 Found 449 hits with Last Name = 'co' and Initial = 'ew'
Found 449 hits with Last Name = 'co' and Initial = 'ew' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1D receptor beta |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1D receptor alpha |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM50469882
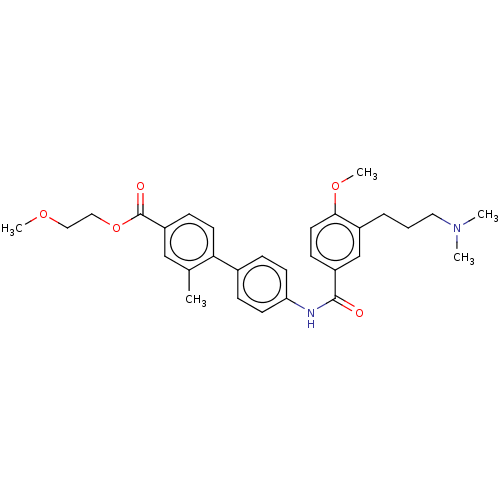
(CHEMBL73446)Show SMILES COCCOC(=O)c1ccc(c(C)c1)-c1ccc(NC(=O)c2ccc(OC)c(CCCN(C)C)c2)cc1 Show InChI InChI=1S/C30H36N2O5/c1-21-19-25(30(34)37-18-17-35-4)10-14-27(21)22-8-12-26(13-9-22)31-29(33)24-11-15-28(36-5)23(20-24)7-6-16-32(2)3/h8-15,19-20H,6-7,16-18H2,1-5H3,(H,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM50469879
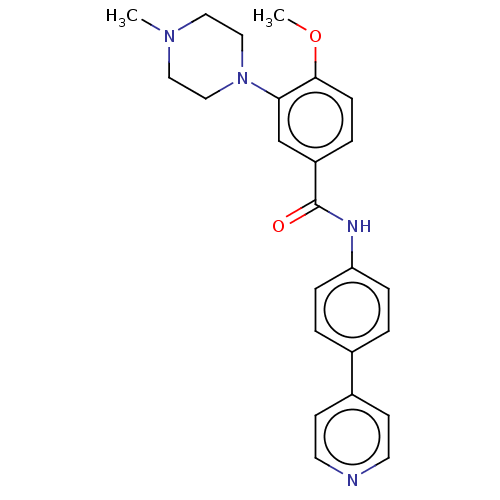
(CHEMBL72088)Show SMILES COc1ccc(cc1N1CCN(C)CC1)C(=O)Nc1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C24H26N4O2/c1-27-13-15-28(16-14-27)22-17-20(5-8-23(22)30-2)24(29)26-21-6-3-18(4-7-21)19-9-11-25-12-10-19/h3-12,17H,13-16H2,1-2H3,(H,26,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM50469881
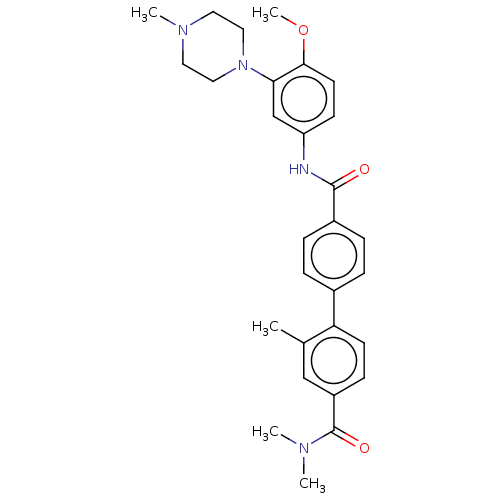
(CHEMBL311150)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)C(=O)N(C)C)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H34N4O3/c1-20-18-23(29(35)31(2)3)10-12-25(20)21-6-8-22(9-7-21)28(34)30-24-11-13-27(36-5)26(19-24)33-16-14-32(4)15-17-33/h6-13,18-19H,14-17H2,1-5H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM50469875
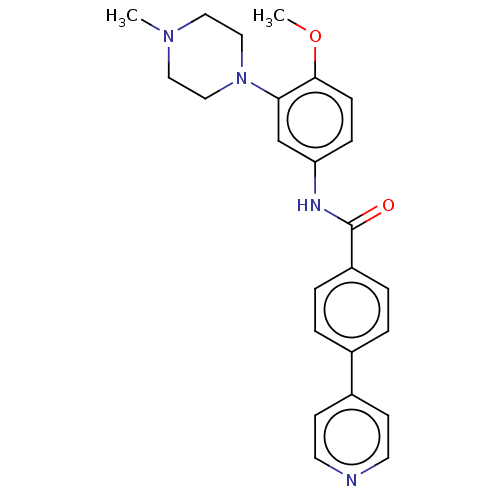
(CHEMBL72981)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccncc2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C24H26N4O2/c1-27-13-15-28(16-14-27)22-17-21(7-8-23(22)30-2)26-24(29)20-5-3-18(4-6-20)19-9-11-25-12-10-19/h3-12,17H,13-16H2,1-2H3,(H,26,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50469881

