 Found 110 hits with Last Name = 'chang' and Initial = 'ty'
Found 110 hits with Last Name = 'chang' and Initial = 'ty' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12985
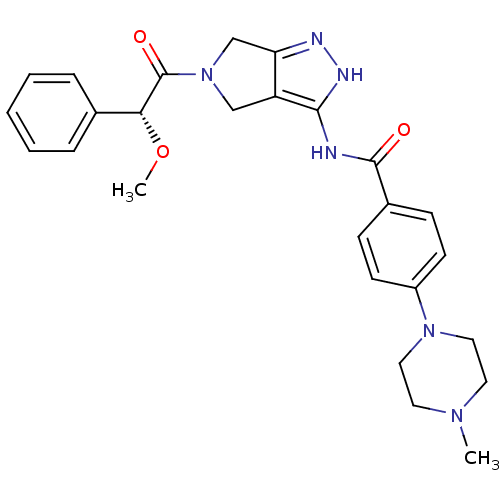
(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233

(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES C[C@H](OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O |r| Show InChI InChI=1S/C32H48N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h6,9-10,13-14,19,21-22,24-27H,5,7-8,11-12,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232
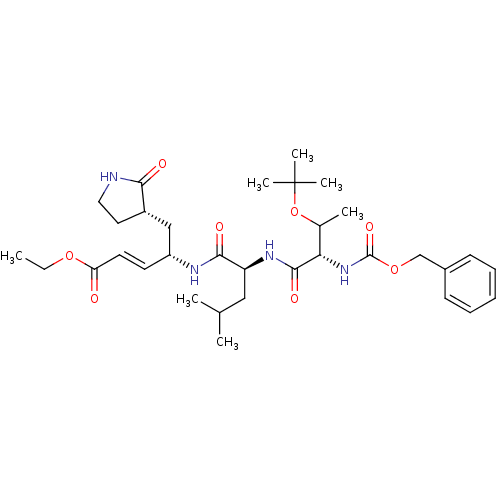
(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)OC(C)(C)C |r| Show InChI InChI=1S/C33H50N4O8/c1-8-43-27(38)15-14-25(19-24-16-17-34-29(24)39)35-30(40)26(18-21(2)3)36-31(41)28(22(4)45-33(5,6)7)37-32(42)44-20-23-12-10-9-11-13-23/h9-15,21-22,24-26,28H,8,16-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b15-14+/t22?,24-,25+,26-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM12985

