 Found 816 hits with Last Name = 'clark' and Initial = 'rd'
Found 816 hits with Last Name = 'clark' and Initial = 'rd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50417287
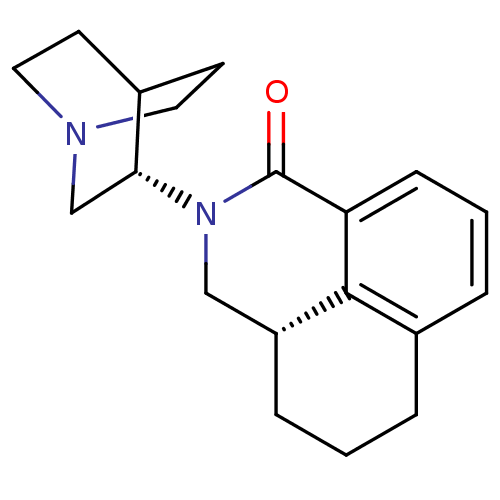
(Aloxi | Aurothioglucose | PALONOSETRON | PALONOSET...)Show SMILES O=C1N(C[C@H]2CCCc3cccc1c23)[C@@H]1CN2CCC1CC2 |wU:4.14,wD:14.16,(.24,-11.26,;.24,-12.8,;1.58,-13.56,;1.58,-15.1,;.24,-15.88,;.24,-17.42,;-1.09,-18.19,;-2.42,-17.42,;-2.42,-15.88,;-3.77,-15.1,;-3.77,-13.56,;-2.43,-12.78,;-1.09,-13.56,;-1.09,-15.1,;2.91,-12.8,;2.91,-11.26,;4.25,-10.49,;5.58,-11.26,;5.58,-12.8,;4.25,-13.56,;4.79,-12.41,;3.77,-11.79,)| Show InChI InChI=1S/C19H24N2O/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20/h2,4,6,13,15,17H,1,3,5,7-12H2/t15-,17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477491

(CHEMBL398034)Show SMILES Clc1cccc(Cl)c1S(=O)(=O)N1CCOc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H19Cl2N3O3S/c19-13-3-1-4-14(20)18(13)27(24,25)23-11-12-26-17-15(5-2-6-16(17)23)22-9-7-21-8-10-22/h1-6,21H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477488
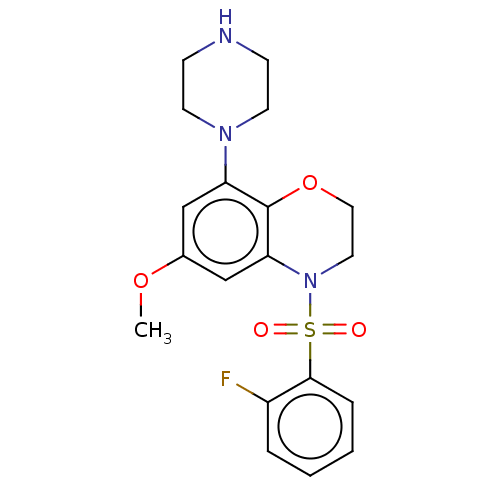
(CHEMBL394690)Show SMILES COc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H22FN3O4S/c1-26-14-12-16(22-8-6-21-7-9-22)19-17(13-14)23(10-11-27-19)28(24,25)18-5-3-2-4-15(18)20/h2-5,12-13,21H,6-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000453
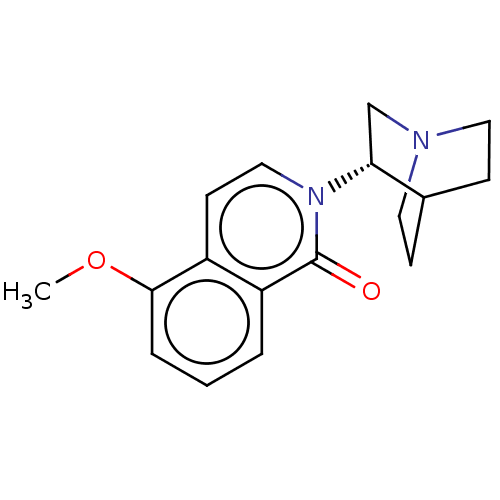
(CHEMBL540055)Show SMILES Cl.COc1cccc2c1ccn([C@@H]1CN3CCC1CC3)c2=O |r,wU:12.11,(5.25,.07,;2.41,-3.7,;1.34,-3.08,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;,.77,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.55,;-3.98,3.16,;-5.37,3.98,;-5.91,2.55,;-4.84,1.88,;-5.42,.72,;-6.81,1.56,;-6.78,3.19,;-1.33,1.54,;-1.33,2.77,)| Show InChI InChI=1S/C17H20N2O2.ClH/c1-21-16-4-2-3-14-13(16)7-10-19(17(14)20)15-11-18-8-5-12(15)6-9-18;/h2-4,7,10,12,15H,5-6,8-9,11H2,1H3;1H/t15-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000523
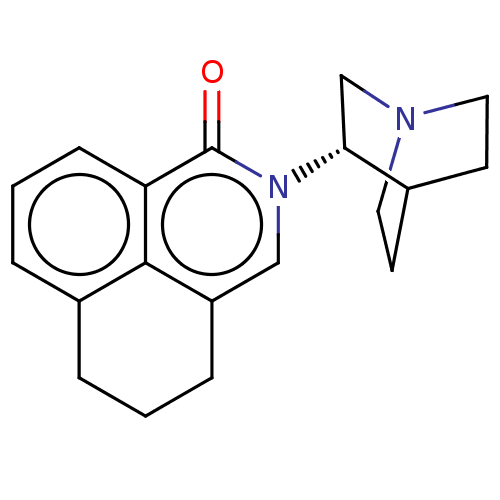
(CHEMBL544784)Show SMILES Cl.O=c1n(cc2CCCc3cccc1c23)[C@@H]1CN2CCC1CC2 |r,wU:15.16,(5.24,-.81,;-3.74,.62,;-2.67,,;-2.67,-1.54,;-1.33,-2.33,;,-1.54,;1.33,-2.33,;2.67,-1.54,;2.67,,;1.33,.77,;1.33,2.31,;,3.1,;-1.33,2.31,;-1.33,.77,;;-4.01,-2.31,;-5.39,-1.47,;-6.79,-2.26,;-5.82,-3.45,;-4.71,-2.85,;-3.99,-3.93,;-5.4,-4.72,;-6.81,-3.88,)| Show InChI InChI=1S/C19H22N2O.ClH/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20;/h2,4,6,11,13,17H,1,3,5,7-10,12H2;1H/t17-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000471

