

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591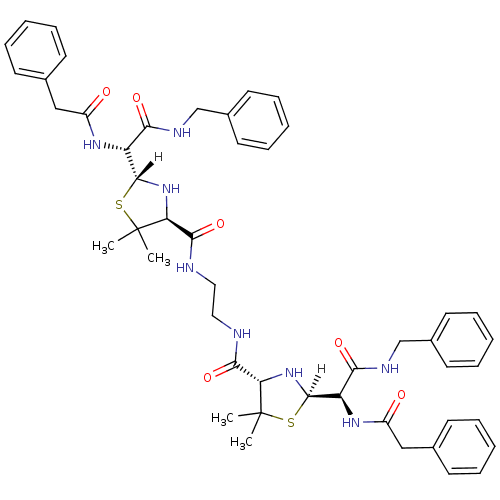 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against HIV-1 Proteinase activity | Bioorg Med Chem Lett 3: 503-508 (1993) Article DOI: 10.1016/S0960-894X(01)81216-6 BindingDB Entry DOI: 10.7270/Q2GH9HXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4942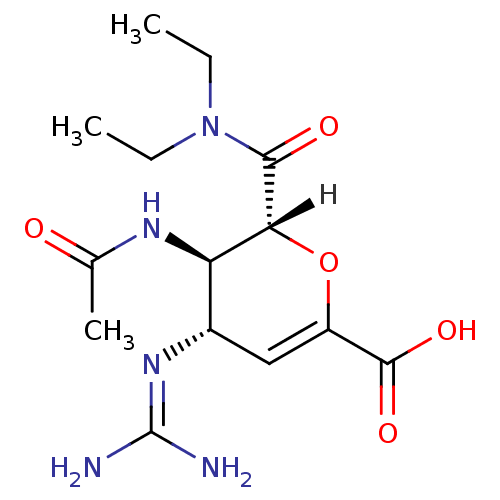 ((2R,3R,4S)-4-carbamimidamido-2-(diethylcarbamoyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4942 ((2R,3R,4S)-4-carbamimidamido-2-(diethylcarbamoyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4972 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(3-phenylphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4940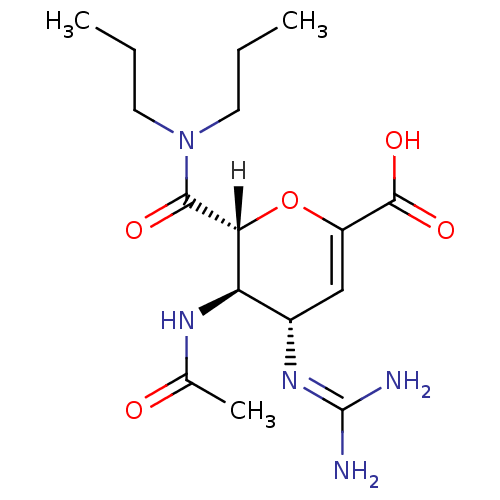 ((2R,3R,4S)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4967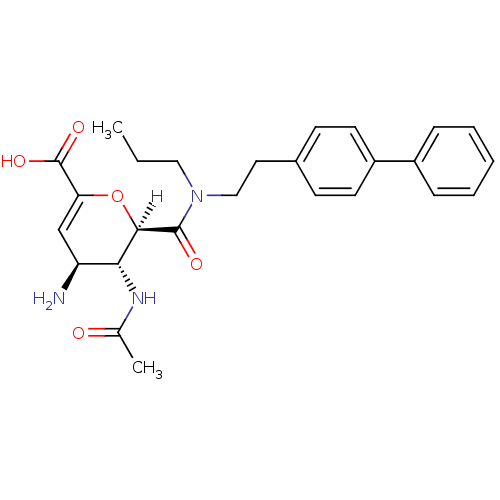 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(4-phenylphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4945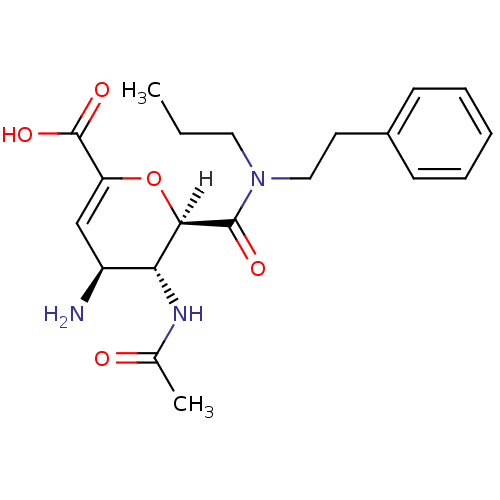 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4940 ((2R,3R,4S)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4937 ((2R,3R,4S)-4-amino-2-(diethylcarbamoyl)-3-acetamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM589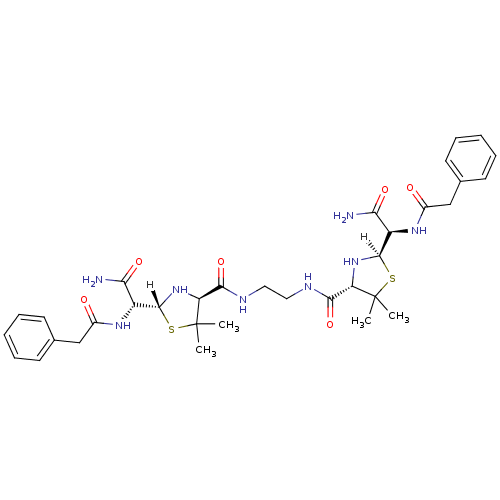 ((2R,4S)-2-[(R)-carbamoyl(1-phenylacetamido)methyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4929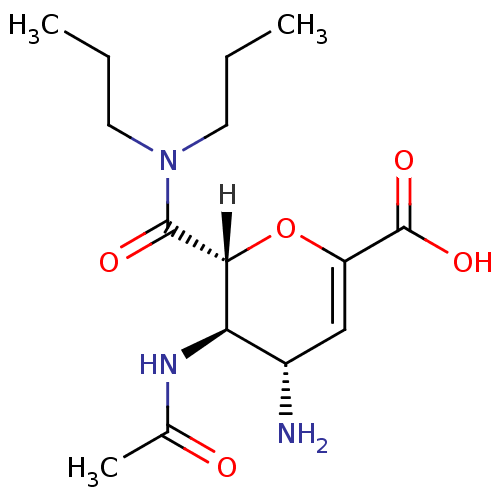 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4937 ((2R,3R,4S)-4-amino-2-(diethylcarbamoyl)-3-acetamid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4941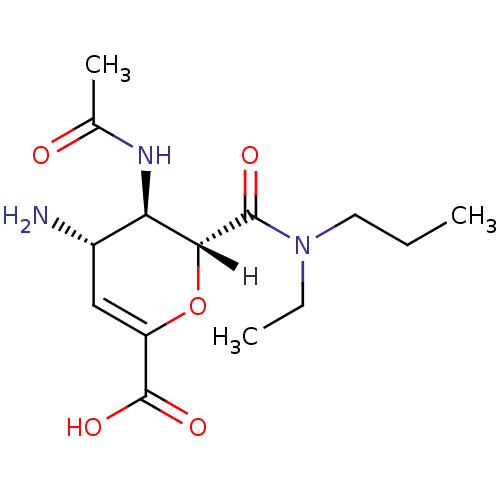 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(propyl)car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4967 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(4-phenylphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4941 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(propyl)car...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4966 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(3-hydroxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4943 ((2R,3R,4S)-4-amino-2-[butyl(propyl)carbamoyl]-3-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus) | BDBM4934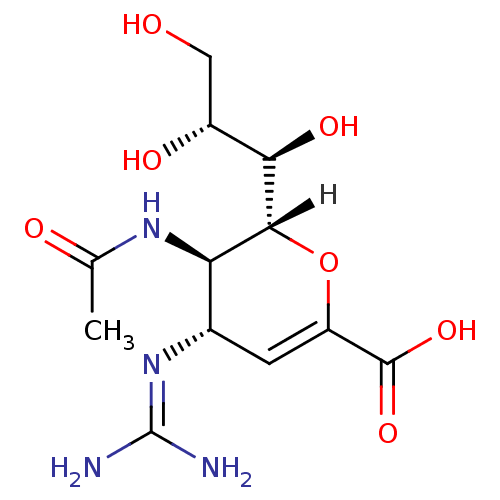 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[(1R,2R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4933 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4943 ((2R,3R,4S)-4-amino-2-[butyl(propyl)carbamoyl]-3-ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza B sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4933 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM590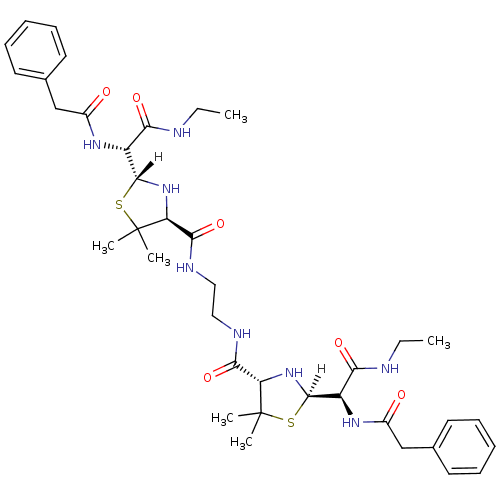 ((2R,4S)-2-[(R)-(ethylcarbamoyl)(1-phenylacetamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM590 ((2R,4S)-2-[(R)-(ethylcarbamoyl)(1-phenylacetamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against HIV-1 Proteinase activity | Bioorg Med Chem Lett 3: 503-508 (1993) Article DOI: 10.1016/S0960-894X(01)81216-6 BindingDB Entry DOI: 10.7270/Q2GH9HXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50288444 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-(phenethyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4965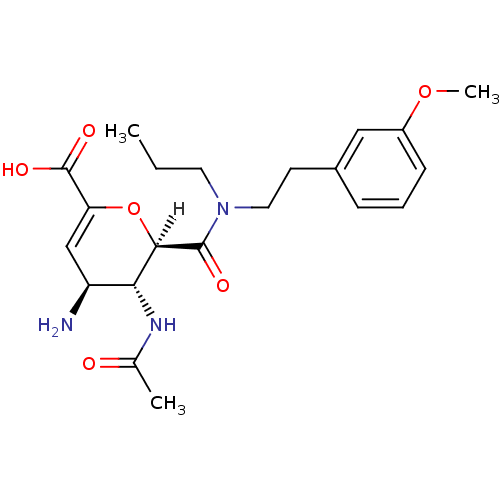 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(3-methoxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4945 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4952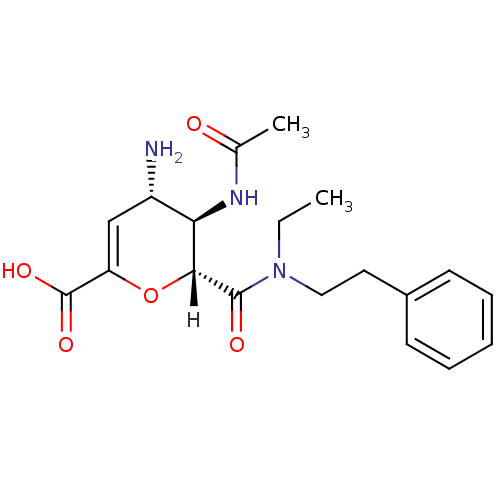 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(2-phenylet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4934 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[(1R,2R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM636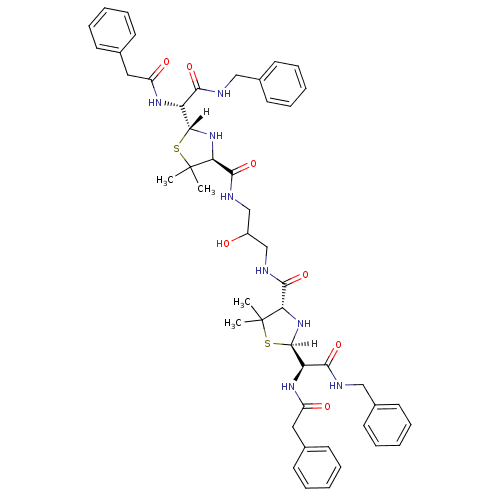 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against HIV-1 Proteinase activity | Bioorg Med Chem Lett 3: 503-508 (1993) Article DOI: 10.1016/S0960-894X(01)81216-6 BindingDB Entry DOI: 10.7270/Q2GH9HXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4952 