(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM12985

(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231

(N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C30H44N4O7/c1-6-40-25(35)13-12-23(17-22-14-15-31-27(22)36)32-28(37)24(16-19(2)3)33-29(38)26(20(4)5)34-30(39)41-18-21-10-8-7-9-11-21/h7-13,19-20,22-24,26H,6,14-18H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H,34,39)/b13-12+/t22-,23+,24-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230
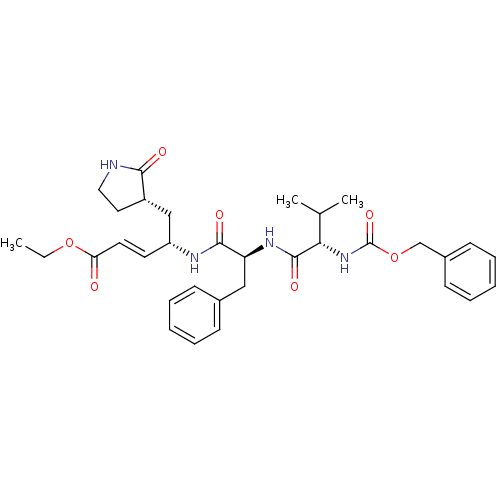
(AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C33H42N4O7/c1-4-43-28(38)16-15-26(20-25-17-18-34-30(25)39)35-31(40)27(19-23-11-7-5-8-12-23)36-32(41)29(22(2)3)37-33(42)44-21-24-13-9-6-10-14-24/h5-16,22,25-27,29H,4,17-21H2,1-3H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b16-15+/t25-,26+,27-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229
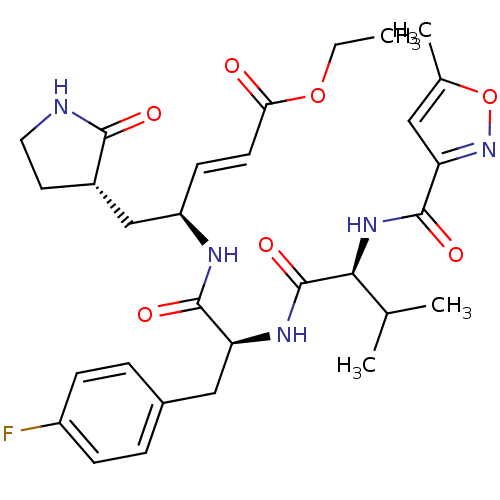
(AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@@H](NC(=O)c1cc(C)on1)C(C)C |r| Show InChI InChI=1S/C30H38FN5O7/c1-5-42-25(37)11-10-22(16-20-12-13-32-27(20)38)33-28(39)23(15-19-6-8-21(31)9-7-19)34-30(41)26(17(2)3)35-29(40)24-14-18(4)43-36-24/h6-11,14,17,20,22-23,26H,5,12-13,15-16H2,1-4H3,(H,32,38)(H,33,39)(H,34,41)(H,35,40)/b11-10+/t20-,22+,23-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330230
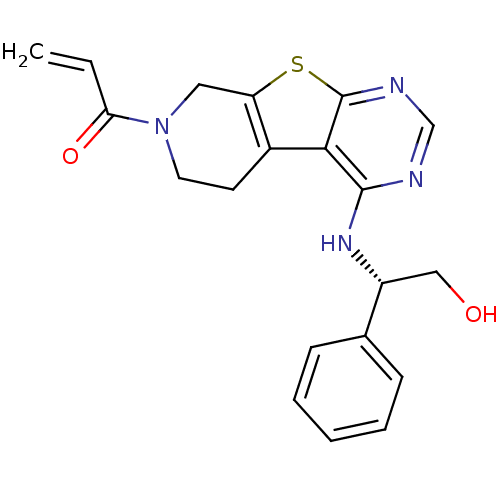
(1-(4-(((1S)-2-Hydroxy-1-phenylethyl)amino)-5,6,7,8...)Show SMILES OC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C20H20N4O2S/c1-2-17(26)24-9-8-14-16(10-24)27-20-18(14)19(21-12-22-20)23-15(11-25)13-6-4-3-5-7-13/h2-7,12,15,25H,1,8-11H2,(H,21,22,23)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330238

((E)-4-(Diethylamino)-1-(4-(((1R)-2-hydroxy-1-pheny...)Show SMILES CCN(CC)C\C=C\C(=O)N1CCc2c(C1)sc1ncnc(N[C@H](CO)c3ccccc3)c21 |r| Show InChI InChI=1S/C25H31N5O2S/c1-3-29(4-2)13-8-11-22(32)30-14-12-19-21(15-30)33-25-23(19)24(26-17-27-25)28-20(16-31)18-9-6-5-7-10-18/h5-11,17,20,31H,3-4,12-16H2,1-2H3,(H,26,27,28)/b11-8+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50564437

