

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059585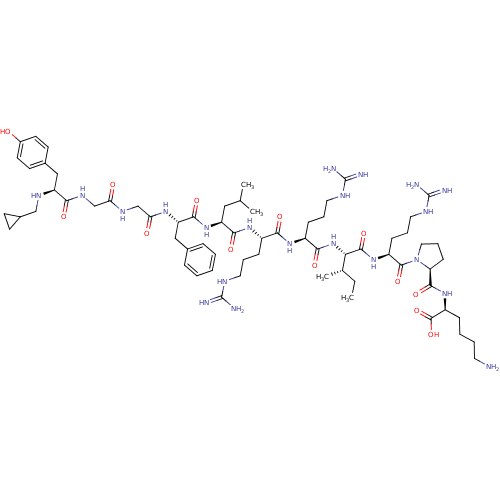 (CHEMBL442323 | N-CPM[D-Pro-10]Dyn A-(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002347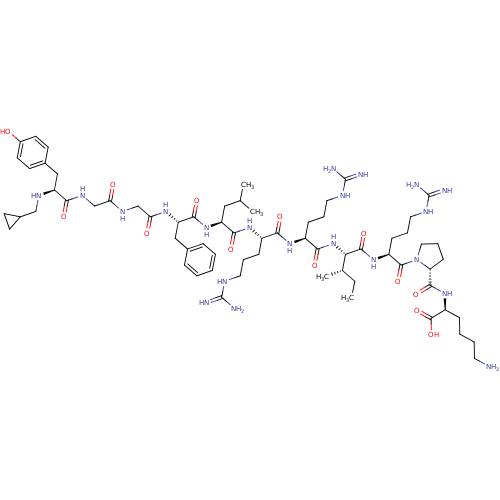 (CHEMBL425806 | [N-cyclopropyl methylTyr1, D-pro10]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002345 (CHEMBL407529 | PhCH2Tyr-Gly-Gly-Phe-Leu-Arg-Arg-ll...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059583 (CHEMBL268818 | N-benzyl[D-Pro-10]Dyn A-(1-11) | Ph...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002346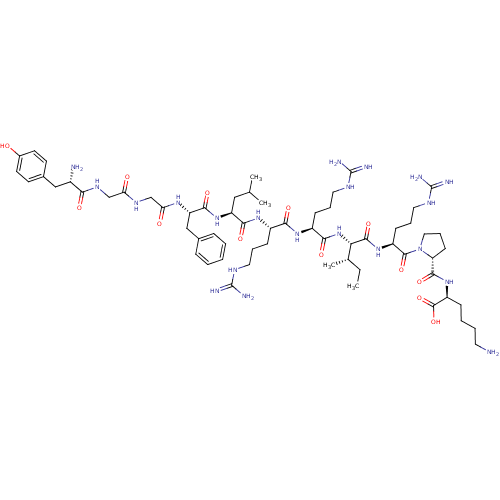 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002346 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620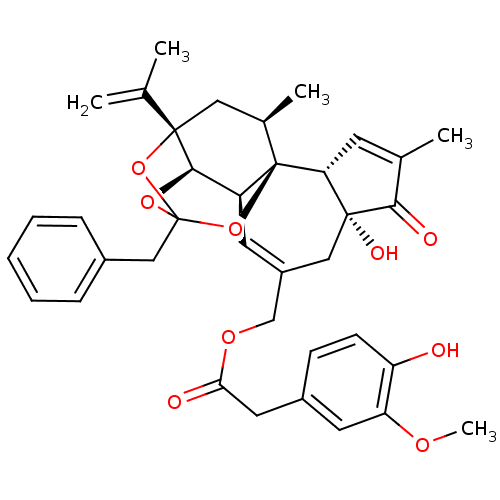 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059586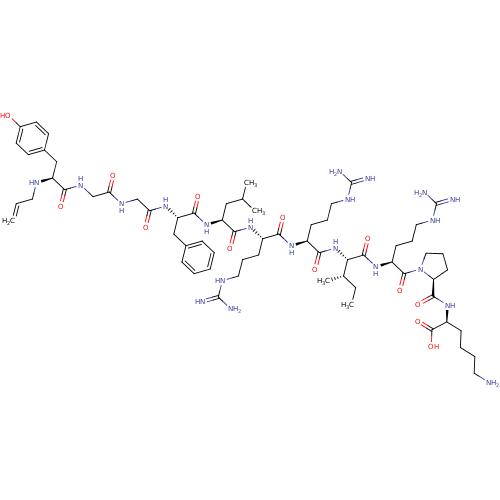 (CHEMBL406338 | N-allyl[D-Pro-10]Dyn A-(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002350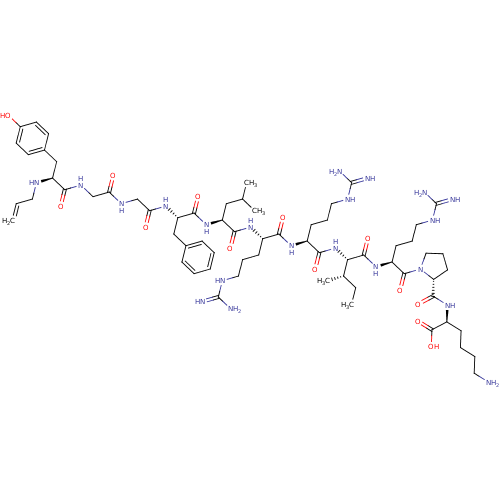 (CHEMBL265387 | [N-allylTyr1, D-pro10]Dynorphin A(1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247744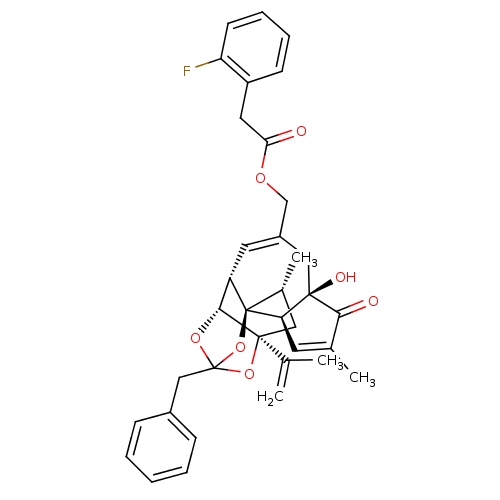 (CHEMBL504725 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284986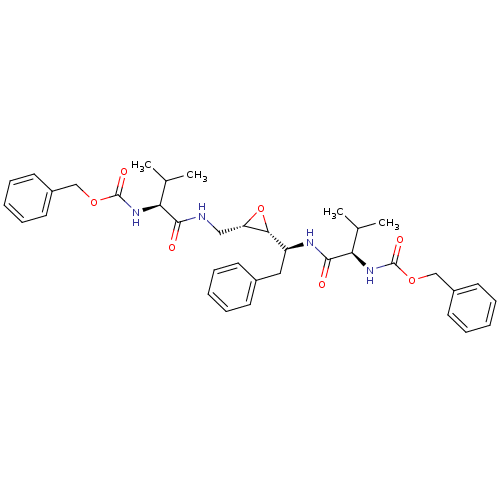 (CHEMBL55197 | [(R)-1-((S)-1-{(2R,3S)-3-[((S)-2-Ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288943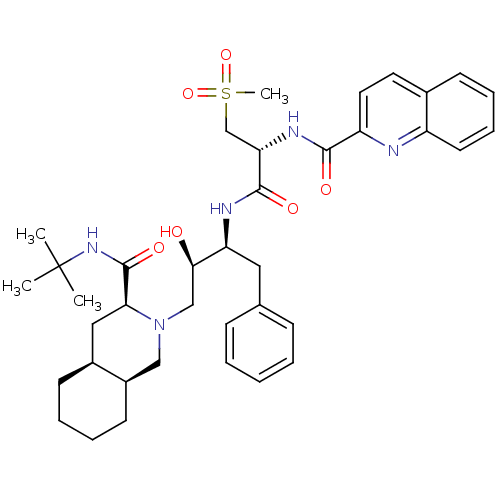 (CHEMBL154519 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052442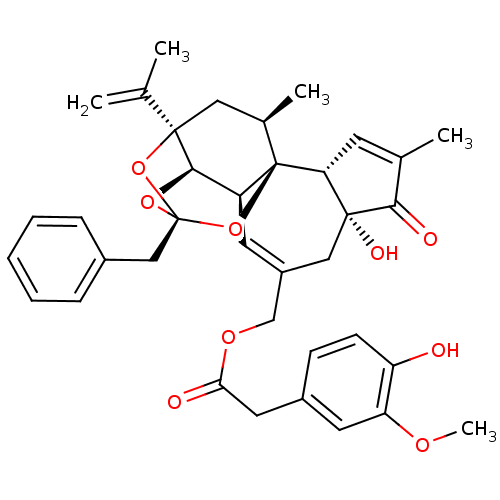 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsacin | J Med Chem 46: 3116-26 (2003) Article DOI: 10.1021/jm030089u BindingDB