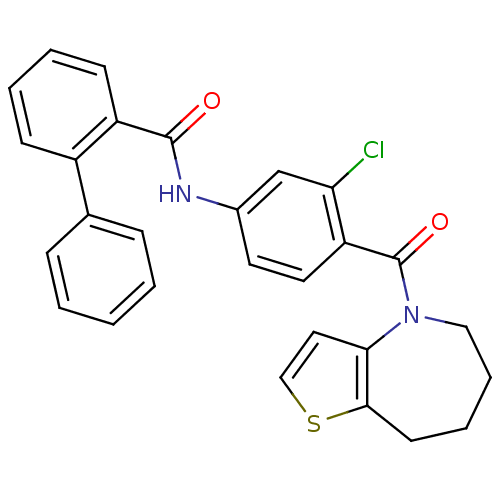 (Biphenyl-2-carboxylic acid [3-chloro-4-(5,6,7,8-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity to rat V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity to human V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087670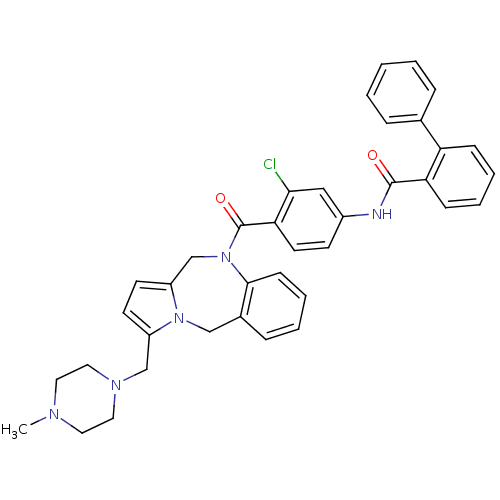 (Biphenyl-2-carboxylic acid {3-chloro-4-[3-(4-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065124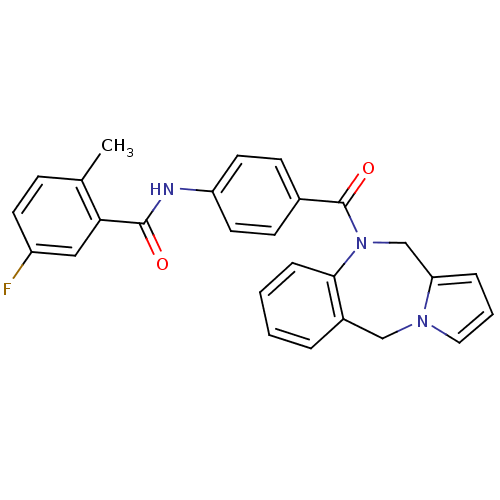 (CHEMBL68085 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50087541 (Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity to rat V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078660 (CHEMBL299532 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of JAK2 by solution phase kinase assay | J Med Chem 53: 8468-84 (2010) Article DOI: 10.1021/jm1004286 BindingDB Entry DOI: 10.7270/Q2154H9D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087551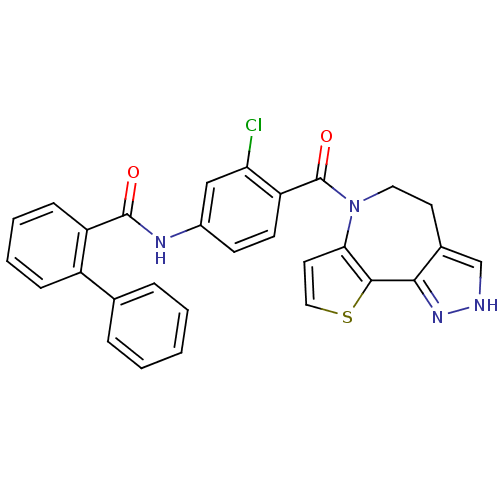 (Biphenyl-2-carboxylic acid [3-chloro-4-(4,5-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity to human V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078651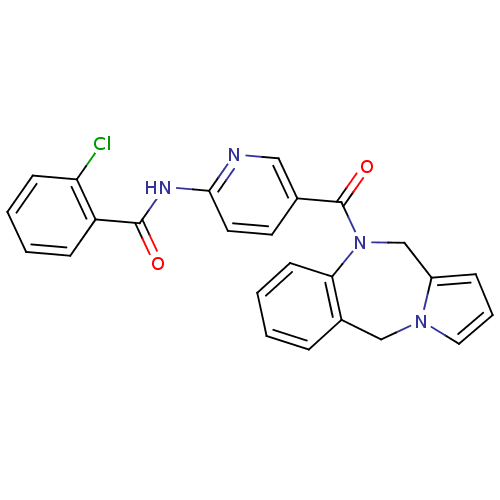 (CHEMBL300963 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065111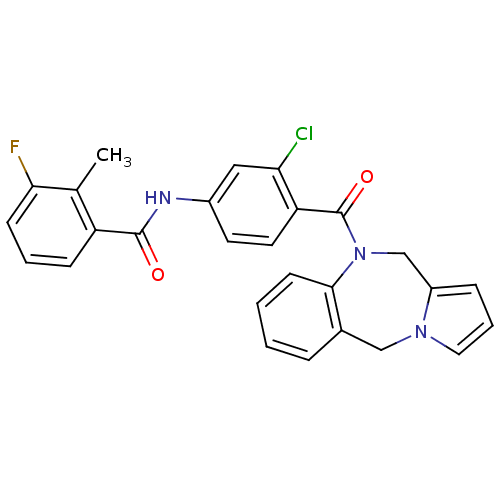 (CHEMBL80029 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065112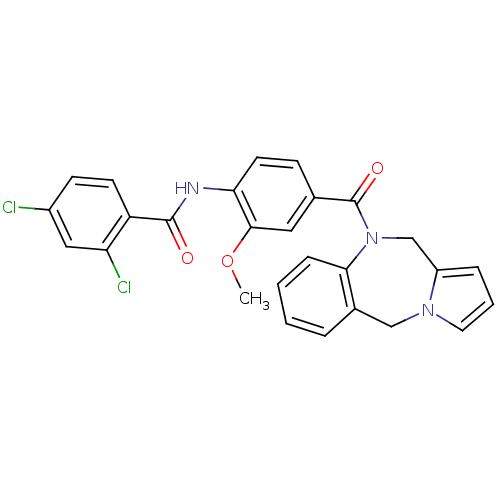 (CHEMBL81269 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50173291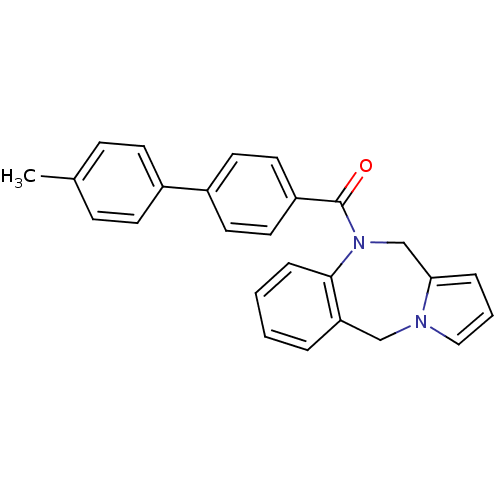 ((5H,11H-Benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]-AVP binding to human vasopressin V1a receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50024948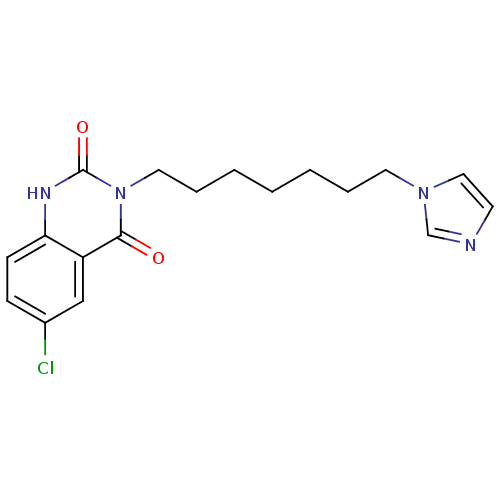 (6-Chloro-3-(7-imidazol-1-yl-heptyl)-1H-quinazoline...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Division of American Cyanamid Company Curated by ChEMBL | Assay Description Tested for inhibition of thromboxane synthetase from spontaneously hypertensive rats | J Med Chem 30: 2277-83 (1988) BindingDB Entry DOI: 10.7270/Q23T9HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50024903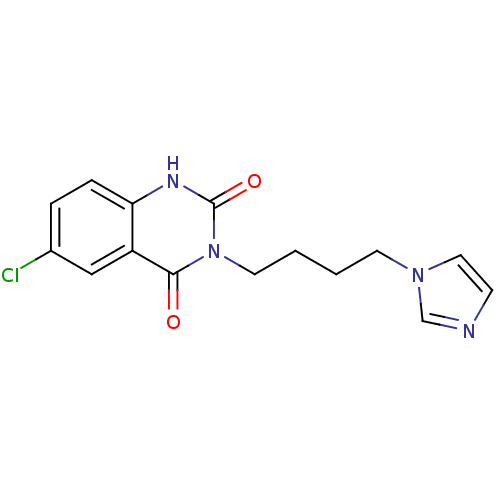 (6-Chloro-3-(4-imidazol-1-yl-butyl)-1H-quinazoline-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Division of American Cyanamid Company Curated by ChEMBL | Assay Description Tested for inhibition of thromboxane synthetase from spontaneously hypertensive rats | J Med Chem 30: 2277-83 (1988) BindingDB Entry DOI: 