(CHEMBL311150)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)C(=O)N(C)C)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H34N4O3/c1-20-18-23(29(35)31(2)3)10-12-25(20)21-6-8-22(9-7-21)28(34)30-24-11-13-27(36-5)26(19-24)33-16-14-32(4)15-17-33/h6-13,18-19H,14-17H2,1-5H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50469876

(CHEMBL306384)Show SMILES COc1ccc(cc1N1CCN(C)CC1)C(=O)Nc1ccc(cc1)-c1ccc(cc1C)-c1noc(C)n1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-22(28-30-20(2)37-32-28)7-11-25(19)21-5-9-24(10-6-21)31-29(35)23-8-12-27(36-4)26(18-23)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM50469878
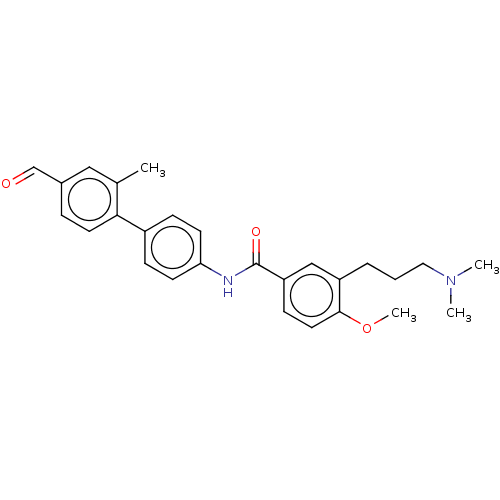
(CHEMBL72700)Show SMILES COc1ccc(cc1CCCN(C)C)C(=O)Nc1ccc(cc1)-c1ccc(C=O)cc1C Show InChI InChI=1S/C27H30N2O3/c1-19-16-20(18-30)7-13-25(19)21-8-11-24(12-9-21)28-27(31)23-10-14-26(32-4)22(17-23)6-5-15-29(2)3/h7-14,16-18H,5-6,15H2,1-4H3,(H,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM50469876

(CHEMBL306384)Show SMILES COc1ccc(cc1N1CCN(C)CC1)C(=O)Nc1ccc(cc1)-c1ccc(cc1C)-c1noc(C)n1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-22(28-30-20(2)37-32-28)7-11-25(19)21-5-9-24(10-6-21)31-29(35)23-8-12-27(36-4)26(18-23)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM50060518
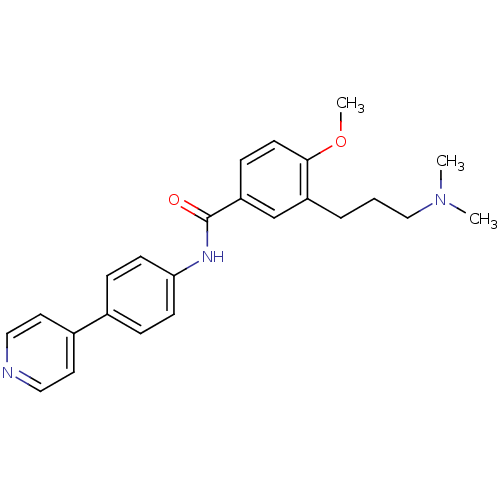
(3-(3-Dimethylamino-propyl)-4-methoxy-N-(4-pyridin-...)Show SMILES COc1ccc(cc1CCCN(C)C)C(=O)Nc1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C24H27N3O2/c1-27(2)16-4-5-20-17-21(8-11-23(20)29-3)24(28)26-22-9-6-18(7-10-22)19-12-14-25-15-13-19/h6-15,17H,4-5,16H2,1-3H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(GUINEA PIG) | BDBM50469877
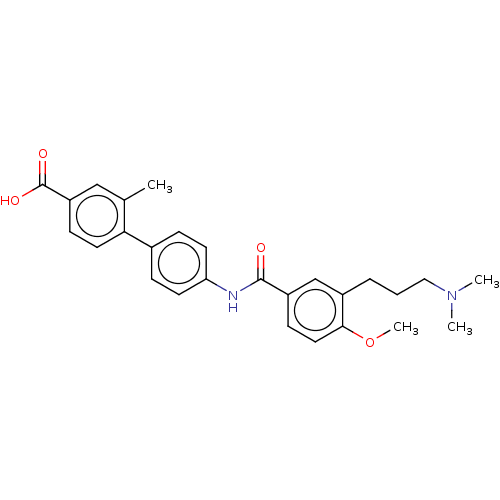
