 Found 658 hits with Last Name = 'colombo' and Initial = 'r'
Found 658 hits with Last Name = 'colombo' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271367
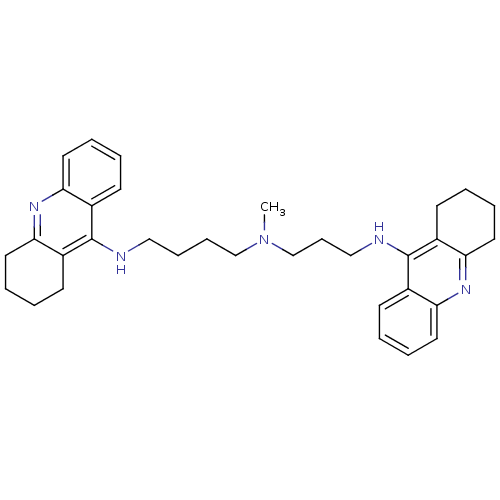
(CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H43N5/c1-39(24-12-22-36-34-27-15-4-8-19-31(27)38-32-20-9-5-16-28(32)34)23-11-10-21-35-33-25-13-2-6-17-29(25)37-30-18-7-3-14-26(30)33/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005193
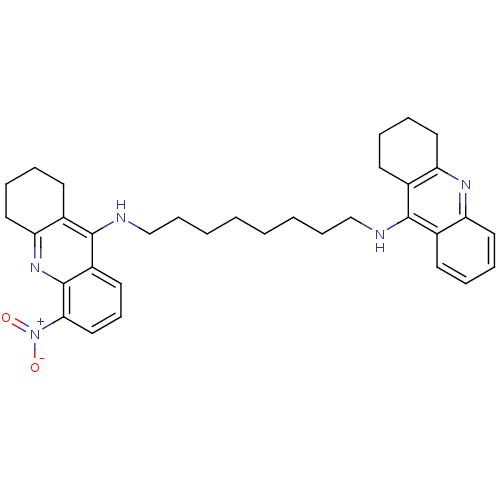
(CHEMBL3099496)Show SMILES [O-][N+](=O)c1cccc2c(NCCCCCCCCNc3c4CCCCc4nc4ccccc34)c3CCCCc3nc12 Show InChI InChI=1S/C34H41N5O2/c40-39(41)31-21-13-17-27-33(26-16-7-10-20-30(26)38-34(27)31)36-23-12-4-2-1-3-11-22-35-32-24-14-5-8-18-28(24)37-29-19-9-6-15-25(29)32/h5,8,13-14,17-18,21H,1-4,6-7,9-12,15-16,19-20,22-23H2,(H,35,37)(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human butyrylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005192
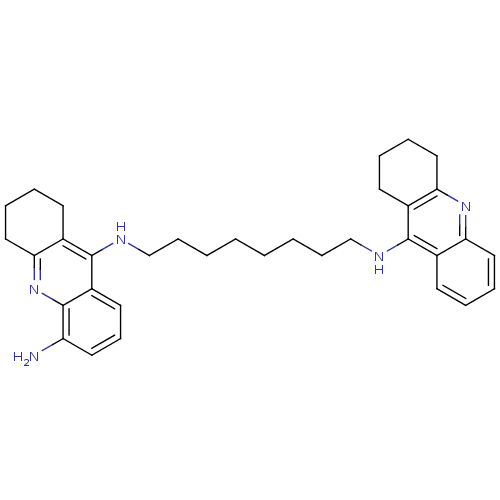
(CHEMBL3099497)Show SMILES Nc1cccc2c(NCCCCCCCCNc3c4CCCCc4nc4ccccc34)c3CCCCc3nc12 Show InChI InChI=1S/C34H43N5/c35-28-18-13-17-27-33(26-16-7-10-21-31(26)39-34(27)28)37-23-12-4-2-1-3-11-22-36-32-24-14-5-8-19-29(24)38-30-20-9-6-15-25(30)32/h5,8,13-14,17-19H,1-4,6-7,9-12,15-16,20-23,35H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005193

(CHEMBL3099496)Show SMILES [O-][N+](=O)c1cccc2c(NCCCCCCCCNc3c4CCCCc4nc4ccccc34)c3CCCCc3nc12 Show InChI InChI=1S/C34H41N5O2/c40-39(41)31-21-13-17-27-33(26-16-7-10-20-30(26)38-34(27)31)36-23-12-4-2-1-3-11-22-35-32-24-14-5-8-18-28(24)37-29-19-9-6-15-25(29)32/h5,8,13-14,17-18,21H,1-4,6-7,9-12,15-16,19-20,22-23H2,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271367

(CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H43N5/c1-39(24-12-22-36-34-27-15-4-8-19-31(27)38-32-20-9-5-16-28(32)34)23-11-10-21-35-33-25-13-2-6-17-29(25)37-30-18-7-3-14-26(30)33/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,37)(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human butyrylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005188
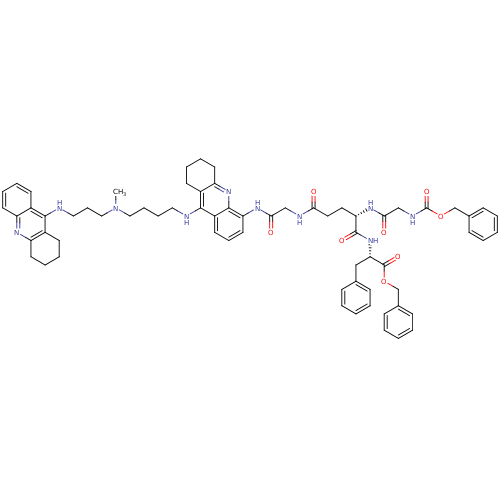
(CHEMBL3099500)Show SMILES CN(CCCCNc1c2CCCCc2nc2c(NC(=O)CNC(=O)CC[C@H](NC(=O)CNC(=O)OCc3ccccc3)C(=O)N[C@@H](Cc3ccccc3)C(=O)OCc3ccccc3)cccc12)CCCNc1c2CCCCc2nc2ccccc12 |r| Show InChI InChI=1S/C67H78N10O8/c1-77(40-20-38-69-62-49-27-11-14-31-53(49)72-54-32-15-12-28-50(54)62)39-18-17-37-68-63-51-29-13-16-33-55(51)75-64-52(63)30-19-34-56(64)73-60(79)42-70-59(78)36-35-57(74-61(80)43-71-67(83)85-45-48-25-9-4-10-26-48)65(81)76-58(41-46-21-5-2-6-22-46)66(82)84-44-47-23-7-3-8-24-47/h2-11,14,19,21-27,30-31,34,57-58H,12-13,15-18,20,28-29,32-33,35-45H2,1H3,(H,68,75)(H,69,72)(H,70,78)(H,71,83)(H,73,79)(H,74,80)(H,76,81)/t57-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8971
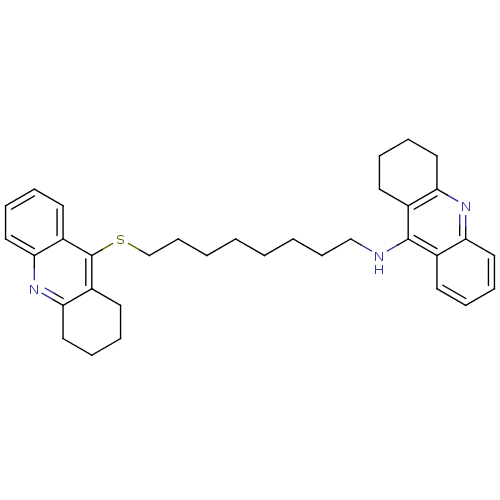
(CHEMBL129108 | N-[8-(1,2,3,4-tetrahydroacridin-9-y...)Show SMILES C(CCCCSc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3S/c1(3-13-23-35-33-25-15-5-9-19-29(25)36-30-20-10-6-16-26(30)33)2-4-14-24-38-34-27-17-7-11-21-31(27)37-32-22-12-8-18-28(32)34/h5,7,9,11,15,17,19,21H,1-4,6,8,10,12-14,16,18,20,22-24H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human butyrylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005191

(CHEMBL3099498)Show SMILES O=C(CC[C@H](NC(=O)CNC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)OCc1ccccc1)NCC(=O)Nc1cccc2c(NCCCCCCCCNc3c4CCCCc4nc4ccccc34)c3CCCCc3nc12 |r| Show InChI InChI=1S/C67H77N9O8/c77-59(38-37-57(74-61(79)43-71-67(82)84-45-48-27-12-7-13-28-48)65(80)76-58(41-46-23-8-5-9-24-46)66(81)83-44-47-25-10-6-11-26-47)70-42-60(78)73-56-36-22-32-52-63(51-31-16-19-35-55(51)75-64(52)56)69-40-21-4-2-1-3-20-39-68-62-49-29-14-17-33-53(49)72-54-34-18-15-30-50(54)62/h5-14,17,22-29,32-33,36,57-58H,1-4,15-16,18-21,30-31,34-35,37-45H2,(H,68,72)(H,69,75)(H,70,77)(H,71,82)(H,73,78)(H,74,79)(H,76,80)/t57-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005189