(CHEMBL4795285)Show SMILES Nc1ccccc1NC(=O)c1ccc(Cn2cnc3c(Nc4ccc(cc4)C(=O)NO)nc(Cl)nc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC6 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330241
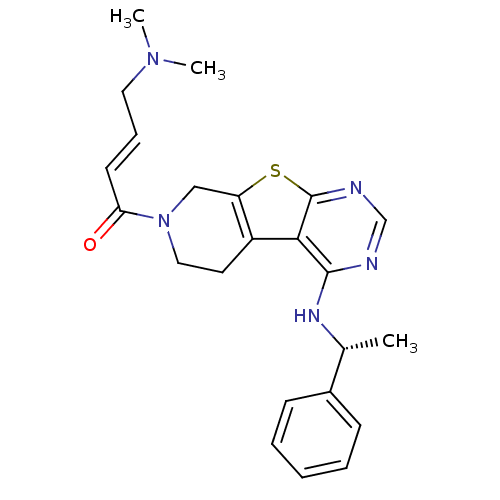
((E)-4-(Dimethylamino)-1-(4-[(1R)-1-phenylethyl]ami...)Show SMILES C[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN(C)C)c1ccccc1 |r| Show InChI InChI=1S/C23H27N5OS/c1-16(17-8-5-4-6-9-17)26-22-21-18-11-13-28(20(29)10-7-12-27(2)3)14-19(18)30-23(21)25-15-24-22/h4-10,15-16H,11-14H2,1-3H3,(H,24,25,26)/b10-7+/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330242
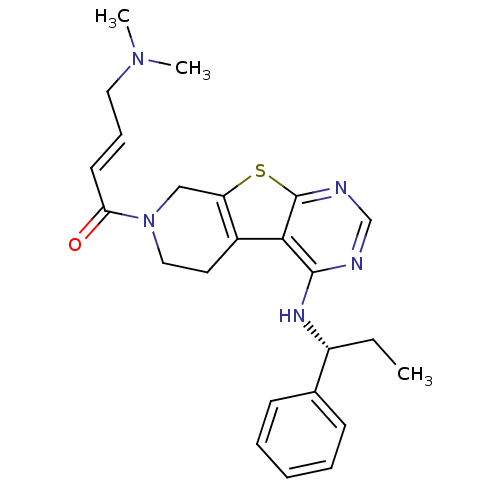
((E)-4-(Dimethylamino)-1-(4-[(1S)-1-phenylpropyl]am...)Show SMILES CC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN(C)C)c1ccccc1 |r| Show InChI InChI=1S/C24H29N5OS/c1-4-19(17-9-6-5-7-10-17)27-23-22-18-12-14-29(21(30)11-8-13-28(2)3)15-20(18)31-24(22)26-16-25-23/h5-11,16,19H,4,12-15H2,1-3H3,(H,25,26,27)/b11-8+/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase tousled-like 2
(Homo sapiens (Human)) | BDBM50599378

(CHEMBL5204542)Show SMILES CN1CCCC1CCO\N=C1\C(\Nc2ccccc\12)=C1\C(=O)Nc2ccccc12 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113904
BindingDB Entry DOI: 10.7270/Q26D5Z1J |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330234

(1-(4-[(1R)-1-Phenylethyl]amino-5,6,7,8-tetrahydrop...)Show SMILES C[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C20H20N4OS/c1-3-17(25)24-10-9-15-16(11-24)26-20-18(15)19(21-12-22-20)23-13(2)14-7-5-4-6-8-14/h3-8,12-13H,1,9-11H2,2H3,(H,21,22,23)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330239
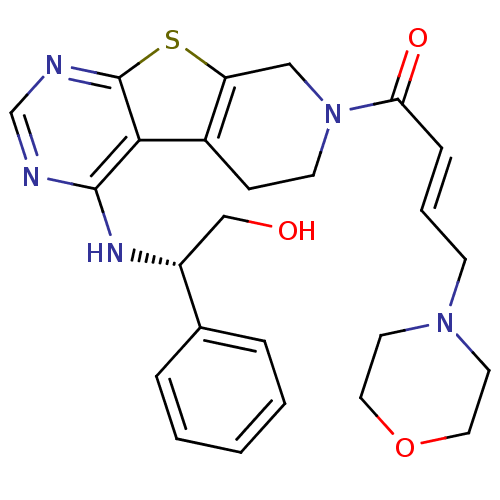
((E)-1-(4-[(1R)-2-Hydroxy-1-phenylethyl]amino-5,6,7...)Show SMILES OC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN1CCOCC1)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3S/c31-16-20(18-5-2-1-3-6-18)28-24-23-19-8-10-30(15-21(19)34-25(23)27-17-26-24)22(32)7-4-9-29-11-13-33-14-12-29/h1-7,17,20,31H,8-16H2,(H,26,27,28)/b7-4+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50564442
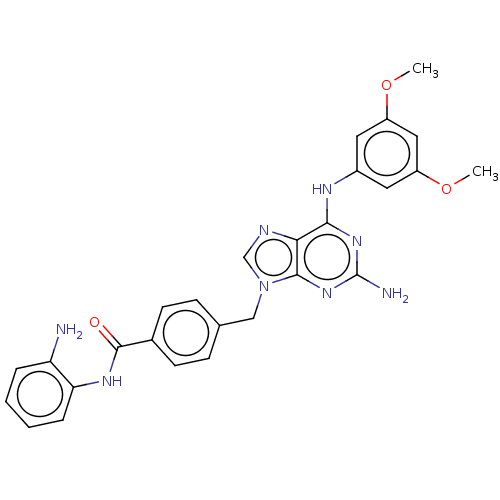
(CHEMBL4789410)Show SMILES COc1cc(Nc2nc(N)nc3n(Cc4ccc(cc4)C(=O)Nc4ccccc4N)cnc23)cc(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase tousled-like 2
(Homo sapiens (Human)) | BDBM50599379