(CHEMBL72092)Show SMILES COc1ccc(cc1CCCN(C)C)C(=O)Nc1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C27H30N2O4/c1-18-16-22(27(31)32)9-13-24(18)19-7-11-23(12-8-19)28-26(30)21-10-14-25(33-4)20(17-21)6-5-15-29(2)3/h7-14,16-17H,5-6,15H2,1-4H3,(H,28,30)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50469881

(CHEMBL311150)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)C(=O)N(C)C)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H34N4O3/c1-20-18-23(29(35)31(2)3)10-12-25(20)21-6-8-22(9-7-21)28(34)30-24-11-13-27(36-5)26(19-24)33-16-14-32(4)15-17-33/h6-13,18-19H,14-17H2,1-5H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus with [3H]8-OH-DPAT |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic M1 receptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenoceptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-2 adrenoceptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic M2 receptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic M3 receptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50469875

(CHEMBL72981)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccncc2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C24H26N4O2/c1-27-13-15-28(16-14-27)22-17-21(7-8-23(22)30-2)26-24(29)20-5-3-18(4-6-20)19-9-11-25-12-10-19/h3-12,17H,13-16H2,1-2H3,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus with [3H]8-OH-DPAT |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50469876

(CHEMBL306384)Show SMILES COc1ccc(cc1N1CCN(C)CC1)C(=O)Nc1ccc(cc1)-c1ccc(cc1C)-c1noc(C)n1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-22(28-30-20(2)37-32-28)7-11-25(19)21-5-9-24(10-6-21)31-29(35)23-8-12-27(36-4)26(18-23)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50469878