(CHEMBL542900)Show SMILES Cl.CCc1cccc2c1ccn([C@@H]1CN3CCC1CC3)c2=O |r,wU:12.11,(5.25,.07,;2.41,-3.7,;1.34,-3.08,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;,.77,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.55,;-3.98,3.16,;-5.37,3.98,;-5.91,2.55,;-4.84,1.88,;-5.42,.72,;-6.81,1.56,;-6.78,3.19,;-1.33,1.54,;-1.33,2.77,)| Show InChI InChI=1S/C18H22N2O.ClH/c1-2-13-4-3-5-16-15(13)8-11-20(18(16)21)17-12-19-9-6-14(17)7-10-19;/h3-5,8,11,14,17H,2,6-7,9-10,12H2,1H3;1H/t17-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000450
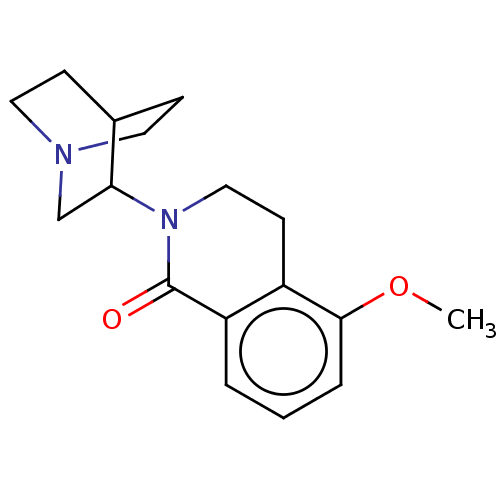
(CHEMBL542669)Show SMILES Cl.COc1cccc2C(=O)N(CCc12)C1CN2CCC1CC2 |(5.25,.07,;2.41,-3.7,;1.34,-3.08,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-1.33,2.77,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;-4.02,1.55,;-3.98,3.16,;-5.37,3.98,;-5.91,2.55,;-4.84,1.88,;-5.42,.72,;-6.81,1.56,;-6.78,3.19,)| Show InChI InChI=1S/C17H22N2O2.ClH/c1-21-16-4-2-3-14-13(16)7-10-19(17(14)20)15-11-18-8-5-12(15)6-9-18;/h2-4,12,15H,5-11H2,1H3;1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000457

(CHEMBL542904)Show SMILES Cl.CCc1cccc2C(=O)N(CCc12)C1CN2CCC1CC2 |(5.25,.07,;2.41,-3.7,;1.34,-3.08,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-1.33,2.77,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;-4.02,1.55,;-3.98,3.16,;-5.37,3.98,;-5.91,2.55,;-4.84,1.88,;-5.42,.72,;-6.81,1.56,;-6.78,3.19,)| Show InChI InChI=1S/C18H24N2O.ClH/c1-2-13-4-3-5-16-15(13)8-11-20(18(16)21)17-12-19-9-6-14(17)7-10-19;/h3-5,14,17H,2,6-12H2,1H3;1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50056419
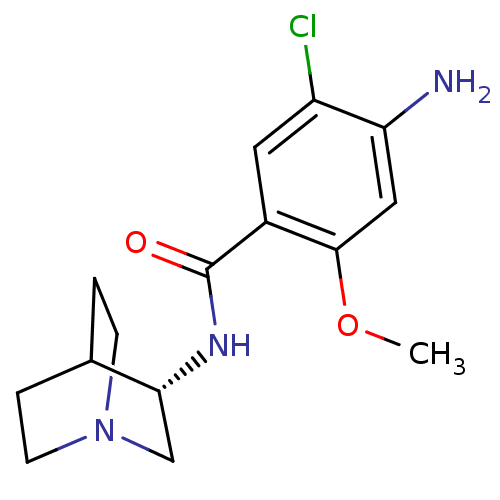
(4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wD:13.13,TLB:12:13:17.16:19.20,(11.11,-14.18,;11.11,-12.63,;9.77,-11.88,;8.43,-12.66,;7.09,-11.89,;5.75,-12.66,;7.09,-10.33,;5.76,-9.57,;8.43,-9.56,;9.75,-10.36,;11.09,-9.59,;11.11,-8.04,;12.44,-10.36,;13.77,-9.59,;14.8,-8.58,;16.63,-8.19,;17.94,-9.45,;16.9,-10.39,;15.57,-9.1,;15.88,-7.77,;16.88,-7.14,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372966
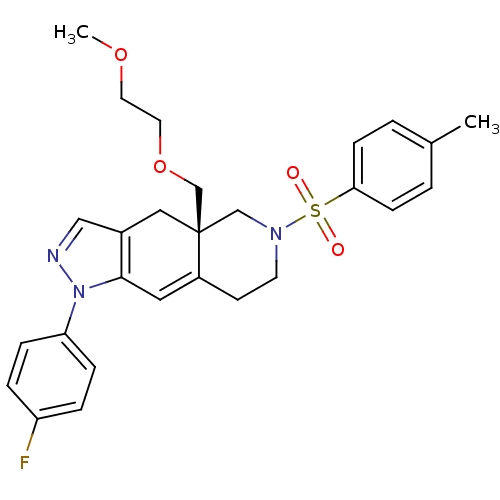
(CHEMBL270668)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(C)cc1 |c:12| Show InChI InChI=1S/C27H30FN3O4S/c1-20-3-9-25(10-4-20)36(32,33)30-12-11-22-15-26-21(16-27(22,18-30)19-35-14-13-34-2)17-29-31(26)24-7-5-23(28)6-8-24/h3-10,15,17H,11-14,16,18-19H2,1-2H3/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505
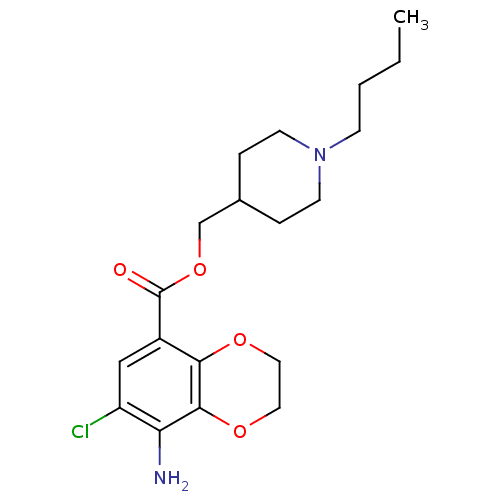
(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477473

