 Found 41 hits with Last Name = 'collins' and Initial = 'ls'
Found 41 hits with Last Name = 'collins' and Initial = 'ls' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268097
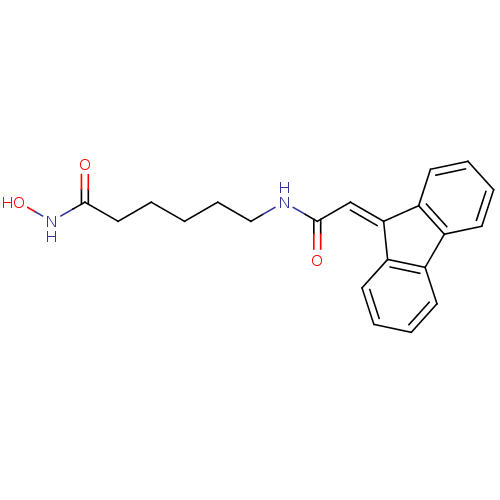
(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268121
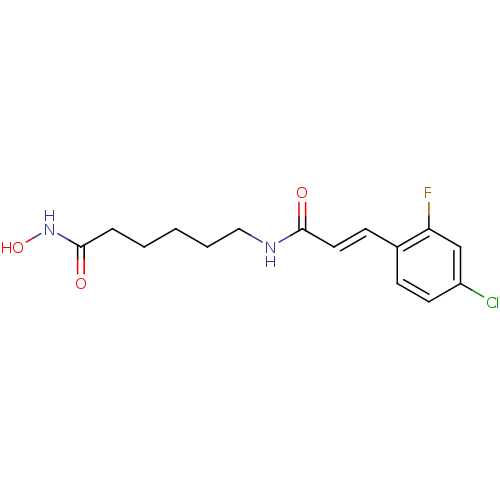
((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268063
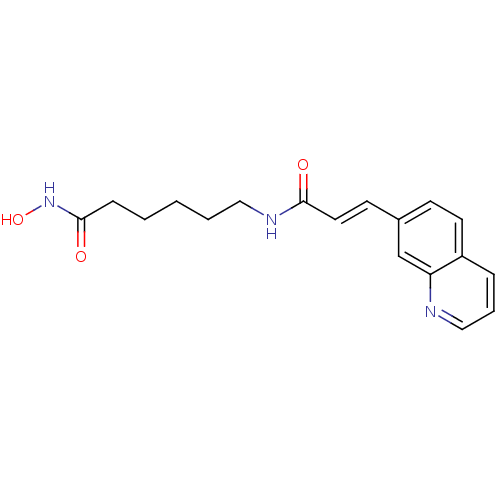
((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268042
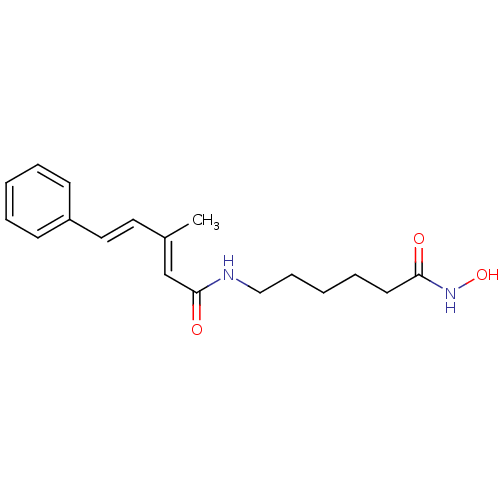
((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC5 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC5 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 779 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data