(CHEMBL72700)Show SMILES COc1ccc(cc1CCCN(C)C)C(=O)Nc1ccc(cc1)-c1ccc(C=O)cc1C Show InChI InChI=1S/C27H30N2O3/c1-19-16-20(18-30)7-13-25(19)21-8-11-24(12-9-21)28-27(31)23-10-14-26(32-4)22(17-23)6-5-15-29(2)3/h7-14,16-18H,5-6,15H2,1-4H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50469880
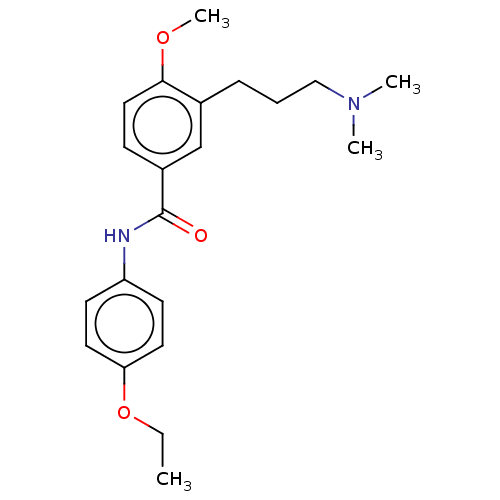
(CHEMBL75771)Show InChI InChI=1S/C21H28N2O3/c1-5-26-19-11-9-18(10-12-19)22-21(24)17-8-13-20(25-4)16(15-17)7-6-14-23(2)3/h8-13,15H,5-7,14H2,1-4H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine D3 receptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50469877

(CHEMBL72092)Show SMILES COc1ccc(cc1CCCN(C)C)C(=O)Nc1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C27H30N2O4/c1-18-16-22(27(31)32)9-13-24(18)19-7-11-23(12-8-19)28-26(30)21-10-14-25(33-4)20(17-21)6-5-15-29(2)3/h7-14,16-17H,5-6,15H2,1-4H3,(H,28,30)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine D2 receptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Mus musculus (Mouse)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for 5-hydroxytryptamine receptor uptake |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine D4 receptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine receptor D1 |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 4 receptor |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50060518

(3-(3-Dimethylamino-propyl)-4-methoxy-N-(4-pyridin-...)Show SMILES COc1ccc(cc1CCCN(C)C)C(=O)Nc1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C24H27N3O2/c1-27(2)16-4-5-20-17-21(8-11-23(20)29-3)24(28)26-22-9-6-18(7-10-22)19-12-14-25-15-13-19/h6-15,17H,4-5,16H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50420996
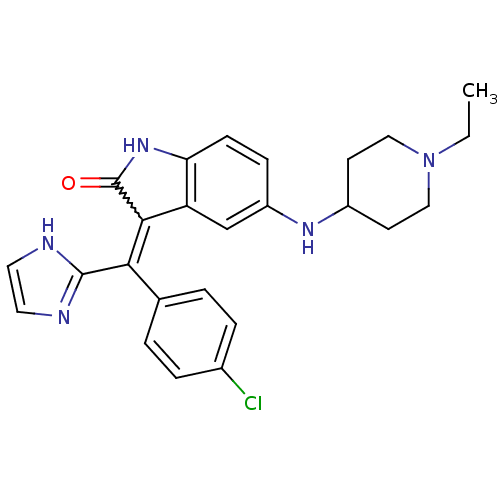
(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380935
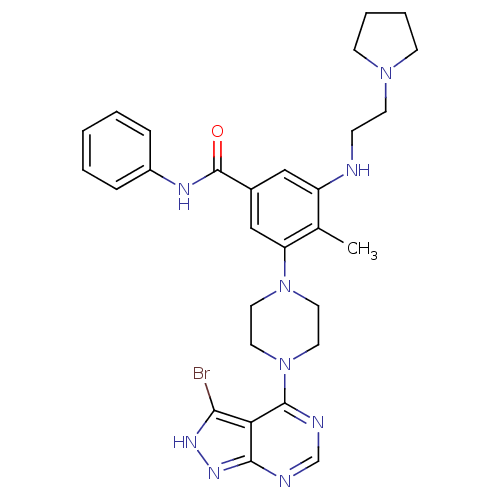