(CHEMBL246918)Show SMILES CC1(C)CN(c2cccc(N3CCNCC3)c2O1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C20H24FN3O3S/c1-20(2)14-24(28(25,26)18-9-4-3-6-15(18)21)17-8-5-7-16(19(17)27-20)23-12-10-22-11-13-23/h3-9,22H,10-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000475
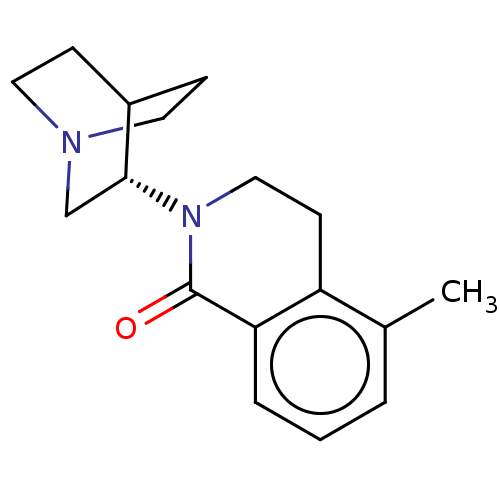
(CHEMBL555068)Show SMILES Cl.Cc1cccc2C(=O)N(CCc12)[C@@H]1CN2CCC1CC2 |r,wU:13.13,(5.24,.45,;1.33,-2.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-1.33,2.77,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;-4.02,1.55,;-3.98,3.16,;-5.37,3.98,;-5.91,2.55,;-4.84,1.88,;-5.42,.72,;-6.81,1.56,;-6.78,3.19,)| Show InChI InChI=1S/C17H22N2O.ClH/c1-12-3-2-4-15-14(12)7-10-19(17(15)20)16-11-18-8-5-13(16)6-9-18;/h2-4,13,16H,5-11H2,1H3;1H/t16-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000460
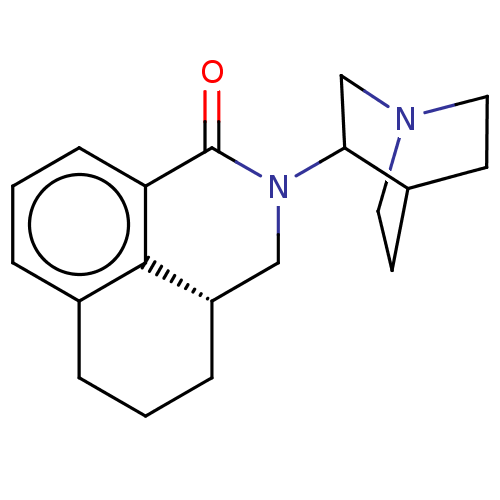
(CHEMBL540057)Show SMILES Cl.[H][C@]12CCCc3cccc(C(=O)N(C1)C1CN4CCC1CC4)c23 |r,wD:2.0,(5.24,-.81,;,-2.57,;,-1.54,;1.33,-2.33,;2.67,-1.54,;2.67,,;1.33,.77,;1.33,2.31,;,3.1,;-1.33,2.31,;-1.33,.77,;-2.67,,;-3.74,.62,;-2.67,-1.54,;-1.33,-2.33,;-4.01,-2.31,;-5.39,-1.47,;-6.79,-2.26,;-5.82,-3.45,;-4.71,-2.85,;-3.99,-3.93,;-5.4,-4.72,;-6.81,-3.88,;)| Show InChI InChI=1S/C19H24N2O.ClH/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20;/h2,4,6,13,15,17H,1,3,5,7-12H2;1H/t15-,17?;/m0./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228318
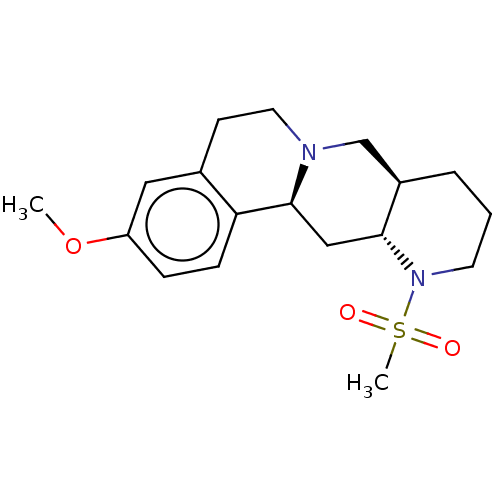
(CHEMBL279807)Show SMILES [H][C@]12CCCN([C@]1([H])C[C@]1([H])N(CCc3cc(OC)ccc13)C2)S(C)(=O)=O Show InChI InChI=1S/C18H26N2O3S/c1-23-15-5-6-16-13(10-15)7-9-19-12-14-4-3-8-20(24(2,21)22)17(14)11-18(16)19/h5-6,10,14,17-18H,3-4,7-9,11-12H2,1-2H3/t14-,17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2034-6 (1989)
BindingDB Entry DOI: 10.7270/Q2WH2S78 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228318