Entry DOI: 10.7270/Q2SB4551 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM92596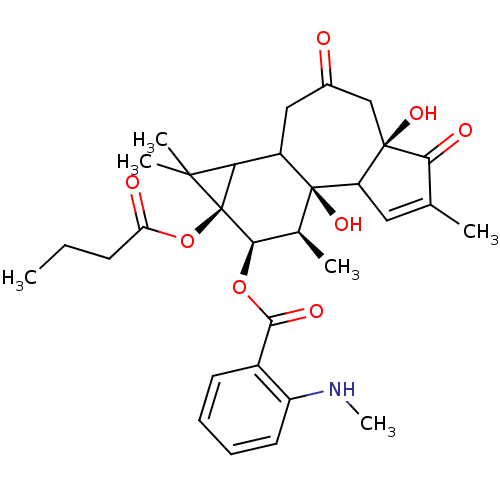 (Sapintoxin D) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288941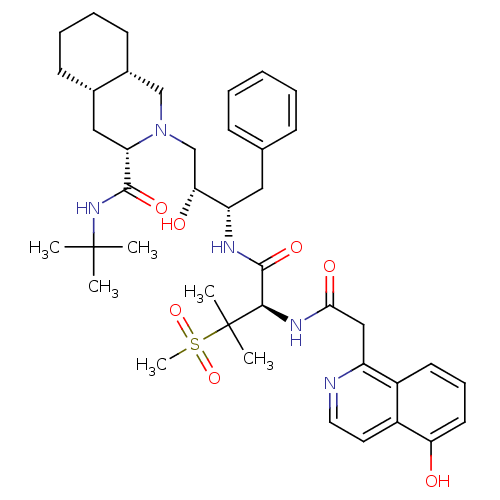 ((3S,4aS,8aS)-2-((2R,3S)-2-Hydroxy-3-{(R)-2-[2-(5-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571836 ((Z)-3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288942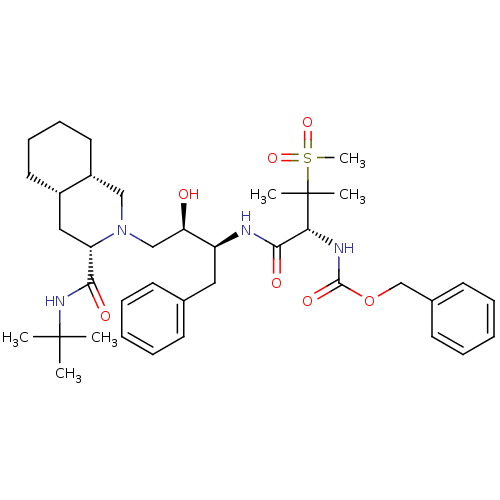 (CHEMBL154692 | {(R)-1-[(1S,2R)-1-Benzyl-3-((3S,4aS...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288939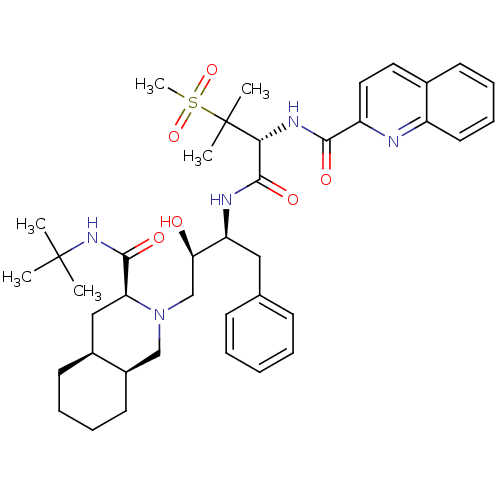 (CHEMBL345187 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059581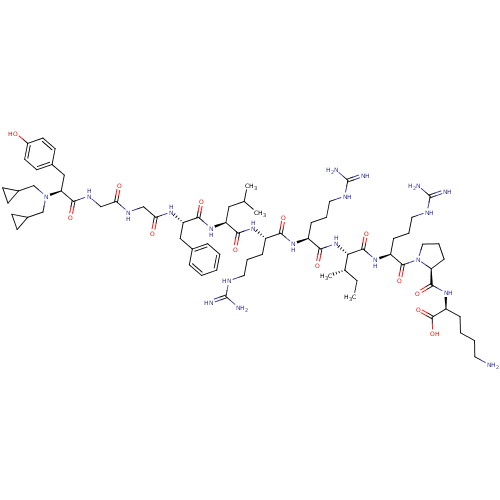 (CHEMBL406893 | N,N-diCPM[D-Pro-10]Dyn A-(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247741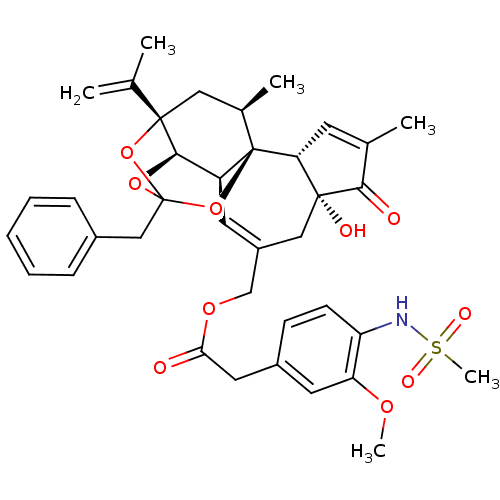 (CHEMBL503101 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002346 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor mu 1 was determined by measuring the inhibition of [3H]-DAMGO binding to rat forebrain membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50002346 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]DAMGO to opioid receptor mu in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266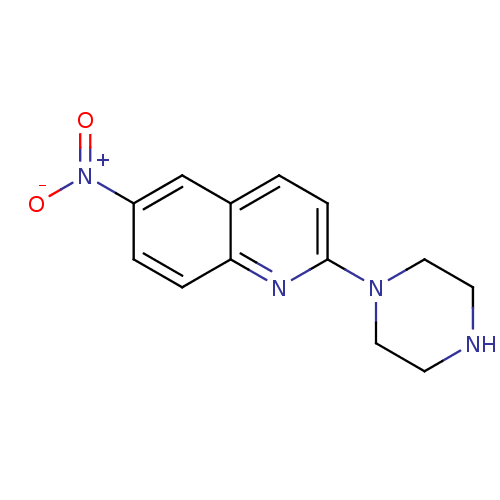 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247749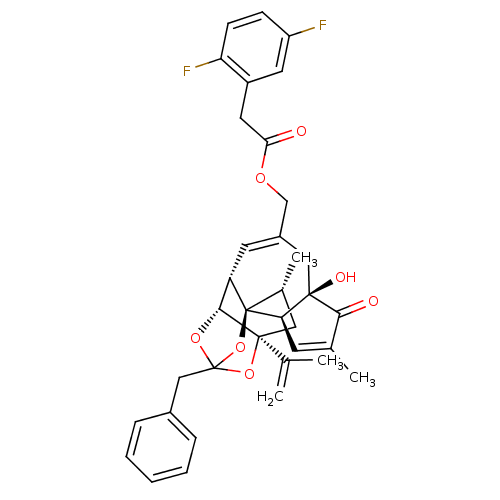 (CHEMBL510583 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208769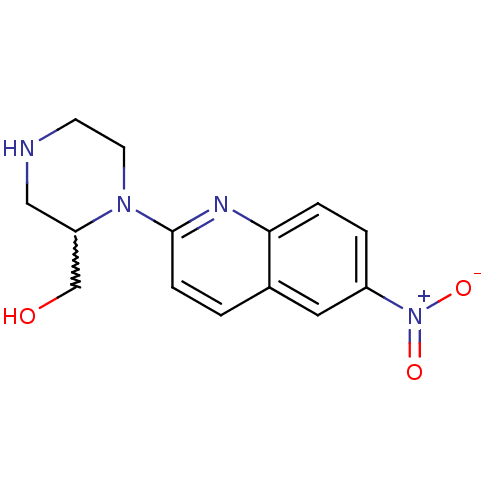 (2-[2-(hydroxymethyl)piperazin-1-yl]-6-nitroquinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208770 (2-[2-(ethoxymethyl)piperazin-1-yl]-6-nitroquinolin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571793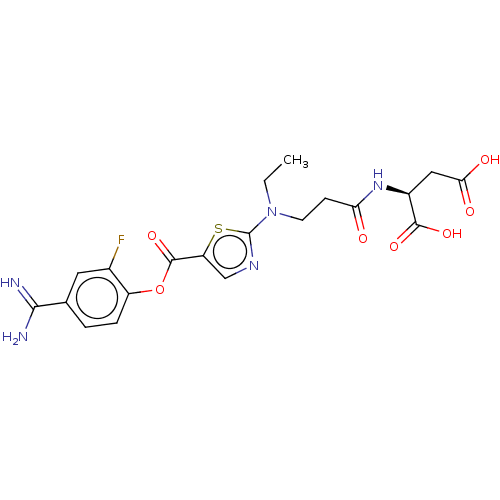 ((3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9294 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571830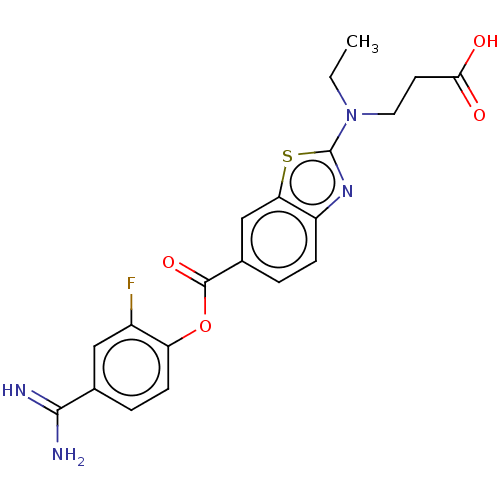 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571797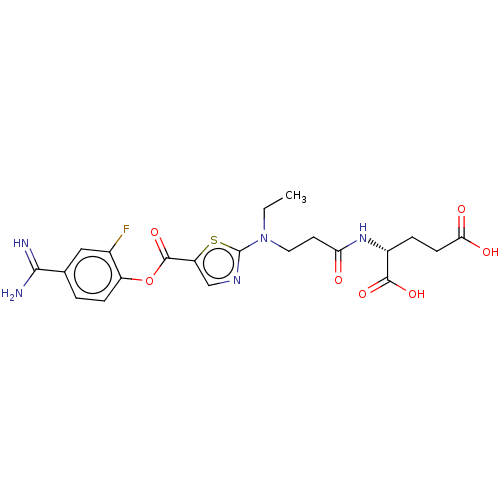 ((3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571817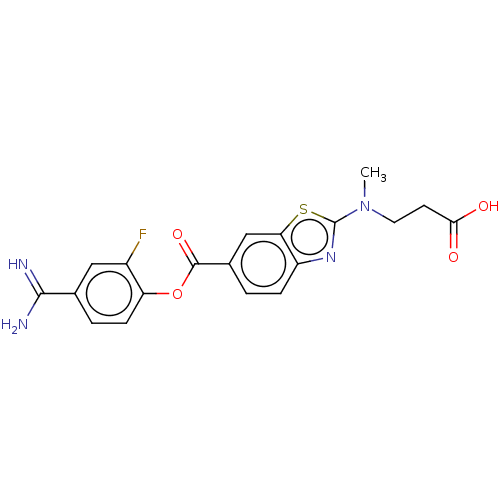 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571802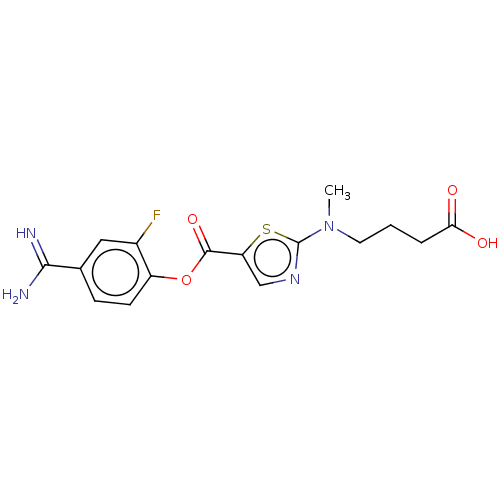 (4-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571815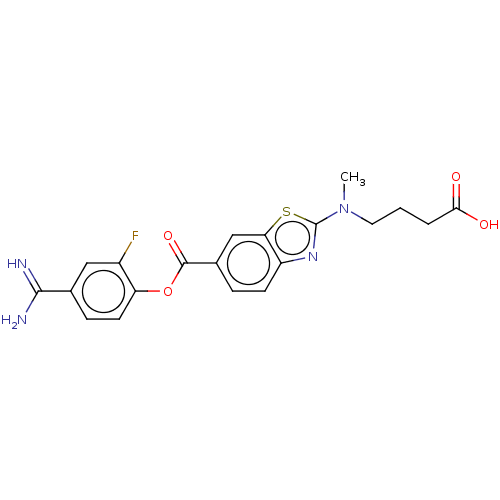 (4-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571814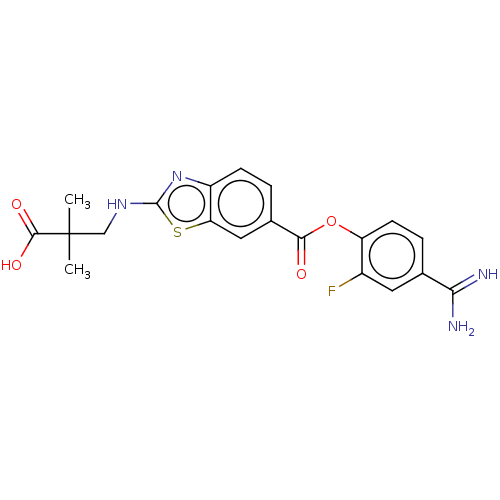 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571772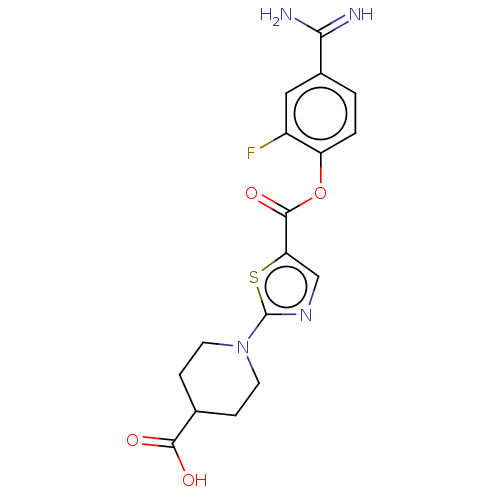 (1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)th...