(CHEMBL5188455)Show SMILES CN1CCC(CC1)O\N=C1\C(\Nc2ccccc\12)=C1\C(=O)Nc2ccccc12 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113904
BindingDB Entry DOI: 10.7270/Q26D5Z1J |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330235
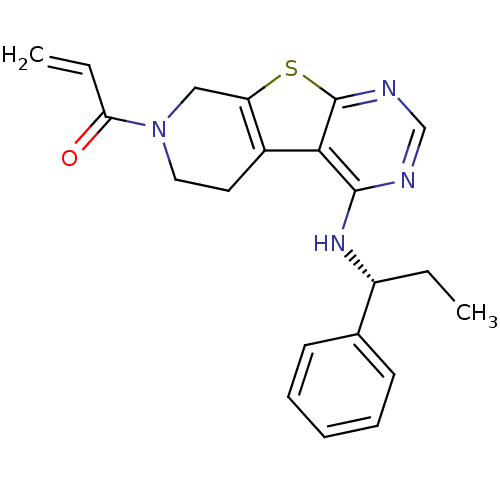
(1-(4-[(1R)-1-Phenylpropyl]amino-5,6,7,8-tetrahydro...)Show SMILES CC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C21H22N4OS/c1-3-16(14-8-6-5-7-9-14)24-20-19-15-10-11-25(18(26)4-2)12-17(15)27-21(19)23-13-22-20/h4-9,13,16H,2-3,10-12H2,1H3,(H,22,23,24)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330237
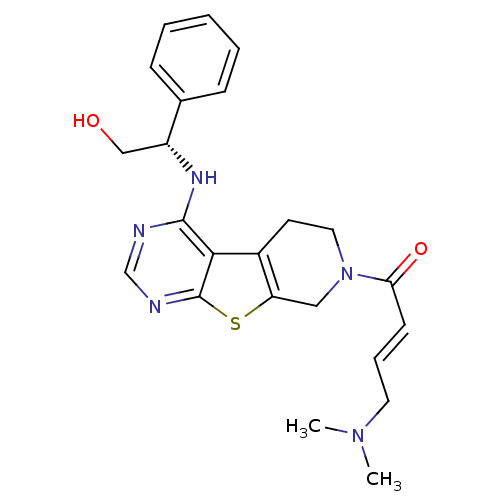
((E)-4-(Dimethylamino)-1-(4-[(1S)-2-hydroxy-1-pheny...)Show SMILES CN(C)C\C=C\C(=O)N1CCc2c(C1)sc1ncnc(N[C@H](CO)c3ccccc3)c21 |r| Show InChI InChI=1S/C23H27N5O2S/c1-27(2)11-6-9-20(30)28-12-10-17-19(13-28)31-23-21(17)22(24-15-25-23)26-18(14-29)16-7-4-3-5-8-16/h3-9,15,18,29H,10-14H2,1-2H3,(H,24,25,26)/b9-6+/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330240
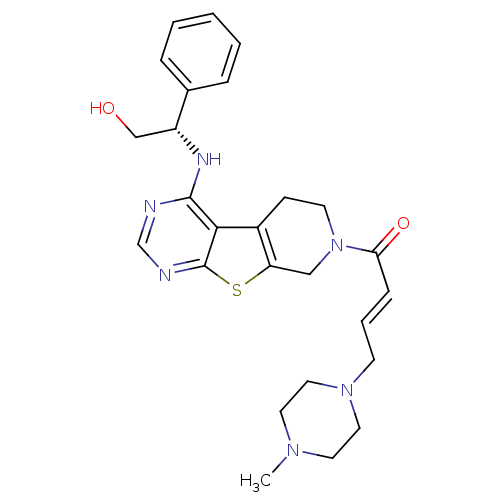
((E)-1-(4-[(1S)-2-Hydroxy-1-phenylethyl]amino-5,6,7...)Show SMILES CN1CCN(C\C=C\C(=O)N2CCc3c(C2)sc2ncnc(N[C@H](CO)c4ccccc4)c32)CC1 |r| Show InChI InChI=1S/C26H32N6O2S/c1-30-12-14-31(15-13-30)10-5-8-23(34)32-11-9-20-22(16-32)35-26-24(20)25(27-18-28-26)29-21(17-33)19-6-3-2-4-7-19/h2-8,18,21,33H,9-17H2,1H3,(H,27,28,29)/b8-5+/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330232