(CHEMBL279807)Show SMILES [H][C@]12CCCN([C@]1([H])C[C@]1([H])N(CCc3cc(OC)ccc13)C2)S(C)(=O)=O Show InChI InChI=1S/C18H26N2O3S/c1-23-15-5-6-16-13(10-15)7-9-19-12-14-4-3-8-20(24(2,21)22)17(14)11-18(16)19/h5-6,10,14,17-18H,3-4,7-9,11-12H2,1-2H3/t14-,17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477475

(CHEMBL246499)Show SMILES Cc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H22FN3O3S/c1-14-12-16(22-8-6-21-7-9-22)19-17(13-14)23(10-11-26-19)27(24,25)18-5-3-2-4-15(18)20/h2-5,12-13,21H,6-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477489

(CHEMBL246705)Show SMILES Fc1ccccc1S(=O)(=O)N1CCOc2c(cc(Cl)cc12)N1CCNCC1 Show InChI InChI=1S/C18H19ClFN3O3S/c19-13-11-15(22-7-5-21-6-8-22)18-16(12-13)23(9-10-26-18)27(24,25)17-4-2-1-3-14(17)20/h1-4,11-12,21H,5-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477479
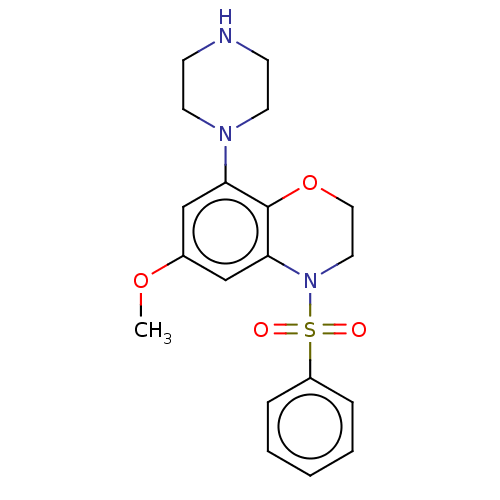
(CHEMBL246706)Show SMILES COc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H23N3O4S/c1-25-15-13-17(21-9-7-20-8-10-21)19-18(14-15)22(11-12-26-19)27(23,24)16-5-3-2-4-6-16/h2-6,13-14,20H,7-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human glucocorticoid receptor expressed in recombinant baculovirus |
Bioorg Med Chem Lett 17: 5704-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.055
BindingDB Entry DOI: 10.7270/Q2FX7B8B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000455
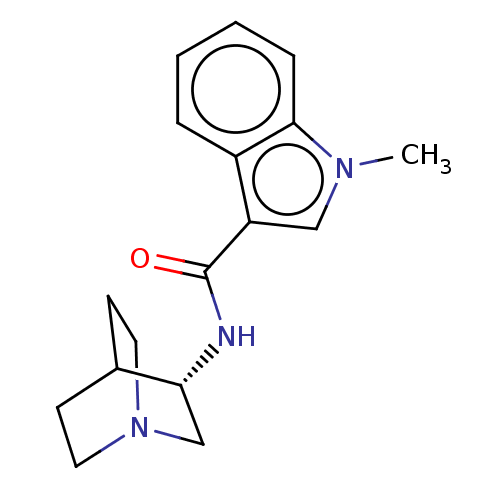
(CHEMBL2093898)Show SMILES Cl.Cn1cc(C(=O)N[C@@H]2CN3CCC2CC3)c2ccccc12 |r,wD:8.6,(8.85,2.41,;2.14,-2.4,;1.76,-1.23,;2.65,.02,;1.76,1.23,;2.24,2.69,;1.42,3.61,;3.74,3.01,;4.22,4.47,;3.12,5.65,;3.61,7.18,;4.97,6.47,;4.61,5.26,;5.81,4.78,;6.29,6.32,;5.19,7.52,;.3,.77,;-1.03,1.55,;-2.37,.77,;-2.37,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C17H21N3O.ClH/c1-19-10-14(13-4-2-3-5-16(13)19)17(21)18-15-11-20-8-6-12(15)7-9-20;/h2-5,10,12,15H,6-9,11H2,1H3,(H,18,21);1H/t15-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000469