(CHEMBL2016887)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C29H34BrN9O/c1-20-23(31-9-12-37-10-5-6-11-37)17-21(29(40)34-22-7-3-2-4-8-22)18-24(20)38-13-15-39(16-14-38)28-25-26(30)35-36-27(25)32-19-33-28/h2-4,7-8,17-19,31H,5-6,9-16H2,1H3,(H,34,40)(H,32,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380930
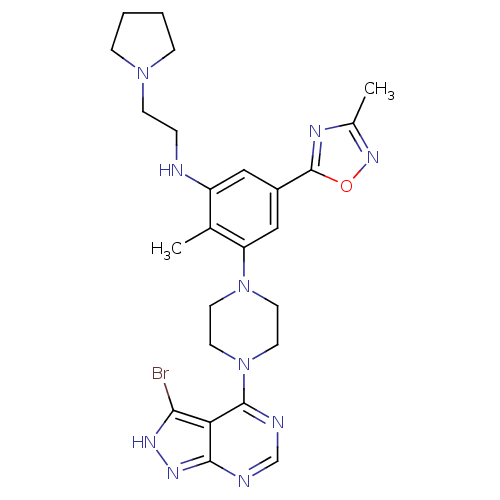
(CHEMBL2016890)Show SMILES Cc1noc(n1)-c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H31BrN10O/c1-16-19(27-5-8-34-6-3-4-7-34)13-18(25-30-17(2)33-37-25)14-20(16)35-9-11-36(12-10-35)24-21-22(26)31-32-23(21)28-15-29-24/h13-15,27H,3-12H2,1-2H3,(H,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50380939
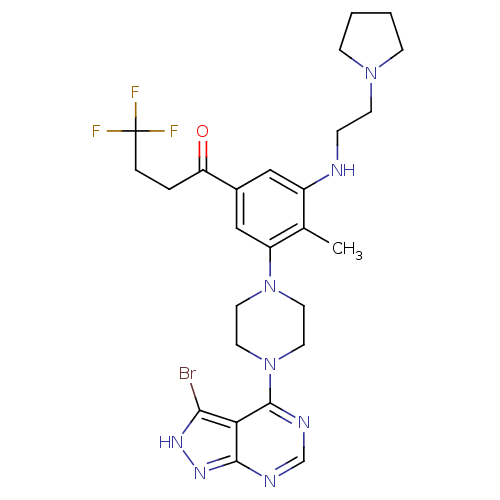
(CHEMBL2016893)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)CCC(F)(F)F Show InChI InChI=1S/C26H32BrF3N8O/c1-17-19(31-6-9-36-7-2-3-8-36)14-18(21(39)4-5-26(28,29)30)15-20(17)37-10-12-38(13-11-37)25-22-23(27)34-35-24(22)32-16-33-25/h14-16,31H,2-13H2,1H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380931
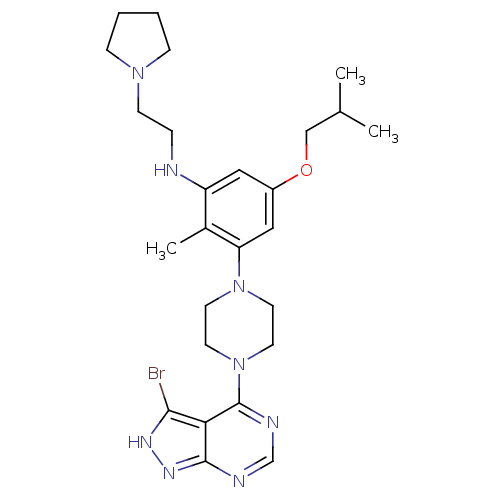
(CHEMBL2016886)Show SMILES CC(C)COc1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C26H37BrN8O/c1-18(2)16-36-20-14-21(28-6-9-33-7-4-5-8-33)19(3)22(15-20)34-10-12-35(13-11-34)26-23-24(27)31-32-25(23)29-17-30-26/h14-15,17-18,28H,4-13,16H2,1-3H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged Flt1 using poly(Glu,Tyr) as substrate after 60 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380929
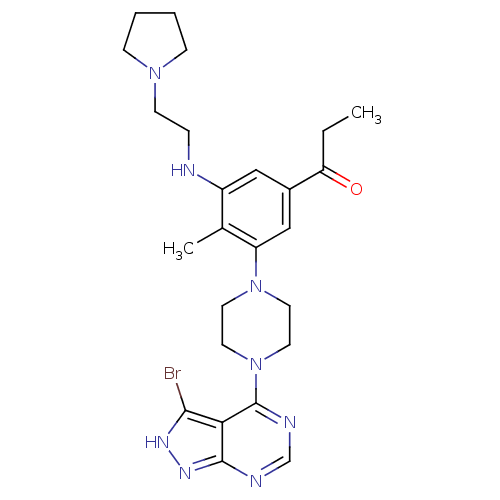
(CHEMBL2016892)Show SMILES CCC(=O)c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H33BrN8O/c1-3-21(35)18-14-19(27-6-9-32-7-4-5-8-32)17(2)20(15-18)33-10-12-34(13-11-33)25-22-23(26)30-31-24(22)28-16-29-25/h14-16,27H,3-13H2,1-2H3,(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Cryptosporidium hominis) | BDBM25826