(CHEMBL3099499)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2c(NC(=O)CNC(=O)CC[C@H](NC(=O)CNC(=O)OCc3ccccc3)C(=O)N[C@@H](Cc3ccccc3)C(=O)OCc3ccccc3)cccc12 |r| Show InChI InChI=1S/C67H78N10O8/c1-77(39-18-17-37-68-62-49-27-11-14-31-53(49)72-54-32-15-12-28-50(54)62)40-20-38-69-63-51-29-13-16-33-55(51)75-64-52(63)30-19-34-56(64)73-60(79)42-70-59(78)36-35-57(74-61(80)43-71-67(83)85-45-48-25-9-4-10-26-48)65(81)76-58(41-46-21-5-2-6-22-46)66(82)84-44-47-23-7-3-8-24-47/h2-11,14,19,21-27,30-31,34,57-58H,12-13,15-18,20,28-29,32-33,35-45H2,1H3,(H,68,72)(H,69,75)(H,70,78)(H,71,83)(H,73,79)(H,74,80)(H,76,81)/t57-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005192

(CHEMBL3099497)Show SMILES Nc1cccc2c(NCCCCCCCCNc3c4CCCCc4nc4ccccc34)c3CCCCc3nc12 Show InChI InChI=1S/C34H43N5/c35-28-18-13-17-27-33(26-16-7-10-21-31(26)39-34(27)28)37-23-12-4-2-1-3-11-22-36-32-24-14-5-8-19-29(24)38-30-20-9-6-15-25(30)32/h5,8,13-14,17-19H,1-4,6-7,9-12,15-16,20-23,35H2,(H,36,38)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human butyrylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005188

(CHEMBL3099500)Show SMILES CN(CCCCNc1c2CCCCc2nc2c(NC(=O)CNC(=O)CC[C@H](NC(=O)CNC(=O)OCc3ccccc3)C(=O)N[C@@H](Cc3ccccc3)C(=O)OCc3ccccc3)cccc12)CCCNc1c2CCCCc2nc2ccccc12 |r| Show InChI InChI=1S/C67H78N10O8/c1-77(40-20-38-69-62-49-27-11-14-31-53(49)72-54-32-15-12-28-50(54)62)39-18-17-37-68-63-51-29-13-16-33-55(51)75-64-52(63)30-19-34-56(64)73-60(79)42-70-59(78)36-35-57(74-61(80)43-71-67(83)85-45-48-25-9-4-10-26-48)65(81)76-58(41-46-21-5-2-6-22-46)66(82)84-44-47-23-7-3-8-24-47/h2-11,14,19,21-27,30-31,34,57-58H,12-13,15-18,20,28-29,32-33,35-45H2,1H3,(H,68,75)(H,69,72)(H,70,78)(H,71,83)(H,73,79)(H,74,80)(H,76,81)/t57-,58-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human butyrylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8971

(CHEMBL129108 | N-[8-(1,2,3,4-tetrahydroacridin-9-y...)Show SMILES C(CCCCSc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3S/c1(3-13-23-35-33-25-15-5-9-19-29(25)36-30-20-10-6-16-26(30)33)2-4-14-24-38-34-27-17-7-11-21-31(27)37-32-22-12-8-18-28(32)34/h5,7,9,11,15,17,19,21H,1-4,6,8,10,12-14,16,18,20,22-24H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005191

(CHEMBL3099498)Show SMILES O=C(CC[C@H](NC(=O)CNC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)OCc1ccccc1)NCC(=O)Nc1cccc2c(NCCCCCCCCNc3c4CCCCc4nc4ccccc34)c3CCCCc3nc12 |r| Show InChI InChI=1S/C67H77N9O8/c77-59(38-37-57(74-61(79)43-71-67(82)84-45-48-27-12-7-13-28-48)65(80)76-58(41-46-23-8-5-9-24-46)66(81)83-44-47-25-10-6-11-26-47)70-42-60(78)73-56-36-22-32-52-63(51-31-16-19-35-55(51)75-64(52)56)69-40-21-4-2-1-3-20-39-68-62-49-29-14-17-33-53(49)72-54-34-18-15-30-50(54)62/h5-14,17,22-29,32-33,36,57-58H,1-4,15-16,18-21,30-31,34-35,37-45H2,(H,68,72)(H,69,75)(H,70,77)(H,71,82)(H,73,78)(H,74,79)(H,76,80)/t57-,58-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human butyrylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005189