(CHEMBL542903)Show SMILES Cl.O=c1n(cc2CCCCc3cccc1c23)[C@@H]1CN2CCC1CC2 |r,wD:16.17,(10.72,2.93,;3,5.97,;2.65,4.82,;3.7,3.7,;3.24,2.21,;1.74,1.87,;1.52,.37,;.18,-.4,;-.81,,;-1.5,1.28,;-.79,2.68,;-1.84,3.81,;-1.39,5.27,;.1,5.61,;1.15,4.48,;.69,3,;5.16,4.04,;5.59,5.56,;7.12,5.92,;7.21,4.43,;6.03,4.11,;6.23,2.87,;7.76,3.25,;8.21,4.78,)| Show InChI InChI=1S/C20H24N2O.ClH/c23-20-17-7-3-6-15-4-1-2-5-16(19(15)17)12-22(20)18-13-21-10-8-14(18)9-11-21;/h3,6-7,12,14,18H,1-2,4-5,8-11,13H2;1H/t18-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229080
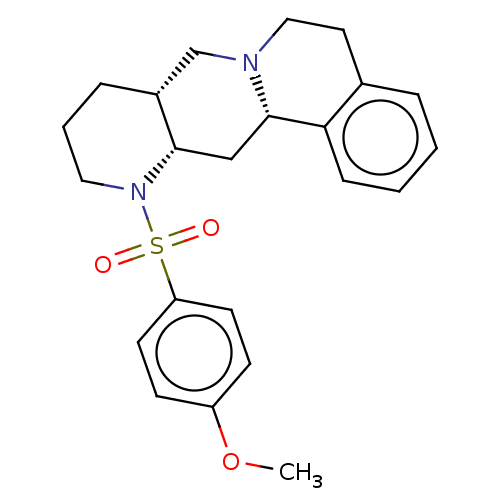
(CHEMBL349665)Show SMILES [H][C@]12CCCN([C@@]1([H])C[C@]1([H])N(CCc3ccccc13)C2)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C23H28N2O3S/c1-28-19-8-10-20(11-9-19)29(26,27)25-13-4-6-18-16-24-14-12-17-5-2-3-7-21(17)23(24)15-22(18)25/h2-3,5,7-11,18,22-23H,4,6,12-16H2,1H3/t18-,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372977
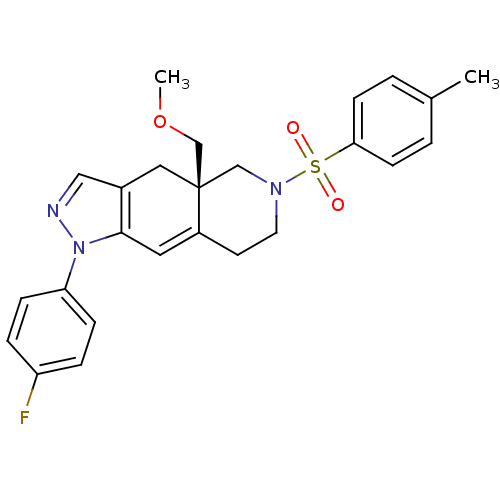
(CHEMBL255369)Show SMILES COC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(C)cc1 |c:9| Show InChI InChI=1S/C25H26FN3O3S/c1-18-3-9-23(10-4-18)33(30,31)28-12-11-20-13-24-19(14-25(20,16-28)17-32-2)15-27-29(24)22-7-5-21(26)6-8-22/h3-10,13,15H,11-12,14,16-17H2,1-2H3/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372975
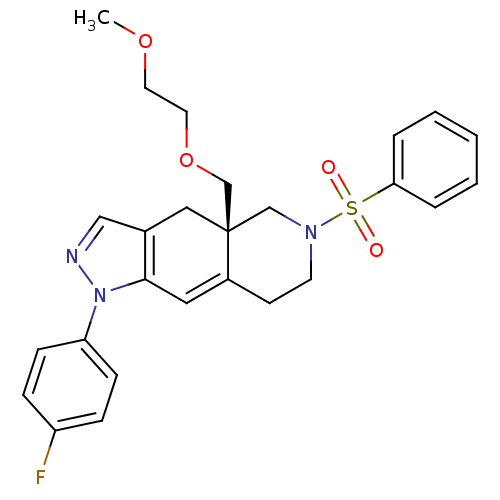
(CHEMBL270667)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccccc1 |c:12| Show InChI InChI=1S/C26H28FN3O4S/c1-33-13-14-34-19-26-16-20-17-28-30(23-9-7-22(27)8-10-23)25(20)15-21(26)11-12-29(18-26)35(31,32)24-5-3-2-4-6-24/h2-10,15,17H,11-14,16,18-19H2,1H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477477
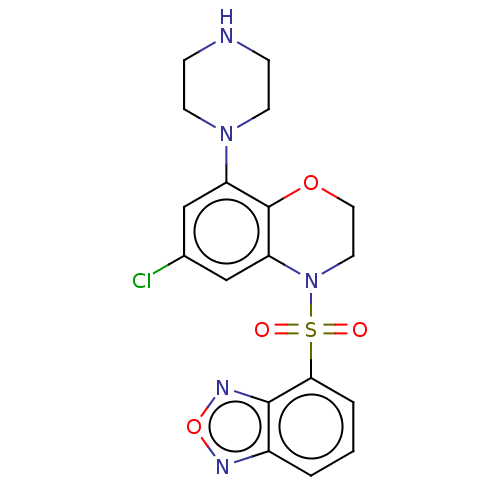
(CHEMBL246291)Show SMILES Clc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1cccc2nonc12 Show InChI InChI=1S/C18H18ClN5O4S/c19-12-10-14(23-6-4-20-5-7-23)18-15(11-12)24(8-9-27-18)29(25,26)16-3-1-2-13-17(16)22-28-21-13/h1-3,10-11,20H,4-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372965
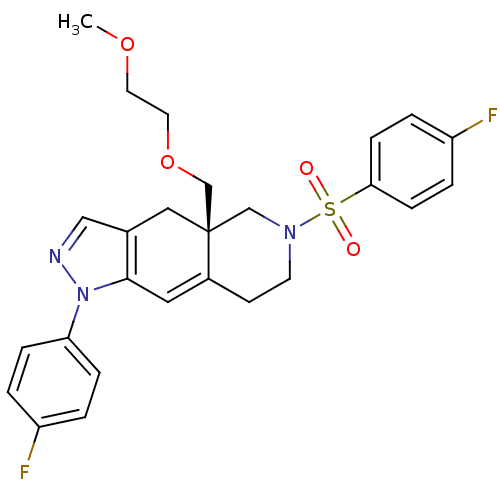
(CHEMBL442803)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(F)cc1 |c:12| Show InChI InChI=1S/C26H27F2N3O4S/c1-34-12-13-35-18-26-15-19-16-29-31(23-6-2-21(27)3-7-23)25(19)14-20(26)10-11-30(17-26)36(32,33)24-8-4-22(28)5-9-24/h2-9,14,16H,10-13,15,17-18H2,1H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372937
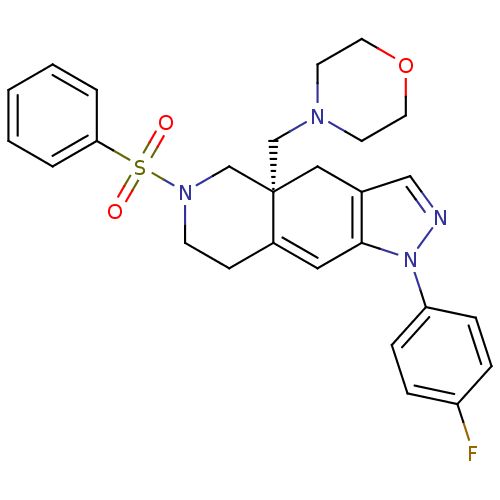
(CHEMBL257461)Show SMILES Fc1ccc(cc1)-n1ncc2C[C@]3(CN4CCOCC4)CN(CCC3=Cc12)S(=O)(=O)c1ccccc1 |c:27| Show InChI InChI=1S/C27H29FN4O3S/c28-23-6-8-24(9-7-23)32-26-16-22-10-11-31(36(33,34)25-4-2-1-3-5-25)20-27(22,17-21(26)18-29-32)19-30-12-14-35-15-13-30/h1-9,16,18H,10-15,17,19-20H2/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113780
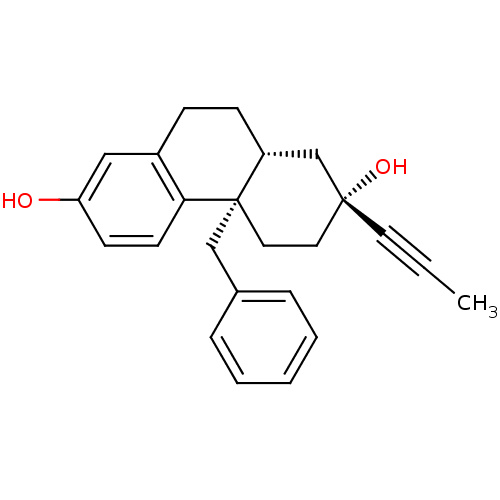
((2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a...)Show SMILES CC#C[C@@]1(O)CC[C@]2(Cc3ccccc3)[C@H](CCc3cc(O)ccc23)C1 Show InChI InChI=1S/C24H26O2/c1-2-12-23(26)13-14-24(16-18-6-4-3-5-7-18)20(17-23)9-8-19-15-21(25)10-11-22(19)24/h3-7,10-11,15,20,25-26H,8-9,13-14,16-17H2,1H3/t20-,23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human glucocorticoid receptor expressed in recombinant baculovirus |
Bioorg Med Chem Lett 17: 5704-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.055
BindingDB Entry DOI: 10.7270/Q2FX7B8B |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372974
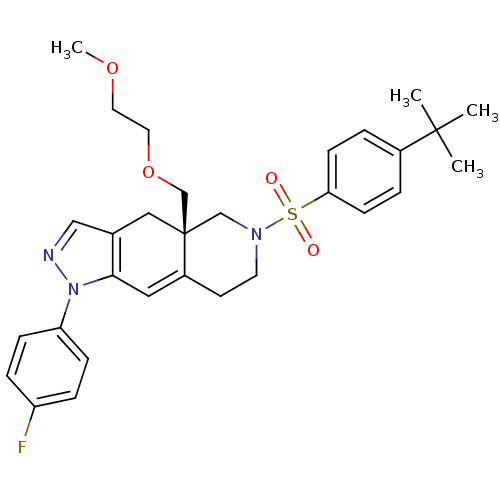
(CHEMBL270575)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(C)(C)C |c:12| Show InChI InChI=1S/C30H36FN3O4S/c1-29(2,3)23-5-11-27(12-6-23)39(35,36)33-14-13-24-17-28-22(18-30(24,20-33)21-38-16-15-37-4)19-32-34(28)26-9-7-25(31)8-10-26/h5-12,17,19H,13-16,18,20-21H2,1-4H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at GR in SW1353/MMTV5 cells assessed as inhibition of dexamethasone-induced luciferase expression |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229162