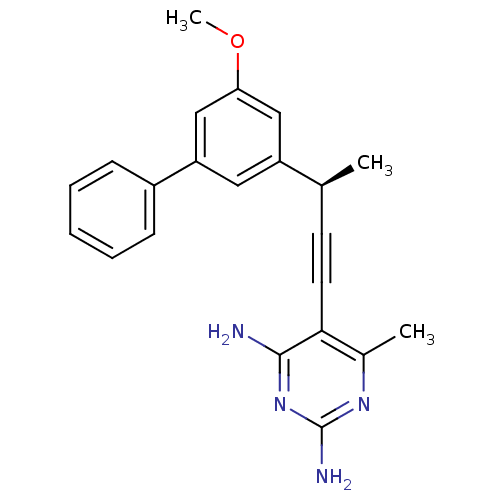
(5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...)Show SMILES COc1cc(cc(c1)-c1ccccc1)[C@@H](C)C#Cc1c(C)nc(N)nc1N |r| Show InChI InChI=1S/C22H22N4O/c1-14(9-10-20-15(2)25-22(24)26-21(20)23)17-11-18(13-19(12-17)27-3)16-7-5-4-6-8-16/h4-8,11-14H,1-3H3,(H4,23,24,25,26)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
US Patent
| Assay Description
Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... |
US Patent US8853228 (2014)
BindingDB Entry DOI: 10.7270/Q2TD9W1T |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Cryptosporidium hominis) | BDBM25818

(5-[3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]-6-me...)Show SMILES COc1cc(cc(c1)-c1ccccc1)C(C)C#Cc1c(C)nc(N)nc1N Show InChI InChI=1S/C22H22N4O/c1-14(9-10-20-15(2)25-22(24)26-21(20)23)17-11-18(13-19(12-17)27-3)16-7-5-4-6-8-16/h4-8,11-14H,1-3H3,(H4,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
US Patent
| Assay Description
Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... |
US Patent US8853228 (2014)
BindingDB Entry DOI: 10.7270/Q2TD9W1T |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380939

(CHEMBL2016893)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)CCC(F)(F)F Show InChI InChI=1S/C26H32BrF3N8O/c1-17-19(31-6-9-36-7-2-3-8-36)14-18(21(39)4-5-26(28,29)30)15-20(17)37-10-12-38(13-11-37)25-22-23(27)34-35-24(22)32-16-33-25/h14-16,31H,2-13H2,1H3,(H,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50379530
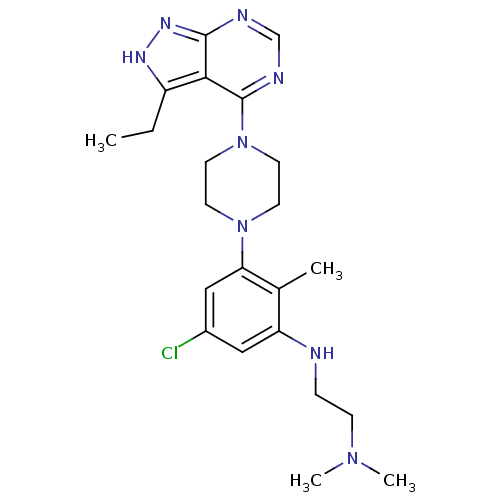
(CHEMBL2012701)Show SMILES CCc1[nH]nc2ncnc(N3CCN(CC3)c3cc(Cl)cc(NCCN(C)C)c3C)c12 Show InChI InChI=1S/C22H31ClN8/c1-5-17-20-21(28-27-17)25-14-26-22(20)31-10-8-30(9-11-31)19-13-16(23)12-18(15(19)2)24-6-7-29(3)4/h12-14,24H,5-11H2,1-4H3,(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380936
