

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50370403 (CHEMBL177829) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to Sigma opioid receptor type 1 in guinea pig brain homogenate with 0.5 nM of [3H](+)-PENT as radioligand | J Med Chem 24: 496-9 (1981) BindingDB Entry DOI: 10.7270/Q2K64JTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50370403 (CHEMBL177829) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to Sigma opioid receptor type 2 in guinea pig brain homogenate with 4 nM of [3H](+)-DTG as radioligand | J Med Chem 24: 496-9 (1981) BindingDB Entry DOI: 10.7270/Q2K64JTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402202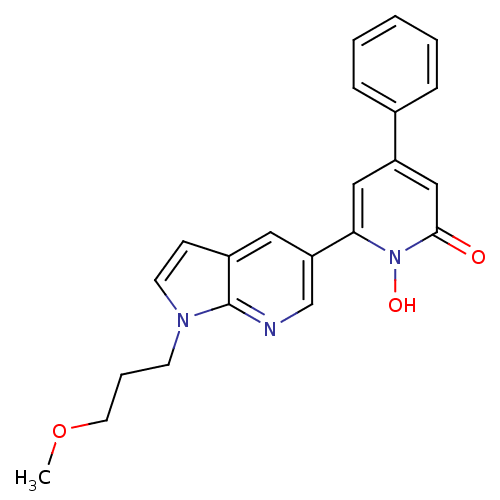 (CHEMBL2203964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825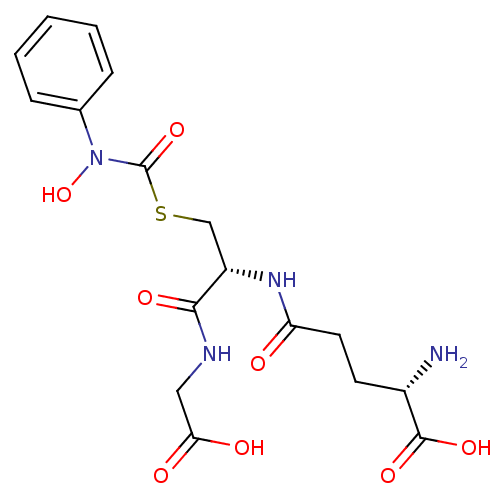 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451155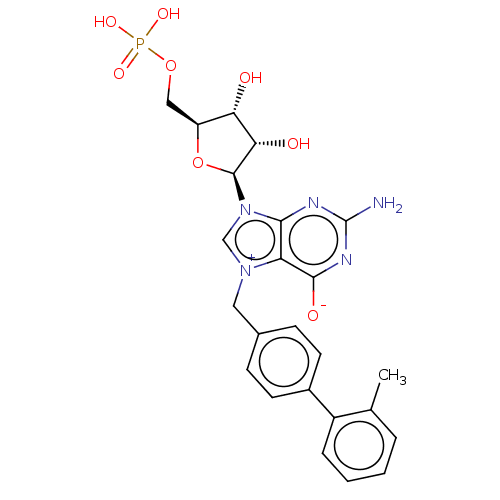 (US10676499, Example 49) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451159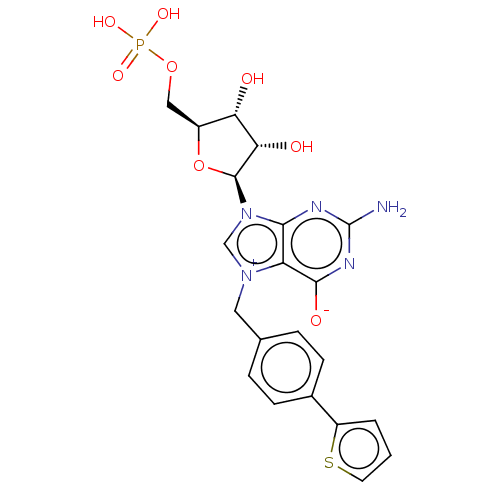 (US10676499, Example 57) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451145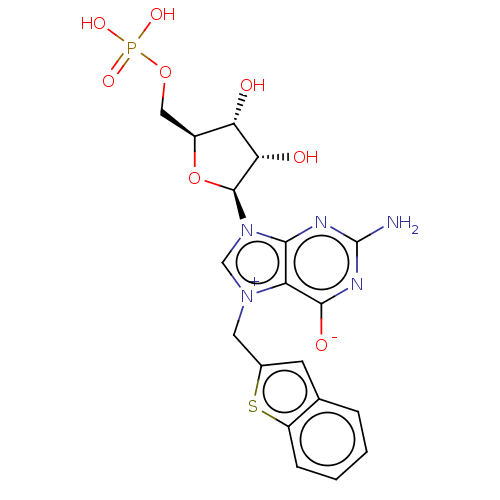 (US10676499, Example 17) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451148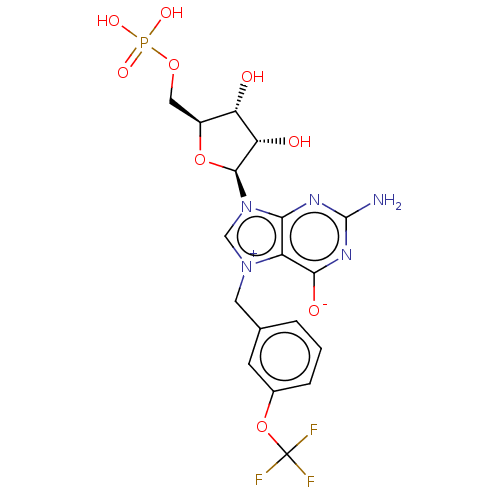 (US10676499, Example 29) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451158 (US10676499, Example 55) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451147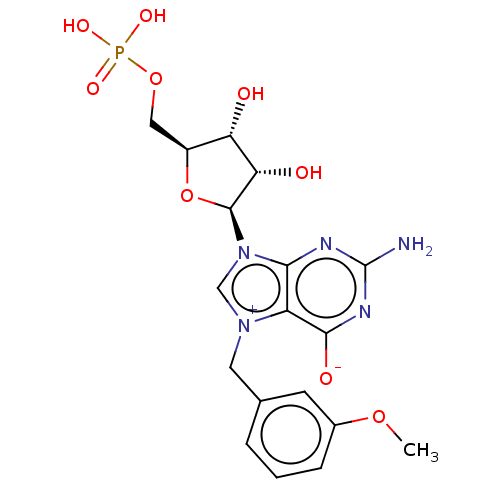 (US10676499, Example 26) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451144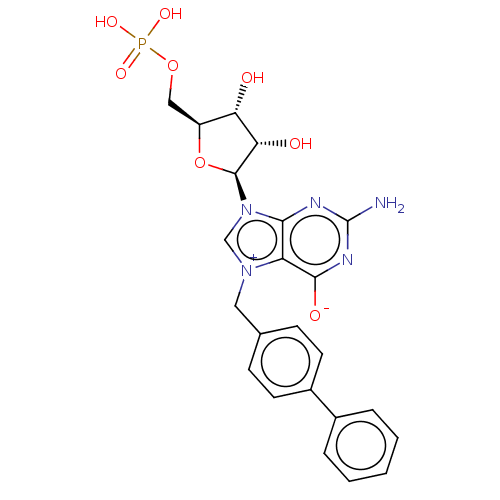 (US10676499, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451150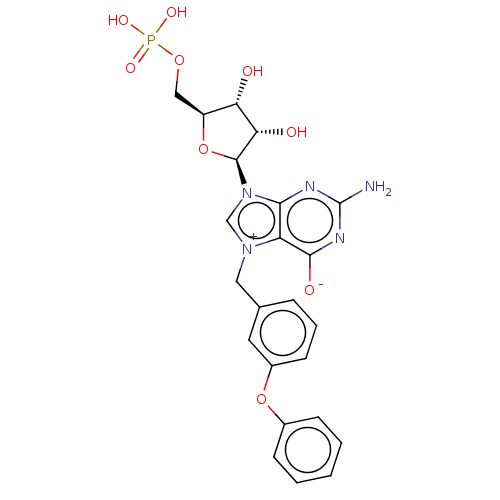 (US10676499, Example 33) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451146 (US10676499, Example 18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451156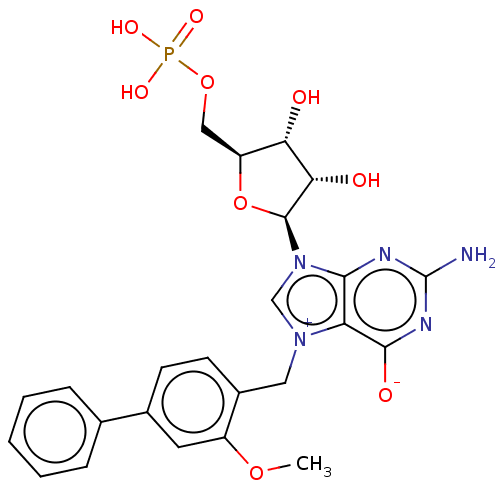 (US10676499, Example 53) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate synthase (Escherichia coli (strain K12)) | BDBM50028881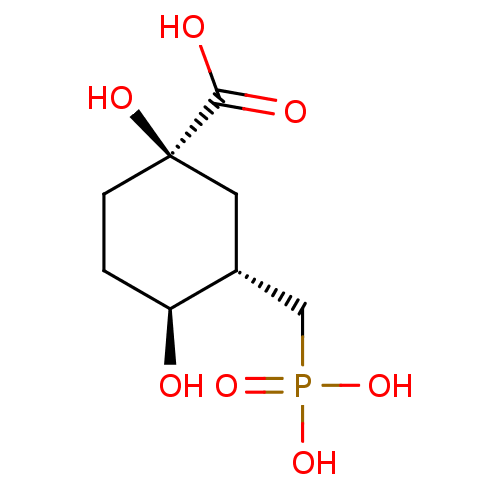 ((1R,3S,4S)-1,4-Dihydroxy-3-phosphonomethyl-cyclohe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory constant against 3-dehydroquinate synthase | J Med Chem 24: 496-9 (1981) BindingDB Entry DOI: 