

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R (unknown origin) | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R (unknown origin) | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against squalene synthase in rat liver squalene synthase (RLSS) enzyme assay | Bioorg Med Chem Lett 4: 1591-1594 (1994) Article DOI: 10.1016/S0960-894X(01)80572-2 BindingDB Entry DOI: 10.7270/Q2TB16T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK114 from neuropeptide Y1 receptor in human SK-N-MC cells | J Med Chem 51: 8168-72 (2008) Article DOI: 10.1021/jm801018u BindingDB Entry DOI: 10.7270/Q2WM1D8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK114 from neuropeptide Y1 receptor in human SK-N-MC cells | J Med Chem 51: 8168-72 (2008) Article DOI: 10.1021/jm801018u BindingDB Entry DOI: 10.7270/Q2WM1D8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50236697 (5-L-isoleucineangiotensin II | 5-isoleucine-angiot...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-Angiotensin 2 from human placental AT1 receptor expressed in African green monkey COS7 cell membranes after 90 mins by gamma cou... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK114 from NPY1R in human SK-N-MC cells by radioligand binding assay | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50532410 (CHEMBL4456247) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]18 from human NPY Y4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ after 90 mins by liquid scintillation counting | J Med Chem 59: 6045-58 (2016) Article DOI: 10.1021/acs.jmedchem.6b00309 BindingDB Entry DOI: 10.7270/Q25T3Q08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50383163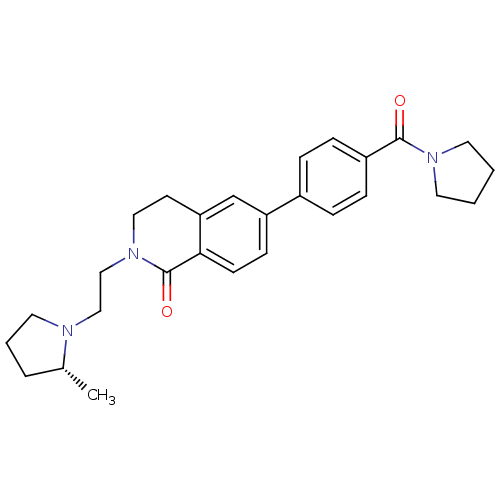 (CHEMBL2031885) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125155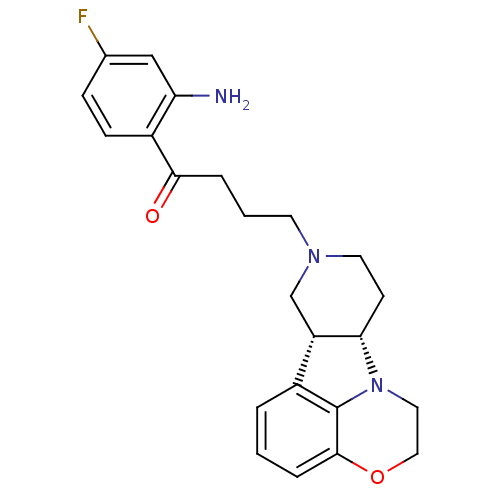 (1-(2-Amino-4-fluoro-phenyl)-4-(6bR,10aS)-1,2,6b,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK114 from NPY1R in human SK-N-MC cells by radioligand binding assay | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human SK-N-MC cells compound treated immediately post radioligand treatment measured after 2 hrs by ... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50334730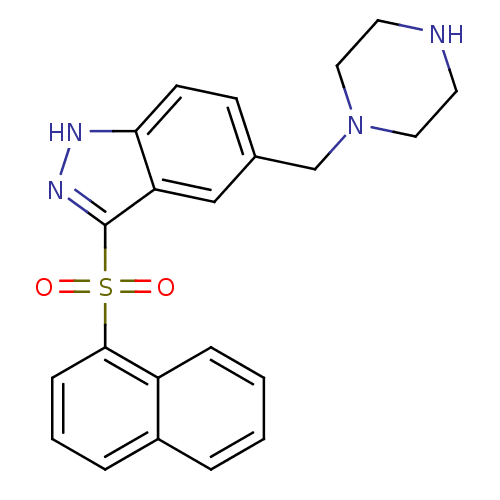 (3-(Naphthalen-1-ylsulfonyl)-5-(piperazin-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells | Bioorg Med Chem 19: 650-62 (2011) Article DOI: 10.1016/j.bmc.2010.10.033 BindingDB Entry DOI: 10.7270/Q29S1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50236697 (5-L-isoleucineangiotensin II | 5-isoleucine-angiot...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-Asp-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-ValTyr-Ile-His-Pro-Phe-OH Tris(hydrotrifluoroacetate) from human AT1 re... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50343731 ((R)-N-alpha-(2,2-Diphenylacetyl)-N-(4-ureidomethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells | Bioorg Med Chem 19: 2859-78 (2011) Article DOI: 10.1016/j.bmc.2011.03.045 BindingDB Entry DOI: 10.7270/Q2F47PG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50214615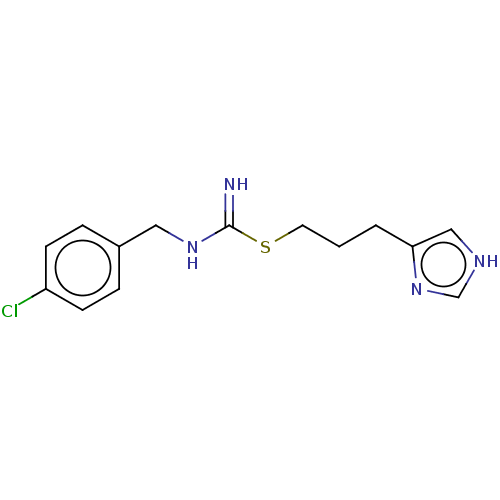 (CHEBI:64177 | Clobenpropit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of clobenpropit-BODIPY-630/650 from recombinant human NLuc-fused H3R expressed in HEK293T cells by Nano-BRET assay | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50159160 (CHEMBL3786779) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50316949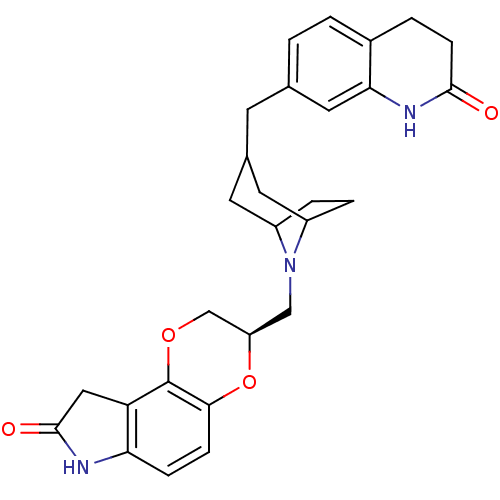 ((3R)-3-((3-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]citalopram form human SRET by liquid scintillation counting | Bioorg Med Chem Lett 20: 2983-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.105 BindingDB Entry DOI: 10.7270/Q2VH5P0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D2L receptor expressed in HEK293T cells co-expressing CRE-Luc incubated for 60 mins by microbeta sci... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113190 BindingDB Entry DOI: 10.7270/Q2TF0245 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human SK-N-MC cells compound treated immediately post radioligand treatment measured after 2 hrs by ... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human SK-N-MC cells compound treated immediately post radioligand treatment measured after 2 hrs by ... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500154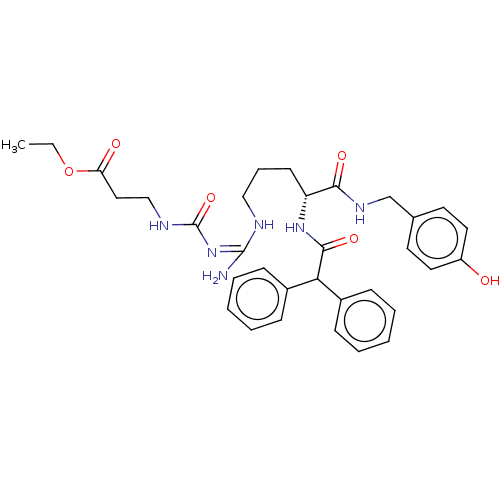 (CHEMBL3746870) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in HEL cells after 60 to 90 mins by flow cytometric analysis | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50084137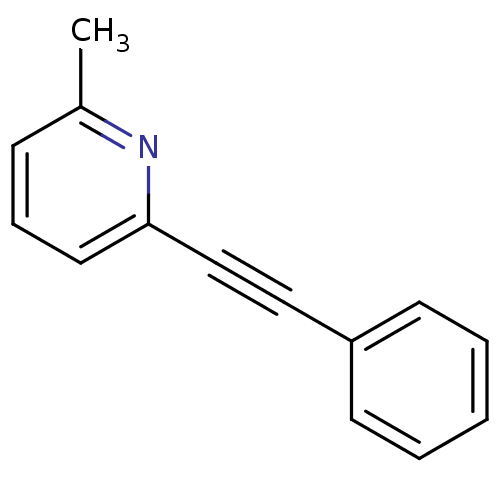 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Life Sci 73: 371-9 (2003) Article DOI: 10.1016/s0024-3205(03)00272-8 BindingDB Entry DOI: 10.7270/Q2KD1WHW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50383144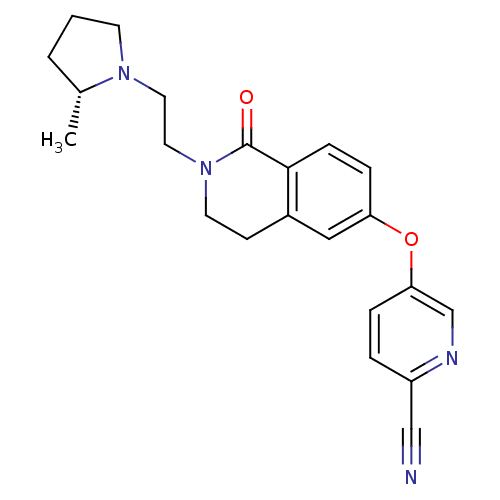 (CHEMBL2031864) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in HEL cells after 60 to 90 mins by flow cytometric analysis | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35226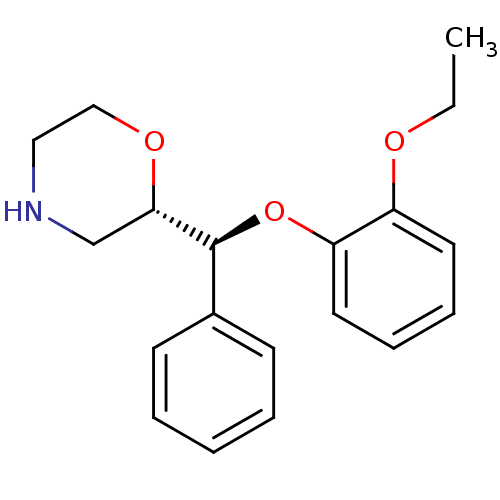 ((S,S)-reboxetine | Reboxetine | Vestra) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... | Bioorg Med Chem 17: 7802-15 (2009) Article DOI: 10.1016/j.bmc.2009.09.023 BindingDB Entry DOI: 10.7270/Q26Q1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300820 (CHEMBL565723 | N-(2-{3-[(3-Chlorophenyl)sulfonyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in HeLa cells | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125161 (1-(2-Amino-4-fluoro-phenyl)-4-(6bR,10aS)-1,2,6b,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK136 from NPY1R in human SK-N-MC cells by radioligand binding assay | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50535849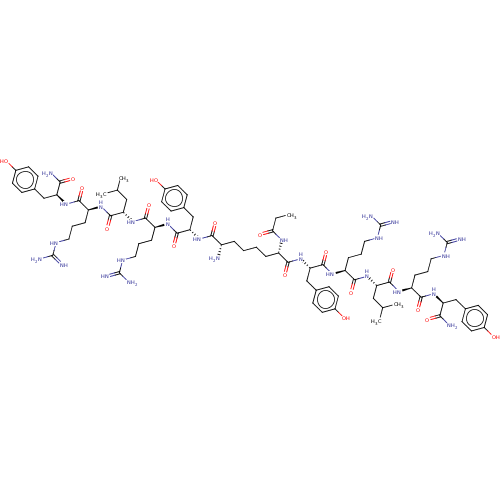 (CHEMBL4522438) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]18 from human NPY Y4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ after 90 mins by liquid scintillation counting | J Med Chem 59: 6045-58 (2016) Article DOI: 10.1021/acs.jmedchem.6b00309 BindingDB Entry DOI: 10.7270/Q25T3Q08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50214615 (CHEBI:64177 | Clobenpropit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of UR-DEBa242 binding to human recombinant NLuc/GPCR-fused H3R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500153 (CHEMBL3746851) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK136 from NPY1R in human SK-N-MC cells by radioligand binding assay | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50159205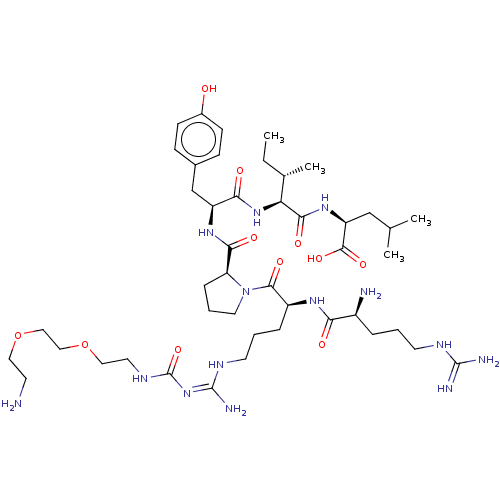 (CHEMBL3785233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50528183 (CHEMBL4540843) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]18 from human NPY Y4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ after 90 mins by liquid scintillation counting | J Med Chem 59: 6045-58 (2016) Article DOI: 10.1021/acs.jmedchem.6b00309 BindingDB Entry DOI: 10.7270/Q25T3Q08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of Aurora B ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human Aurora B ATP binding site by rapid dilution method | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK136 from NPY1R in human SK-N-MC cells by radioligand binding assay | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316677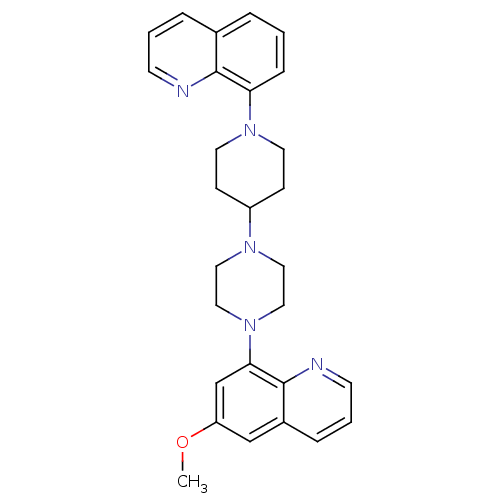 (6-Methoxy-8-{4-[1-(8-quinolinyl)-4-piperidinyl]-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50354216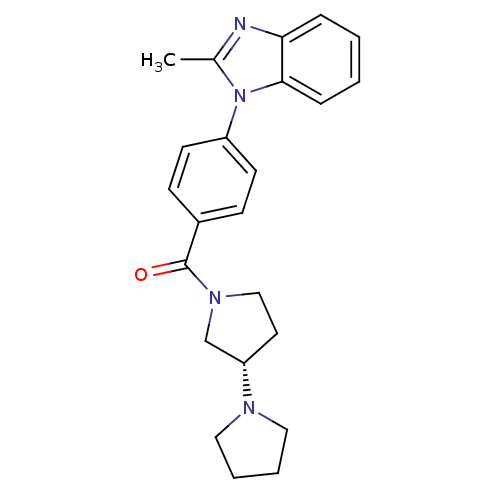 (CHEMBL1836004) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)alpha-methylhistamine from human histamine H3 receptor expressed in human HEK293T cells | Bioorg Med Chem Lett 21: 5957-60 (2011) Article DOI: 10.1016/j.bmcl.2011.07.061 BindingDB Entry DOI: 10.7270/Q2QN6753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50302220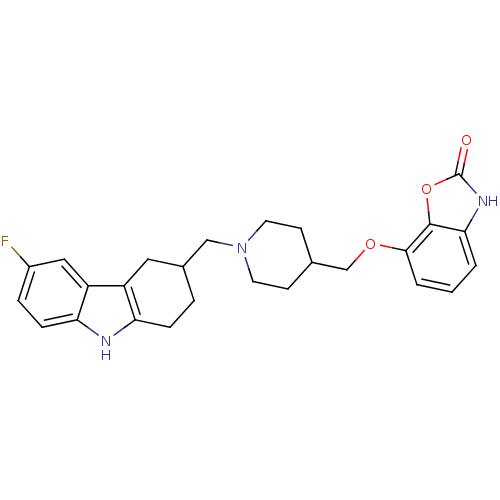 (7-((1-((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay | Bioorg Med Chem Lett 19: 5552-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.050 BindingDB Entry DOI: 10.7270/Q2Z89DCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316673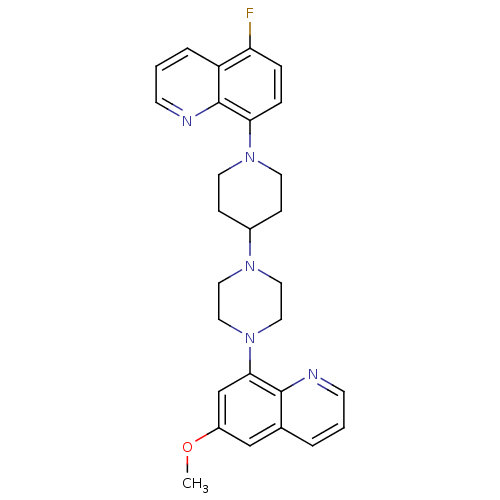 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50354216 (CHEMBL1836004) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125173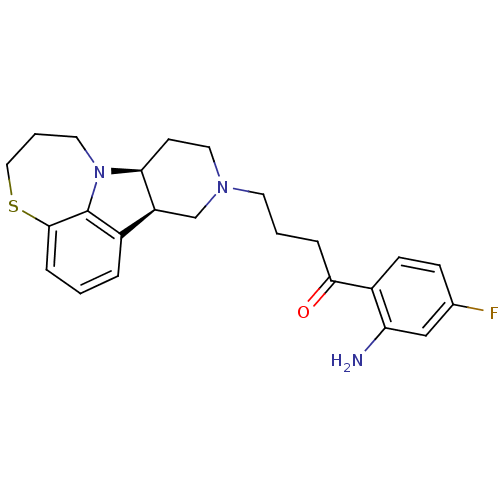 (1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-6,7,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50159164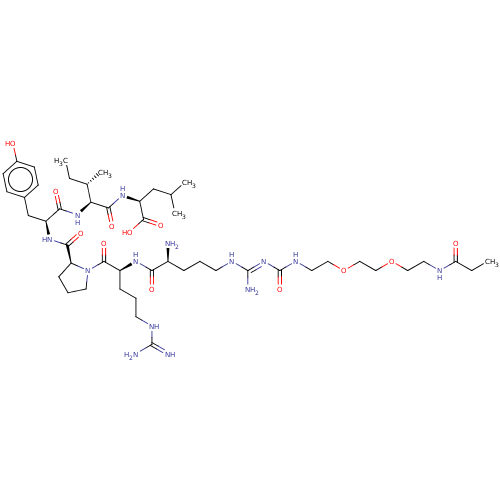 (CHEMBL3787200) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9811 total ) | Next | Last >> |