(CHEMBL349747)Show SMILES [H][C@]12CCCN([C@@]1([H])C[C@]1([H])N(CCc3cc(OC)ccc13)C2)S(=O)(=O)CCOC Show InChI InChI=1S/C20H30N2O4S/c1-25-10-11-27(23,24)22-8-3-4-16-14-21-9-7-15-12-17(26-2)5-6-18(15)20(21)13-19(16)22/h5-6,12,16,19-20H,3-4,7-11,13-14H2,1-2H3/t16-,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for alpha-1 adrenoceptor of rat cerebral cortex was determined by ligand binding using [3H]prazosin |
J Med Chem 26: 855-61 (1983)
BindingDB Entry DOI: 10.7270/Q2TH8PW6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000462
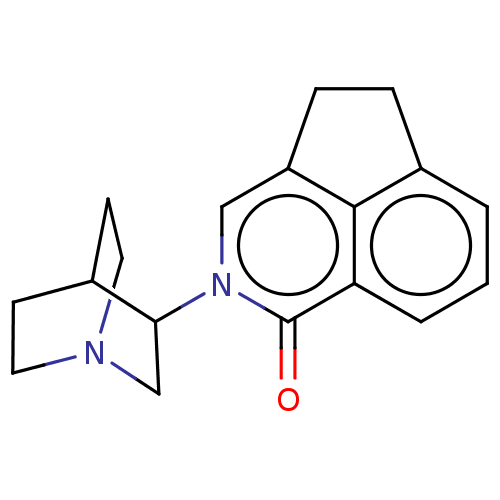
(CHEMBL545719)Show SMILES Cl.O=c1n(cc2CCc3cccc1c23)C1CN2CCC1CC2 |(5.29,-.99,;-1.31,-3.3,;-1.32,-2.04,;-2.66,-1.28,;-2.66,.25,;-1.32,1.01,;-1.01,2.56,;.94,2.54,;1.34,1.01,;2.67,.25,;2.67,-1.28,;1.34,-2.04,;,-1.28,;,.25,;-4.01,-2.07,;-3.97,-3.71,;-5.39,-4.55,;-5.94,-3.08,;-4.85,-2.41,;-5.43,-1.23,;-6.85,-2.08,;-6.82,-3.74,)| Show InChI InChI=1S/C18H20N2O.ClH/c21-18-15-3-1-2-13-4-5-14(17(13)15)10-20(18)16-11-19-8-6-12(16)7-9-19;/h1-3,10,12,16H,4-9,11H2;1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000445
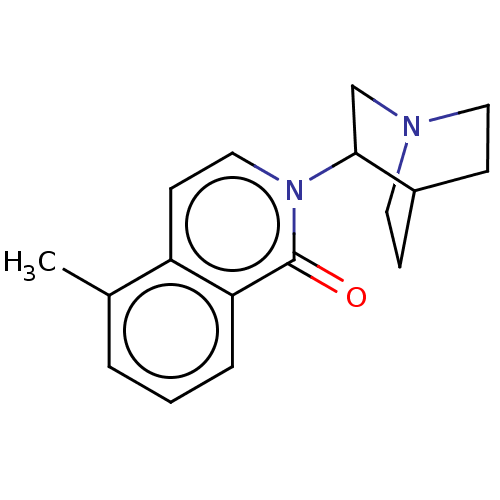
(CHEMBL555038)Show SMILES Cl.Cc1cccc2c1ccn(C1CN3CCC1CC3)c2=O |(5.24,.45,;1.33,-2.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;,.77,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.55,;-3.98,3.16,;-5.37,3.98,;-5.91,2.55,;-4.84,1.88,;-5.42,.72,;-6.81,1.56,;-6.78,3.19,;-1.33,1.54,;-1.33,2.77,)| Show InChI InChI=1S/C17H20N2O.ClH/c1-12-3-2-4-15-14(12)7-10-19(17(15)20)16-11-18-8-5-13(16)6-9-18;/h2-4,7,10,13,16H,5-6,8-9,11H2,1H3;1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477497
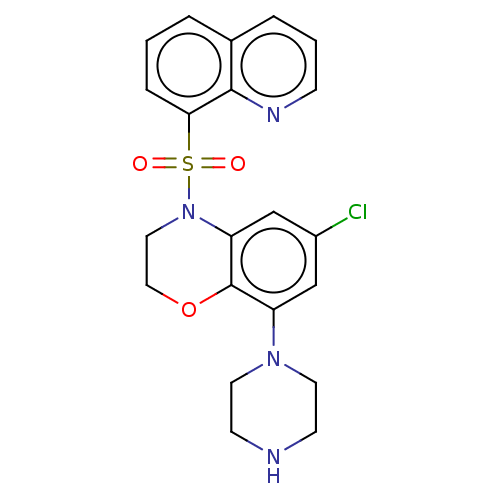
(CHEMBL246498)Show SMILES Clc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1cccc2cccnc12 Show InChI InChI=1S/C21H21ClN4O3S/c22-16-13-17(25-9-7-23-8-10-25)21-18(14-16)26(11-12-29-21)30(27,28)19-5-1-3-15-4-2-6-24-20(15)19/h1-6,13-14,23H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477490
(CHEMBL394691)Show SMILES Fc1ccccc1S(=O)(=O)N1CC2(CCC2)Oc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C21H24FN3O3S/c22-16-5-1-2-8-19(16)29(26,27)25-15-21(9-4-10-21)28-20-17(6-3-7-18(20)25)24-13-11-23-12-14-24/h1-3,5-8,23H,4,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477487