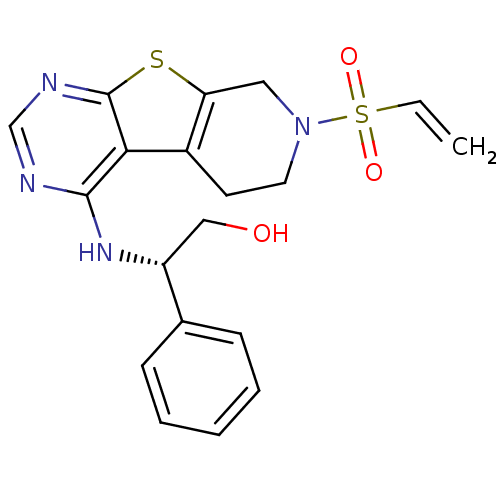
((2S)-2-Phenyl-2-[7-(vinylsulfonyl)-5,6,7,8-tetrahy...)Show SMILES OC[C@@H](Nc1ncnc2sc3CN(CCc3c12)S(=O)(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C19H20N4O3S2/c1-2-28(25,26)23-9-8-14-16(10-23)27-19-17(14)18(20-12-21-19)22-15(11-24)13-6-4-3-5-7-13/h2-7,12,15,24H,1,8-11H2,(H,20,21,22)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50564443
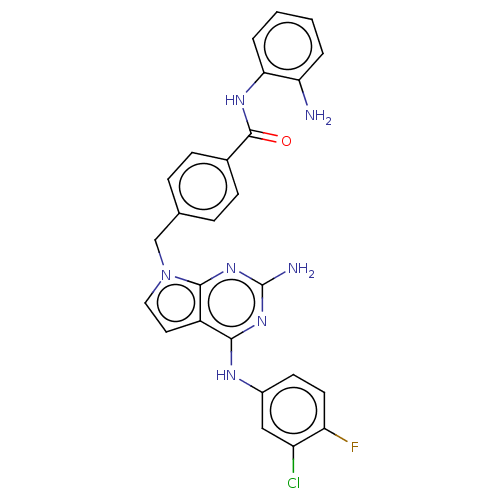
(CHEMBL4782132)Show SMILES Nc1nc(Nc2ccc(F)c(Cl)c2)c2ccn(Cc3ccc(cc3)C(=O)Nc3ccccc3N)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50564441
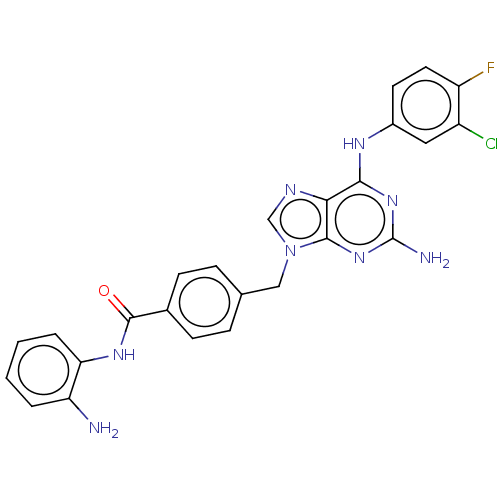
(CHEMBL4790678)Show SMILES Nc1ccccc1NC(=O)c1ccc(Cn2cnc3c(Nc4ccc(F)c(Cl)c4)nc(N)nc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330243
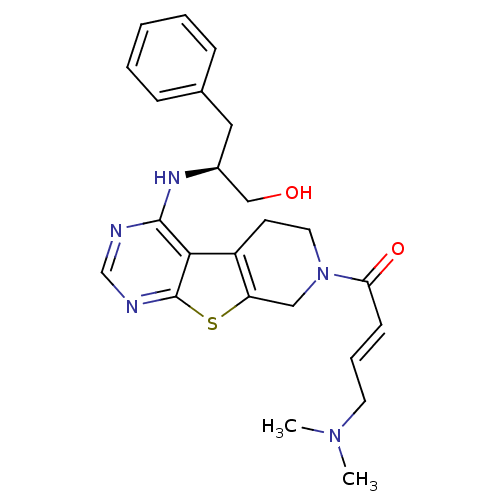
((E)-1-(4-[(1R)-1-Benzyl-2-hydroxyethyl]amino-5,6,7...)Show SMILES CN(C)C\C=C\C(=O)N1CCc2c(C1)sc1ncnc(N[C@H](CO)Cc3ccccc3)c21 |r| Show InChI InChI=1S/C24H29N5O2S/c1-28(2)11-6-9-21(31)29-12-10-19-20(14-29)32-24-22(19)23(25-16-26-24)27-18(15-30)13-17-7-4-3-5-8-17/h3-9,16,18,30H,10-15H2,1-2H3,(H,25,26,27)/b9-6+/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50563419