10.7270/Q23T9HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065129 (CHEMBL312036 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087664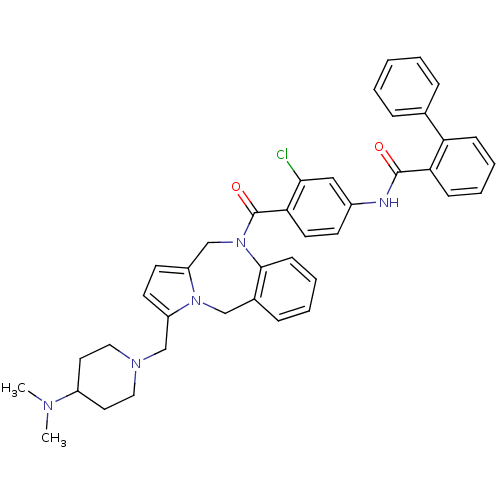 (Biphenyl-2-carboxylic acid {3-chloro-4-[3-(4-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065125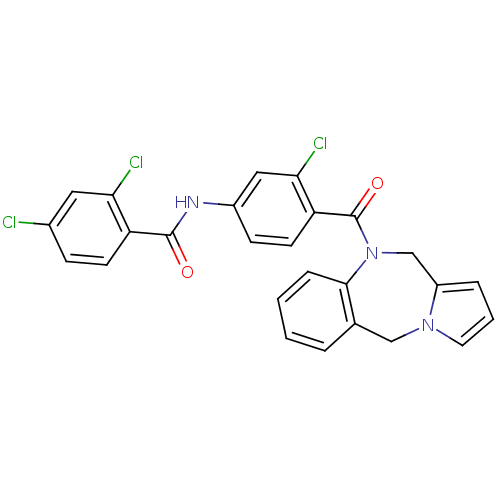 (CHEMBL311230 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50332622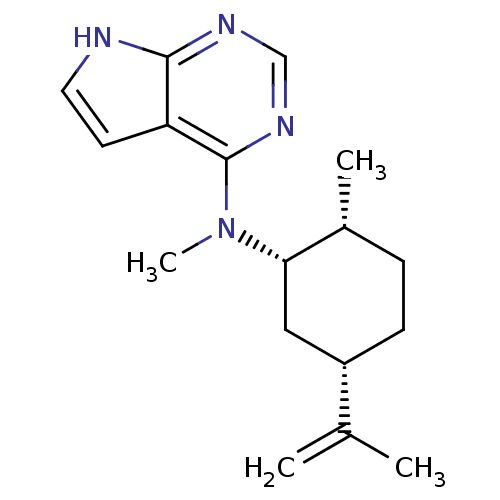 (CHEMBL1630782 | N-Methyl-N-((1S,2R,5S)-2-methyl-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA | J Med Chem 53: 8468-84 (2010) Article DOI: 10.1021/jm1004286 BindingDB Entry DOI: 10.7270/Q2154H9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065128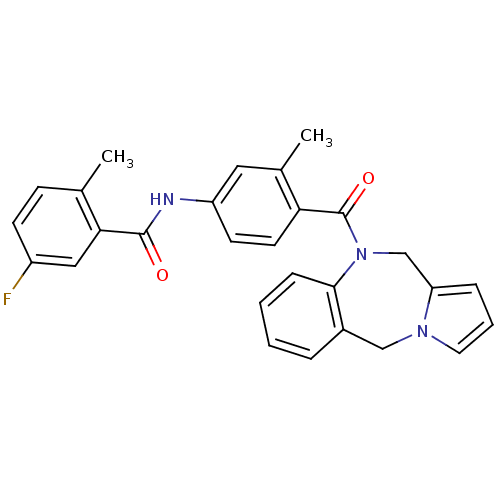 (CHEMBL81133 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087675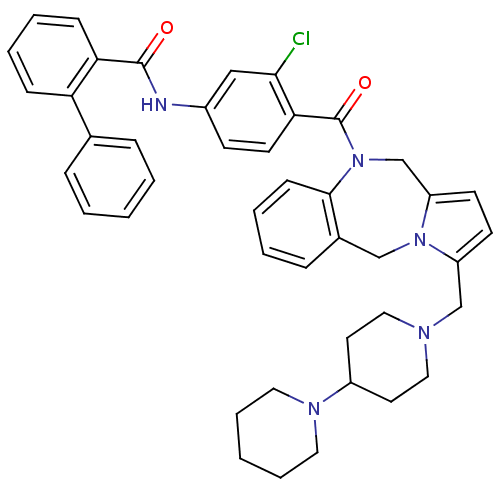 (Biphenyl-2-carboxylic acid [4-(3-[1,4']bipiperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity to rat V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from vasopressin V2 receptor of rat kidney medulla. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50087552 (Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity to rat V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087674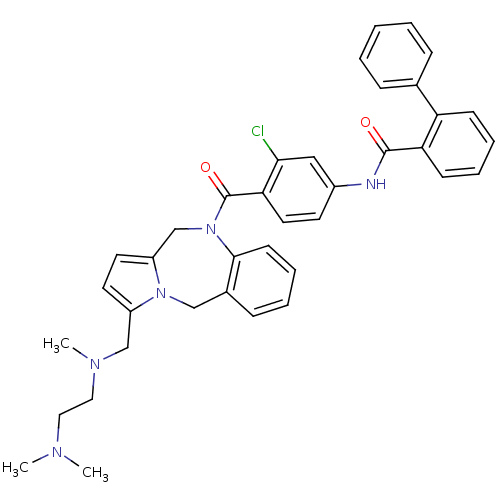 (Biphenyl-2-carboxylic acid [3-chloro-4-(3-{[(2-dim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087541 (Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity to human V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50024906 (3-(3-Imidazol-1-yl-2-methyl-propyl)-6-methyl-1H-qu...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Division of American Cyanamid Company Curated by ChEMBL | Assay Description Tested for inhibition of thromboxane synthetase from spontaneously hypertensive rats | J Med Chem 30: 2277-83 (1988) BindingDB Entry DOI: 10.7270/Q23T9HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078654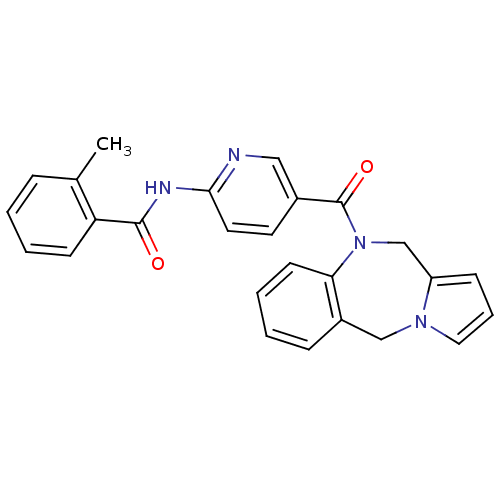 (CHEMBL297990 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of (P[3H]-AVP binding towards isolated rat kidney medullary Vasopre ssin V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50024934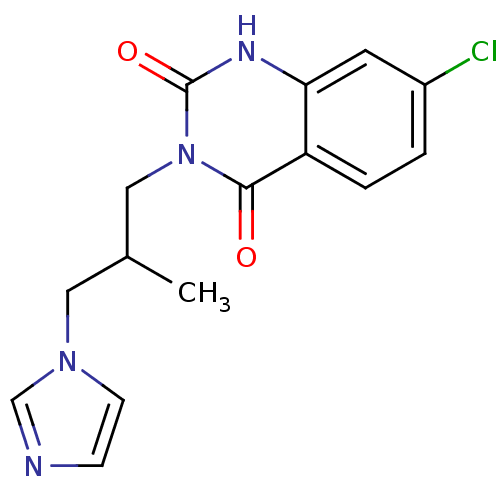 (7-Chloro-3-(3-imidazol-1-yl-2-methyl-propyl)-1H-qu...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Division of American Cyanamid Company Curated by ChEMBL | Assay Description Tested for inhibition of thromboxane synthetase from spontaneously hypertensive rats | J Med Chem 30: 2277-83 (1988) BindingDB Entry DOI: 