(CHEMBL246704)Show SMILES Fc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C18H19F2N3O3S/c19-13-11-15(22-7-5-21-6-8-22)18-16(12-13)23(9-10-26-18)27(24,25)17-4-2-1-3-14(17)20/h1-4,11-12,21H,5-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229147
(CHEMBL348793)Show SMILES [H][C@]12CCCN([C@]1([H])C[C@]1([H])N(CCc3cc4OCOc4cc13)C2)S(C)(=O)=O Show InChI InChI=1S/C18H24N2O4S/c1-25(21,22)20-5-2-3-13-10-19-6-4-12-7-17-18(24-11-23-17)8-14(12)16(19)9-15(13)20/h7-8,13,15-16H,2-6,9-11H2,1H3/t13-,15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229143
(CHEMBL162971)Show SMILES [H][C@]12CCCN([C@@]1([H])C[C@]1([H])N(CCc3cc4OCOc4cc13)C2)S(C)(=O)=O Show InChI InChI=1S/C18H24N2O4S/c1-25(21,22)20-5-2-3-13-10-19-6-4-12-7-17-18(24-11-23-17)8-14(12)16(19)9-15(13)20/h7-8,13,15-16H,2-6,9-11H2,1H3/t13-,15+,16+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229179

(Delequamine)Show SMILES [H][C@]12CCCN([C@@]1([H])C[C@]1([H])N(CCc3cc(OC)ccc13)C2)S(C)(=O)=O Show InChI InChI=1S/C18H26N2O3S/c1-23-15-5-6-16-13(10-15)7-9-19-12-14-4-3-8-20(24(2,21)22)17(14)11-18(16)19/h5-6,10,14,17-18H,3-4,7-9,11-12H2,1-2H3/t14-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228316
(CHEMBL62912)Show InChI InChI=1S/C18H26N2O3S/c1-23-15-5-6-16-13(10-15)7-9-19-12-14-4-3-8-20(24(2,21)22)17(14)11-18(16)19/h5-6,10,14,17-18H,3-4,7-9,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Escherichia coli dihydrofolate reductase. |
J Med Chem 32: 2034-6 (1989)
BindingDB Entry DOI: 10.7270/Q2WH2S78 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372974