(CHEMBL4764787)Show SMILES COc1cc(CNc2nc(NCc3ccc(cc3)C(=O)Nc3ccccc3N)nc3[nH]ccc23)cc(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) using fluorogenic-(RHKKAc) as substrate by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113169
BindingDB Entry DOI: 10.7270/Q2833WRW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase tousled-like 2
(Homo sapiens (Human)) | BDBM50599380
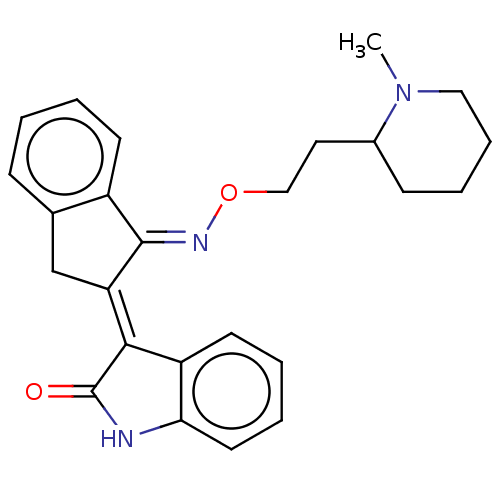
(CHEMBL5173181)Show SMILES CN1CCCCC1CCO\N=C1\C(\Cc2ccccc\12)=C1\C(=O)Nc2ccccc12 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113904
BindingDB Entry DOI: 10.7270/Q26D5Z1J |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50564438
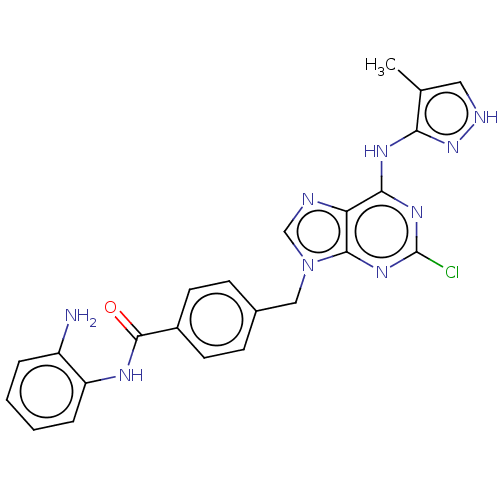
(CHEMBL4781354)Show SMILES Cc1c[nH]nc1Nc1nc(Cl)nc2n(Cc3ccc(cc3)C(=O)Nc3ccccc3N)cnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50564442