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(2-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4946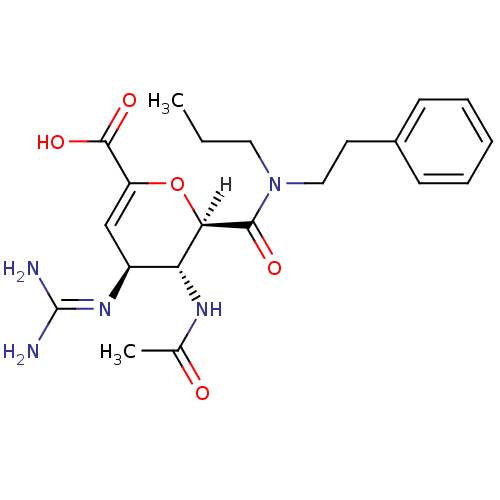 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[(2-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4935 ((2R,3R,4S)-4-amino-2-[(2,5-dimethylpyrrolidin-1-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4968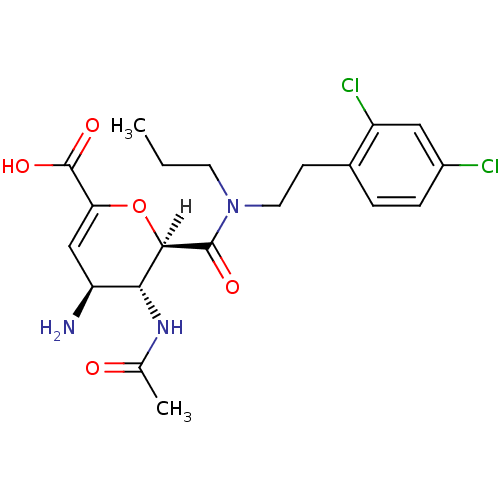 ((2R,3R,4S)-4-amino-2-{[2-(2,4-dichlorophenyl)ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4949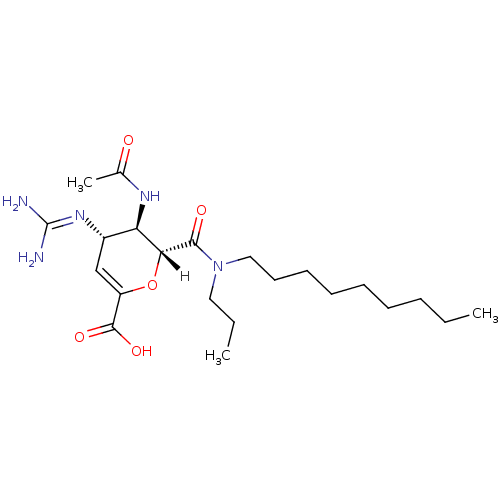 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[nonyl(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4963 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(4-methoxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4959 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4956 ((2R,3R,4S)-4-amino-2-[(2-azidoethyl)(2-phenylethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4971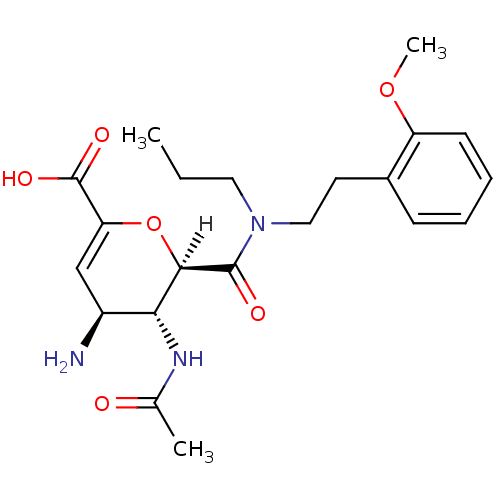 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(2-methoxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4964 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(4-hydroxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281566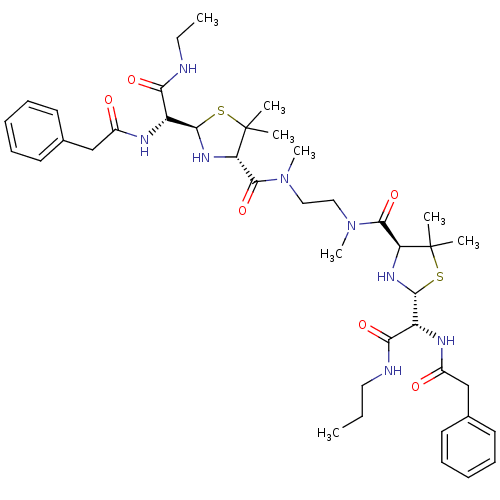 (1N-propyl-2-benzylcarboxamido-2-[4-[2-[2-benzylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against HIV-1 Proteinase activity | Bioorg Med Chem Lett 3: 503-508 (1993) Article DOI: 10.1016/S0960-894X(01)81216-6 BindingDB Entry DOI: 10.7270/Q2GH9HXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4969 ((2R,3R,4S)-4-amino-3-acetamido-2-({2-[4-(propan-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4957 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-hydroxyethyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4958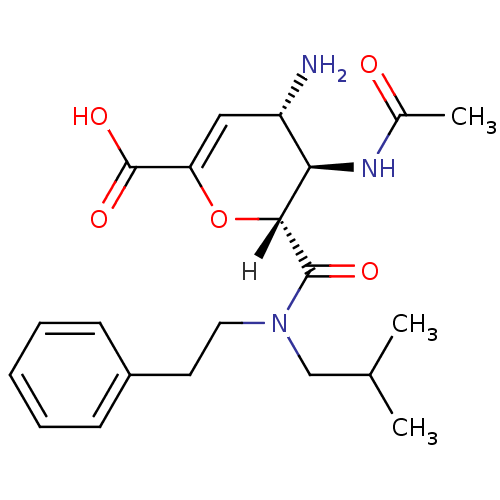 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-methylpropyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281571 (1N-benzyl-2-[4-[4-[2-benzylcarbamoyl(benzylcarboxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against HIV-1 Proteinase activity | Bioorg Med Chem Lett 3: 503-508 (1993) Article DOI: 10.1016/S0960-894X(01)81216-6 BindingDB Entry DOI: 10.7270/Q2GH9HXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281568 (1N-benzyl-2-[4-[4-[2-benzylcarbamoyl(benzylcarboxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against HIV-1 Proteinase activity | Bioorg Med Chem Lett 3: 503-508 (1993) Article DOI: 10.1016/S0960-894X(01)81216-6 BindingDB Entry DOI: 10.7270/Q2GH9HXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50288445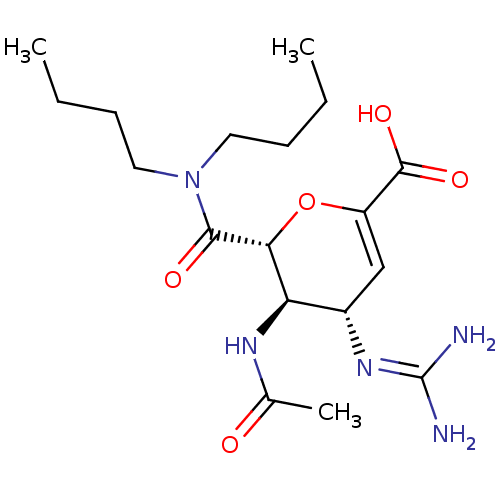 ((4S,5R,6R)-5-Acetylamino-6-dibutylcarbamoyl-4-guan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 177 total ) | Next | Last >> |