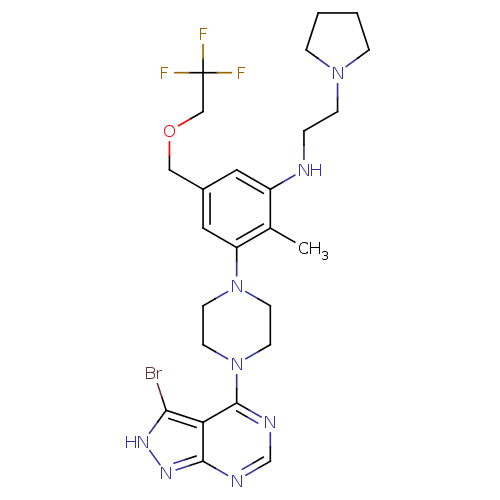
(CHEMBL2016888)Show SMILES Cc1c(NCCN2CCCC2)cc(COCC(F)(F)F)cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H32BrF3N8O/c1-17-19(30-4-7-35-5-2-3-6-35)12-18(14-38-15-25(27,28)29)13-20(17)36-8-10-37(11-9-36)24-21-22(26)33-34-23(21)31-16-32-24/h12-13,16,30H,2-11,14-15H2,1H3,(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50379531
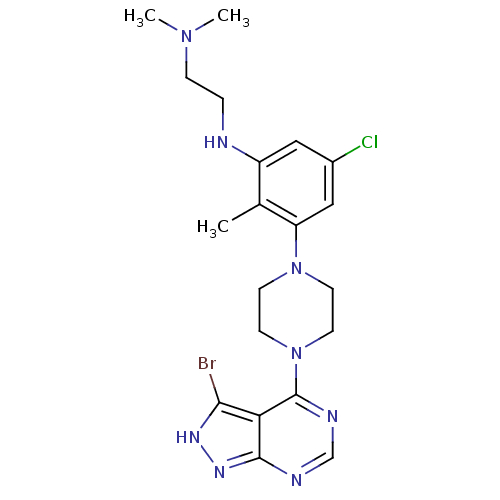
(CHEMBL2012702)Show SMILES CN(C)CCNc1cc(Cl)cc(N2CCN(CC2)c2ncnc3n[nH]c(Br)c23)c1C Show InChI InChI=1S/C20H26BrClN8/c1-13-15(23-4-5-28(2)3)10-14(22)11-16(13)29-6-8-30(9-7-29)20-17-18(21)26-27-19(17)24-12-25-20/h10-12,23H,4-9H2,1-3H3,(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380932
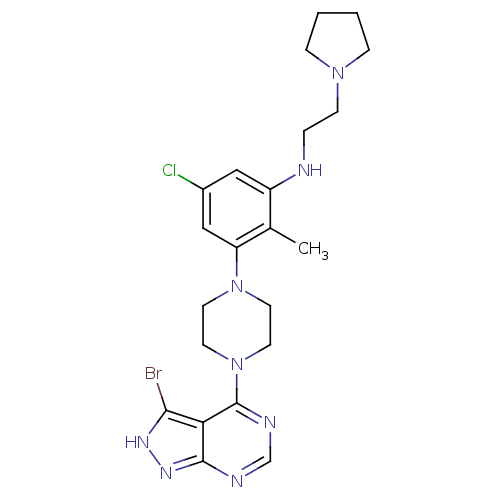
(CHEMBL2016771)Show SMILES Cc1c(NCCN2CCCC2)cc(Cl)cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C22H28BrClN8/c1-15-17(25-4-7-30-5-2-3-6-30)12-16(24)13-18(15)31-8-10-32(11-9-31)22-19-20(23)28-29-21(19)26-14-27-22/h12-14,25H,2-11H2,1H3,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380934
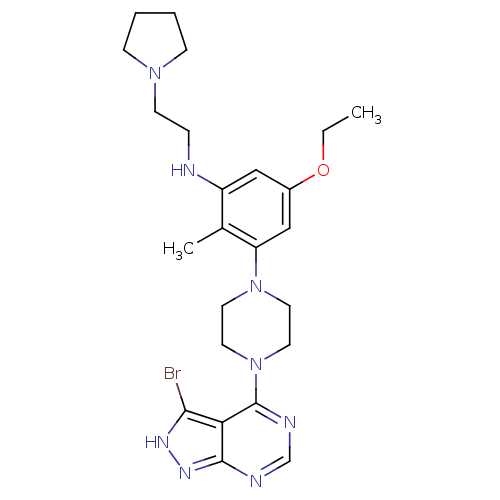
(CHEMBL2016885)Show SMILES CCOc1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C24H33BrN8O/c1-3-34-18-14-19(26-6-9-31-7-4-5-8-31)17(2)20(15-18)32-10-12-33(13-11-32)24-21-22(25)29-30-23(21)27-16-28-24/h14-16,26H,3-13H2,1-2H3,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421033
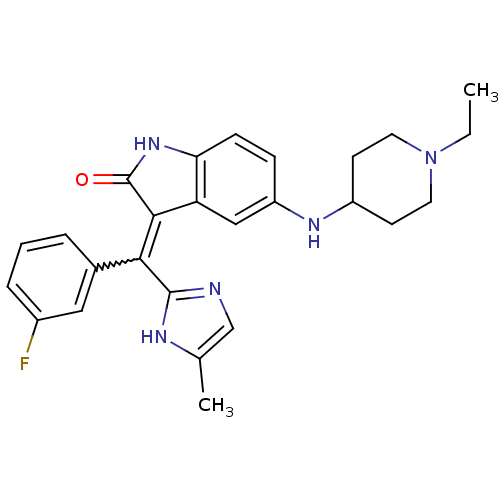
(CHEMBL2087167)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cccc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-11-9-19(10-12-32)30-20-7-8-22-21(14-20)24(26(33)31-22)23(25-28-15-16(2)29-25)17-5-4-6-18(27)13-17/h4-8,13-15,19,30H,3,9-12H2,1-2H3,(H,28,29)(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data