(CHEMBL4789410)Show SMILES COc1cc(Nc2nc(N)nc3n(Cc4ccc(cc4)C(=O)Nc4ccccc4N)cnc23)cc(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC2 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50564437

(CHEMBL4795285)Show SMILES Nc1ccccc1NC(=O)c1ccc(Cn2cnc3c(Nc4ccc(cc4)C(=O)NO)nc(Cl)nc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50563418

(CHEMBL4796132)Show SMILES COc1ccc(CNc2nc(N)nc3n(Cc4ccc(cc4)C(=O)Nc4ccccc4N)ccc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) using fluorogenic-(RHKKAc) as substrate by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113169
BindingDB Entry DOI: 10.7270/Q2833WRW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322820
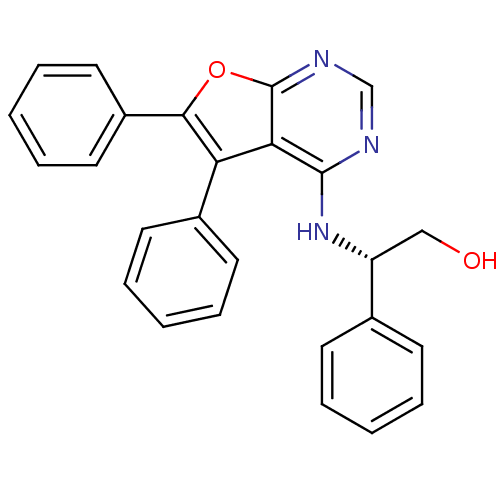
(CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...)Show SMILES OC[C@@H](Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H21N3O2/c30-16-21(18-10-4-1-5-11-18)29-25-23-22(19-12-6-2-7-13-19)24(20-14-8-3-9-15-20)31-26(23)28-17-27-25/h1-15,17,21,30H,16H2,(H,27,28,29)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50564442

(CHEMBL4789410)Show SMILES COc1cc(Nc2nc(N)nc3n(Cc4ccc(cc4)C(=O)Nc4ccccc4N)cnc23)cc(OC)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50564440

(CHEMBL4781490)Show SMILES Nc1nc(Cl)c2ncn(Cc3ccc(cc3)C(=O)Nc3ccccc3N)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50564437

(CHEMBL4795285)Show SMILES Nc1ccccc1NC(=O)c1ccc(Cn2cnc3c(Nc4ccc(cc4)C(=O)NO)nc(Cl)nc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC8 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50563418

(CHEMBL4796132)Show SMILES COc1ccc(CNc2nc(N)nc3n(Cc4ccc(cc4)C(=O)Nc4ccccc4N)ccc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC2 (unknown origin) using fluorogenic-(RHKKAc) as substrate by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113169
BindingDB Entry DOI: 10.7270/Q2833WRW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50563419

(CHEMBL4764787)Show SMILES COc1cc(CNc2nc(NCc3ccc(cc3)C(=O)Nc3ccccc3N)nc3[nH]ccc23)cc(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 481 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC2 (unknown origin) using fluorogenic-(RHKKAc) as substrate by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113169
BindingDB Entry DOI: 10.7270/Q2833WRW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 544 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 544 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) using fluorogenic-(RHKKAc) as substrate by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113169
BindingDB Entry DOI: 10.7270/Q2833WRW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50564441

(CHEMBL4790678)Show SMILES Nc1ccccc1NC(=O)c1ccc(Cn2cnc3c(Nc4ccc(F)c(Cl)c4)nc(N)nc23)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 563 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50564441

(CHEMBL4790678)Show SMILES Nc1ccccc1NC(=O)c1ccc(Cn2cnc3c(Nc4ccc(F)c(Cl)c4)nc(N)nc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 585 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC2 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 613 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC2 (unknown origin) using fluorogenic-(RHKKAc) as substrate by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113169
BindingDB Entry DOI: 10.7270/Q2833WRW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 613 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC2 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 624 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) using fluorogenic-(RHKKAc) as substrate by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113169
BindingDB Entry DOI: 10.7270/Q2833WRW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 624 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50564436
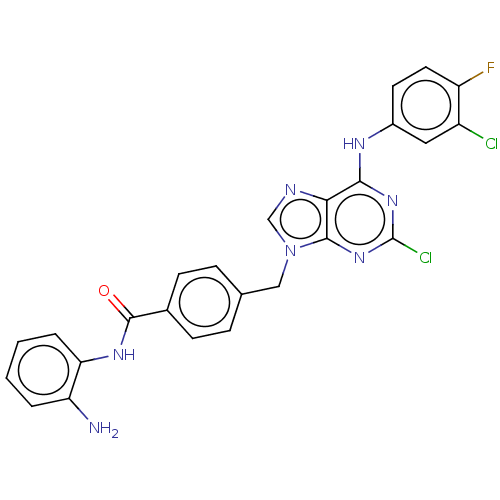
(CHEMBL4784684)Show SMILES Nc1ccccc1NC(=O)c1ccc(Cn2cnc3c(Nc4ccc(F)c(Cl)c4)nc(Cl)nc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 685 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate by Fluoresce... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112291
BindingDB Entry DOI: 10.7270/Q26W9FT0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data