10.7270/Q2K64JTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517464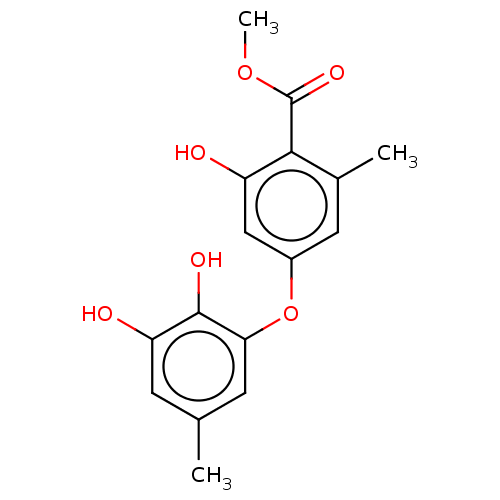 (CHEMBL1234300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657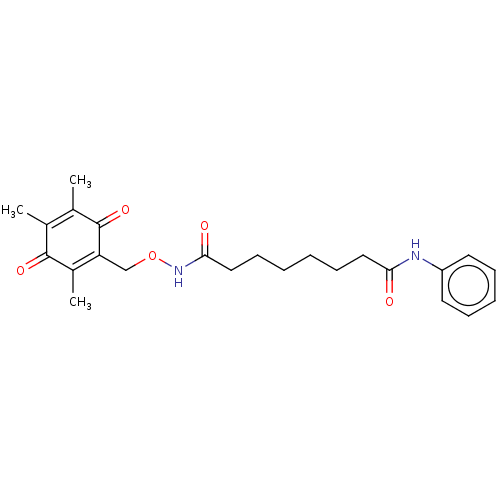 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to human Zn2+-HDAC8 assessed as loss of activity by Fluor-de-Lys activity assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50409932 (CHEMBL178537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) | J Med Chem 24: 496-9 (1981) BindingDB Entry DOI: 10.7270/Q2K64JTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Clostridium botulinum BoNT/A using SNAP-25 (141-206) as substrate by HPLC analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451160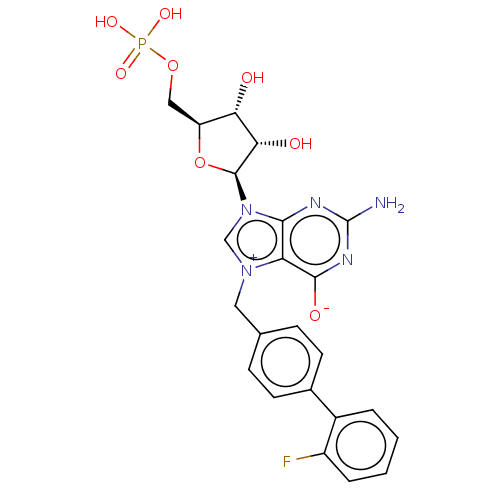 (US10676499, Example 58) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546871 (CHEMBL4745069) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451151 (US10676499, Example 40) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451157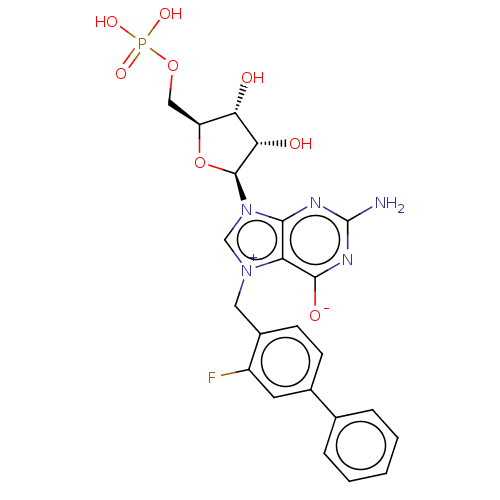 (US10676499, Example 54) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451154 (US10676499, Example 