(CHEMBL270575)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(C)(C)C |c:12| Show InChI InChI=1S/C30H36FN3O4S/c1-29(2,3)23-5-11-27(12-6-23)39(35,36)33-14-13-24-17-28-22(18-30(24,20-33)21-38-16-15-37-4)19-32-34(28)26-9-7-25(31)8-10-26/h5-12,17,19H,13-16,18,20-21H2,1-4H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229152

(CHEMBL349004)Show SMILES [H][C@]12CCCN([C@@]1([H])C[C@]1([H])N(CCc3cc(C)ccc13)C2)S(C)(=O)=O Show InChI InChI=1S/C18H26N2O2S/c1-13-5-6-16-14(10-13)7-9-19-12-15-4-3-8-20(23(2,21)22)17(15)11-18(16)19/h5-6,10,15,17-18H,3-4,7-9,11-12H2,1-2H3/t15-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM82273

(CAS_167710-87-4 | RS 39604)Show SMILES COc1cc(COc2cc(N)c(Cl)cc2C(=O)CCC2CCN(CCNS(C)(=O)=O)CC2)cc(OC)c1 Show InChI InChI=1S/C26H36ClN3O6S/c1-34-20-12-19(13-21(14-20)35-2)17-36-26-16-24(28)23(27)15-22(26)25(31)5-4-18-6-9-30(10-7-18)11-8-29-37(3,32)33/h12-16,18,29H,4-11,17,28H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1087-95 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15922.x
BindingDB Entry DOI: 10.7270/Q2707ZX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000446

(CHEMBL88565)Show SMILES CN1C2CCC1CC(C2)n1cc2CCCc3cccc(c23)c1=O |THB:9:7:1:3.4| Show InChI InChI=1S/C20H24N2O/c1-21-15-8-9-16(21)11-17(10-15)22-12-14-6-2-4-13-5-3-7-18(19(13)14)20(22)23/h3,5,7,12,15-17H,2,4,6,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50449636
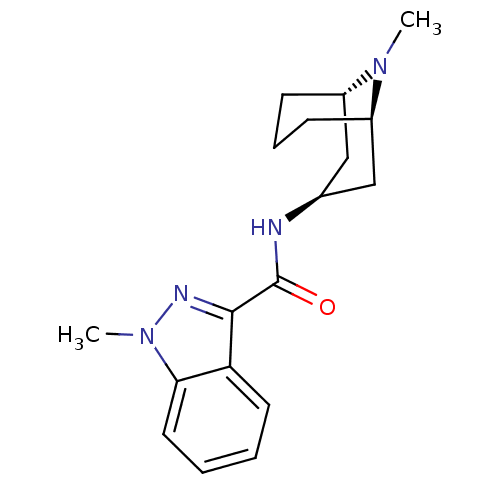
(BRL-43694 | GRANISETRON | Kytril | LY-278584 | San...)Show SMILES [H][C@]12CCC[C@]([H])(C[C@H](C1)NC(=O)c1nn(C)c3ccccc13)N2C |r,TLB:24:23:8.9.7:2.4.3| Show InChI InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23)/t12-,13-,14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477472
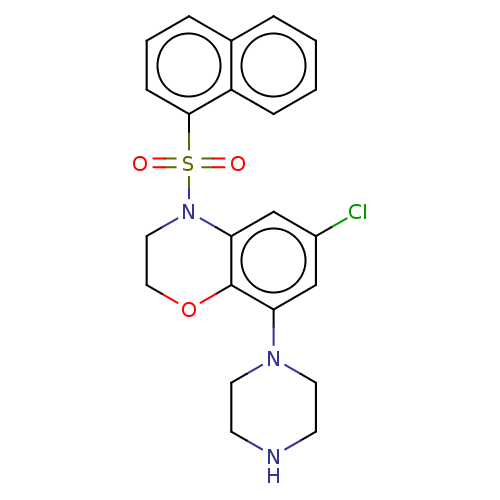
(CHEMBL398036)Show SMILES Clc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22ClN3O3S/c23-17-14-19(25-10-8-24-9-11-25)22-20(15-17)26(12-13-29-22)30(27,28)21-7-3-5-16-4-1-2-6-18(16)21/h1-7,14-15,24H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372931
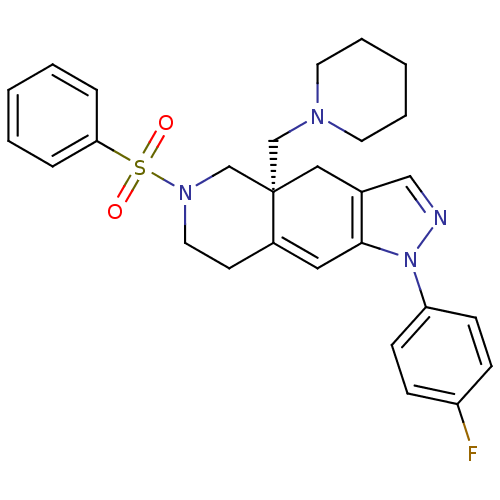
(CHEMBL409835)Show SMILES Fc1ccc(cc1)-n1ncc2C[C@]3(CN4CCCCC4)CN(CCC3=Cc12)S(=O)(=O)c1ccccc1 |c:27| Show InChI InChI=1S/C28H31FN4O2S/c29-24-9-11-25(12-10-24)33-27-17-23-13-16-32(36(34,35)26-7-3-1-4-8-26)21-28(23,18-22(27)19-30-33)20-31-14-5-2-6-15-31/h1,3-4,7-12,17,19H,2,5-6,13-16,18,20-21H2/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data