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20286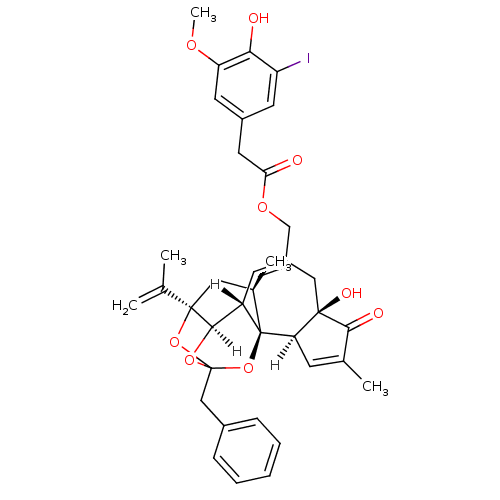 (5-I-RTX | 5-iodoresiniferatoxin | [(1R,2R,6R,10S,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -54.7 | n/a | n/a | 12.2 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571760 (3-((5-((4-carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208771 (2-[(2-methoxymethyl)piperazin-1-yl]-6-nitroquinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290520 (((R)-1-{(S)-1-[(2R,3S)-3-(2-tert-Butylsulfamoyl-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571843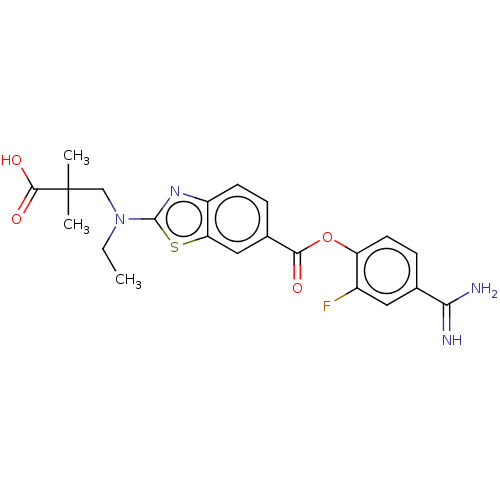 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290527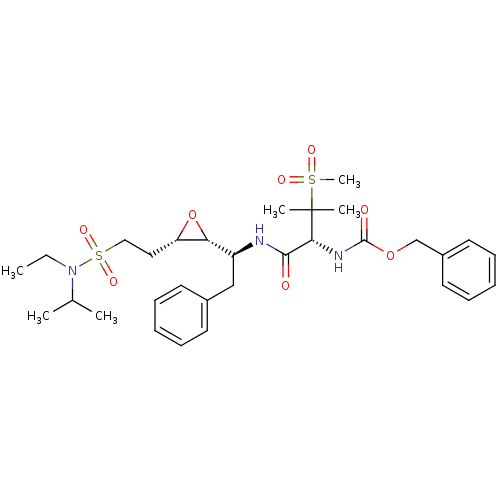 (CHEMBL86971 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247742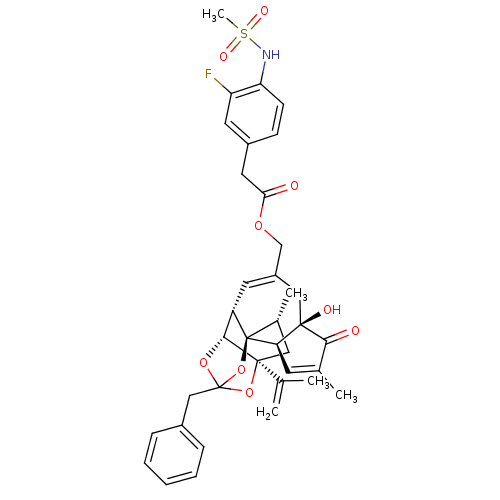 (CHEMBL509154 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247748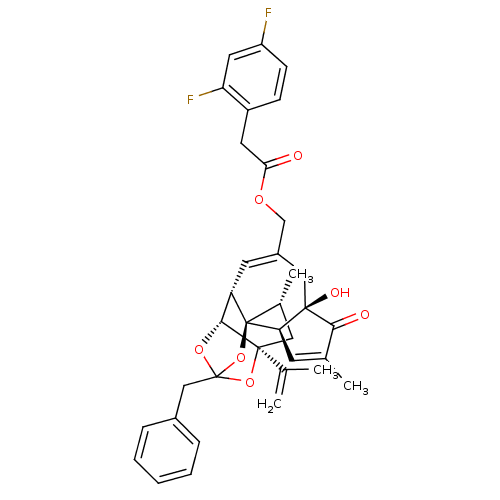 (CHEMBL510228 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571841 (3-((6-((4-Carbamimidoylphenoxy)carbonyl)benzo[d]th...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571766 (4-Carbamimidoyl-2-fluorophenyl 2-(4-(methoxycarbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247751 (CHEMBL449201 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571783 ((1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50009242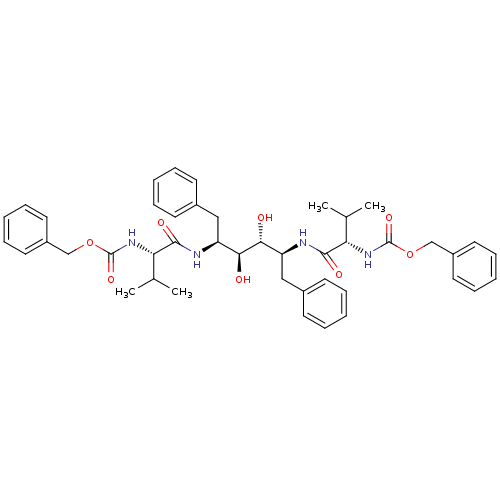 (CHEMBL48565 | {(S)-1-[(1S,2S,3R,4S)-1-Benzyl-4-((S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4238 total ) | Next | Last >> |