43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451153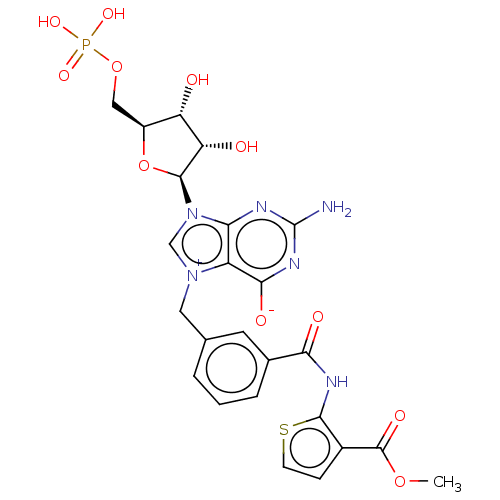 (US10676499, Example 42) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Reversible-time dependent inhibition of human wild type HDAC8 by Fluor-de-Lys activity assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50048539 (CHEMBL3309328) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of recombinant Clostridium botulinum N-terminal 6His-tagged BoNT/A (Met1 to Phe425 residues) catalytic domain expressed in Es... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451152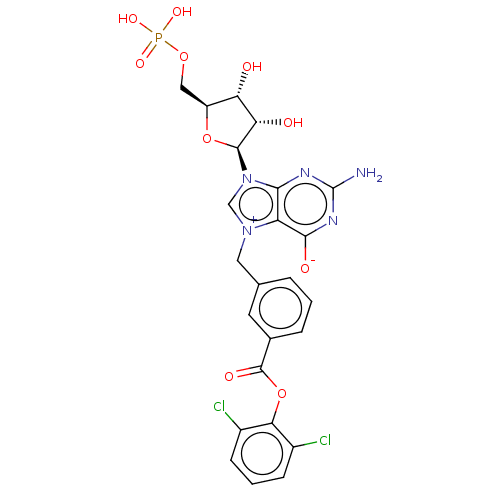 (US10676499, Example 41) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546870 (CHEMBL4790141) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057418 (CHEMBL3326435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546872 (CHEMBL4761825) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate by me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546874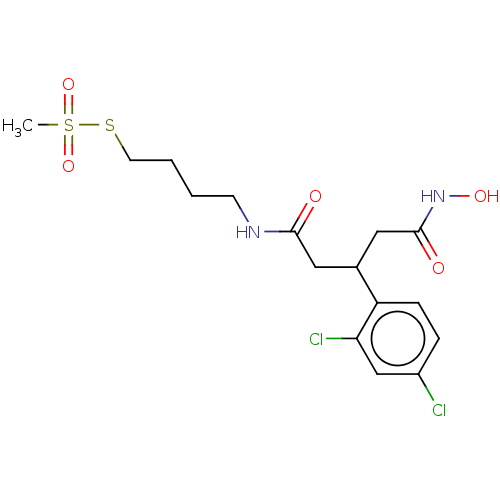 (CHEMBL4795025) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate by me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546876 (CHEMBL4787837) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate by me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057411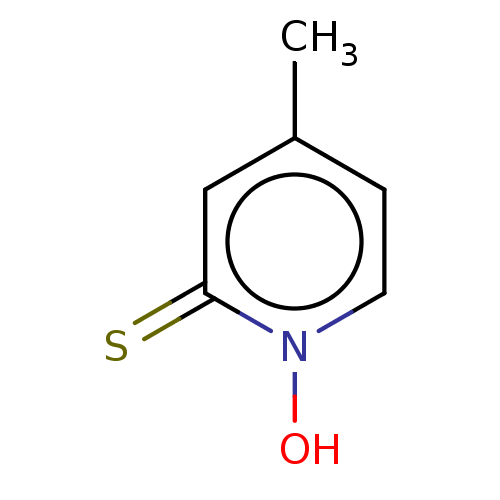 (CHEMBL3326430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546880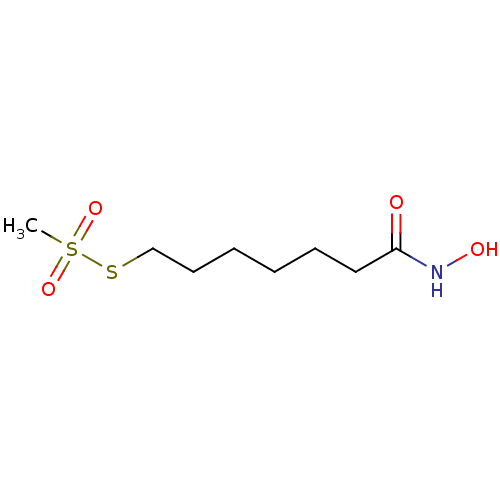 (CHEMBL4799810) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide as substrate preincubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546875 (CHEMBL4746123) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate by me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057412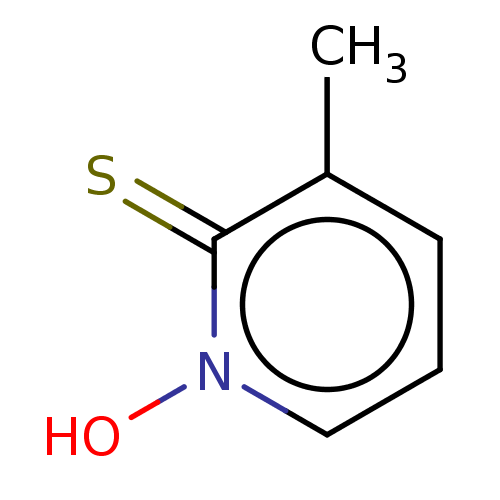 (CHEMBL3326429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50048539 (CHEMBL3309328) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057406 (CHEMBL3326434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546878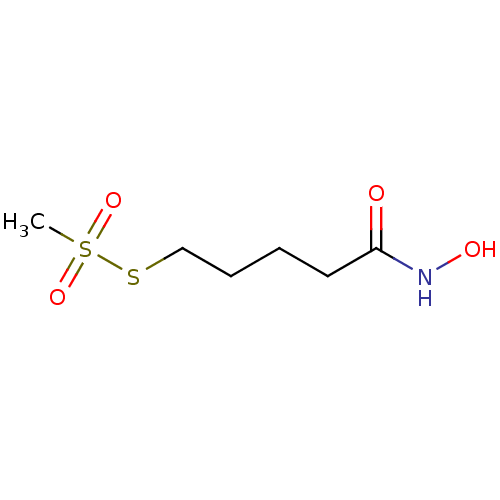 (CHEMBL4790780) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide as substrate preincubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546879 (CHEMBL4787587) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide as substrate preincubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546877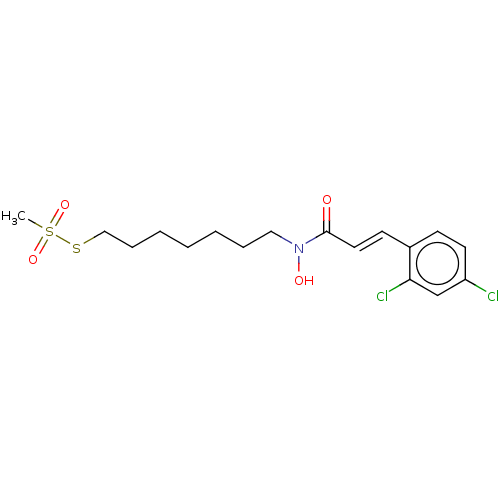 (CHEMBL4743480) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451149 (US10676499, Example 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057410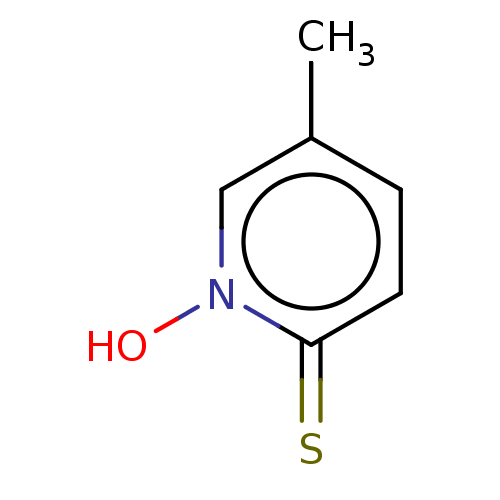 (CHEMBL3326431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057417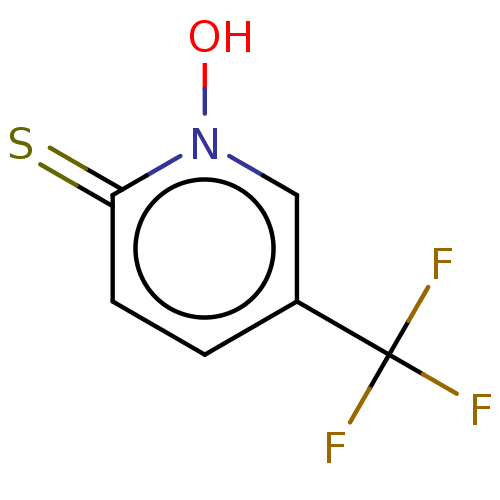 (CHEMBL3326436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026220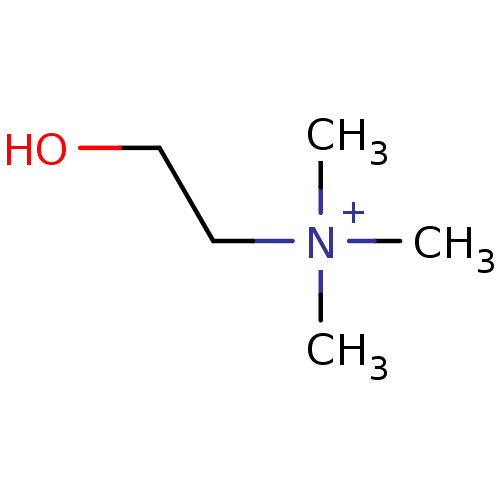 (2-hydroxy-N,N,N-trimethylethanaminium | CHEMBL2824...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(noncompetitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026470 ((2-Hydroxy-ethyl)-dimethyl-sulfonium; iodide | CHE...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM60985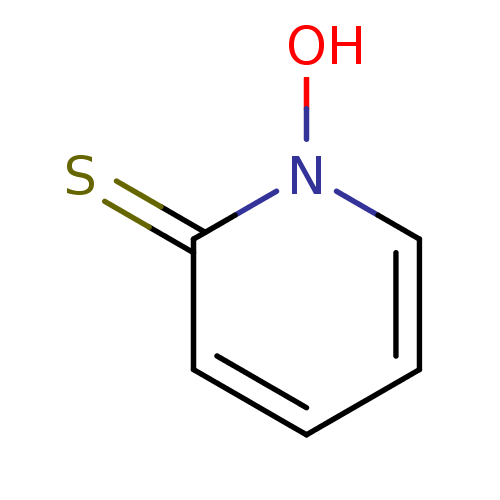 (1-hydroxy-2-pyridinethione | 1-hydroxypyridine-2-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057414 (CHEMBL3326439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057408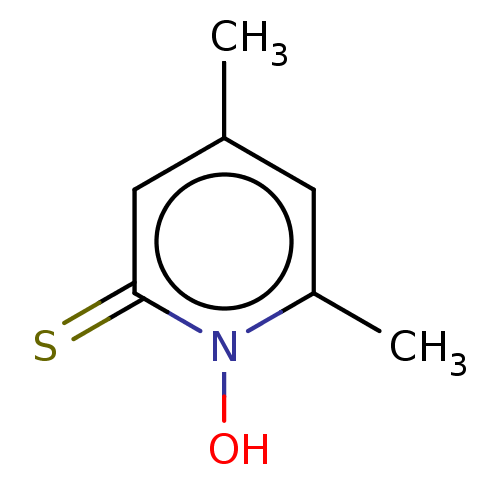 (CHEMBL3326433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1516 total ) | Next | Last >> |