10.7270/Q23T9HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50173297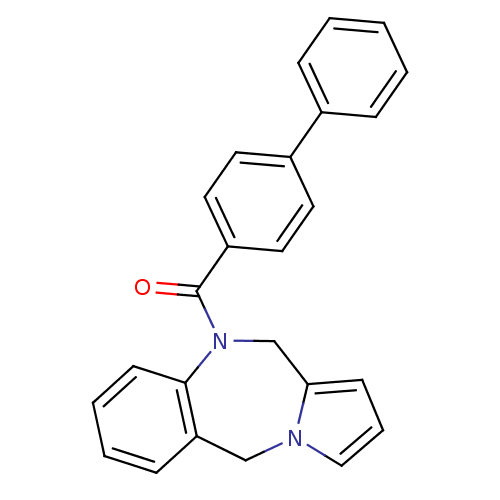 ((5H,11H-Benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]-AVP binding to human vasopressin V1a receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from vasopressin V2 receptor of rat kidney medulla. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity to rat V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity to human V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of JAK3 by solution phase kinase assay | J Med Chem 53: 8468-84 (2010) Article DOI: 10.1021/jm1004286 BindingDB Entry DOI: 10.7270/Q2154H9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50087543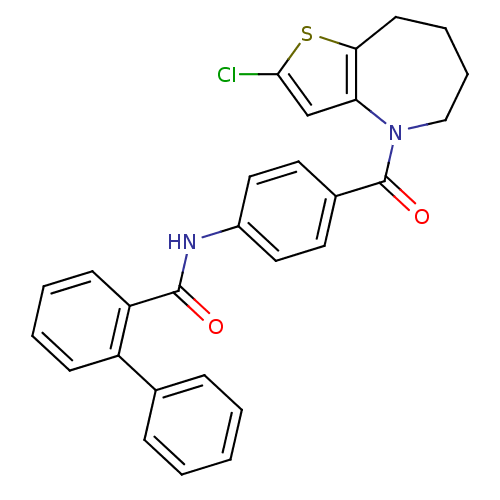 (Biphenyl-2-carboxylic acid [4-(2-chloro-5,6,7,8-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity to rat V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50087552 (Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity for rat V1a receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087542 (CHEMBL350089 | N-[3-Chloro-4-(5,6,7,8-tetrahydro-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity to human V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 505 total ) | Next | Last >> |