(CHEMBL3099499)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2c(NC(=O)CNC(=O)CC[C@H](NC(=O)CNC(=O)OCc3ccccc3)C(=O)N[C@@H](Cc3ccccc3)C(=O)OCc3ccccc3)cccc12 |r| Show InChI InChI=1S/C67H78N10O8/c1-77(39-18-17-37-68-62-49-27-11-14-31-53(49)72-54-32-15-12-28-50(54)62)40-20-38-69-63-51-29-13-16-33-55(51)75-64-52(63)30-19-34-56(64)73-60(79)42-70-59(78)36-35-57(74-61(80)43-71-67(83)85-45-48-25-9-4-10-26-48)65(81)76-58(41-46-21-5-2-6-22-46)66(82)84-44-47-23-7-3-8-24-47/h2-11,14,19,21-27,30-31,34,57-58H,12-13,15-18,20,28-29,32-33,35-45H2,1H3,(H,68,72)(H,69,75)(H,70,78)(H,71,83)(H,73,79)(H,74,80)(H,76,81)/t57-,58-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human butyrylcholine esterase |
ACS Med Chem Lett 4: 1178-82 (2013)
Article DOI: 10.1021/ml4002908
BindingDB Entry DOI: 10.7270/Q2TQ6318 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399922
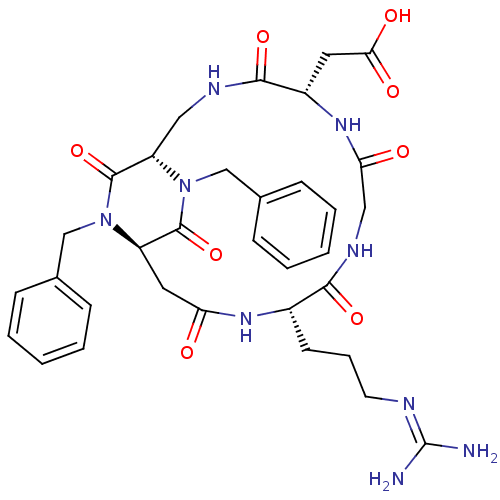
(CHEMBL2180974)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@H]-2-[#7](-[#6]-c3ccccc3)-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7](-[#6]-c1ccccc1)-[#6]-2=O |r| Show InChI InChI=1S/C33H41N9O8/c34-33(35)36-13-7-12-22-29(47)38-17-27(44)40-23(14-28(45)46)30(48)37-16-25-32(50)41(18-20-8-3-1-4-9-20)24(15-26(43)39-22)31(49)42(25)19-21-10-5-2-6-11-21/h1-6,8-11,22-25H,7,12-19H2,(H,37,48)(H,38,47)(H,39,43)(H,40,44)(H,45,46)(H4,34,35,36)/t22-,23-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399922

(CHEMBL2180974)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@H]-2-[#7](-[#6]-c3ccccc3)-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7](-[#6]-c1ccccc1)-[#6]-2=O |r| Show InChI InChI=1S/C33H41N9O8/c34-33(35)36-13-7-12-22-29(47)38-17-27(44)40-23(14-28(45)46)30(48)37-16-25-32(50)41(18-20-8-3-1-4-9-20)24(15-26(43)39-22)31(49)42(25)19-21-10-5-2-6-11-21/h1-6,8-11,22-25H,7,12-19H2,(H,37,48)(H,38,47)(H,39,43)(H,40,44)(H,45,46)(H4,34,35,36)/t22-,23-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399923

(CHEMBL2180979)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@@H]-2-[#7](-[#6]-c3ccccc3)-[#6](=O)-[#6@@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7](-[#6]-c1ccccc1)-[#6]-2=O |r| Show InChI InChI=1S/C33H41N9O8/c34-33(35)36-13-7-12-22-29(47)38-17-27(44)40-23(14-28(45)46)30(48)37-16-25-32(50)41(18-20-8-3-1-4-9-20)24(15-26(43)39-22)31(49)42(25)19-21-10-5-2-6-11-21/h1-6,8-11,22-25H,7,12-19H2,(H,37,48)(H,38,47)(H,39,43)(H,40,44)(H,45,46)(H4,34,35,36)/t22-,23-,24-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399919
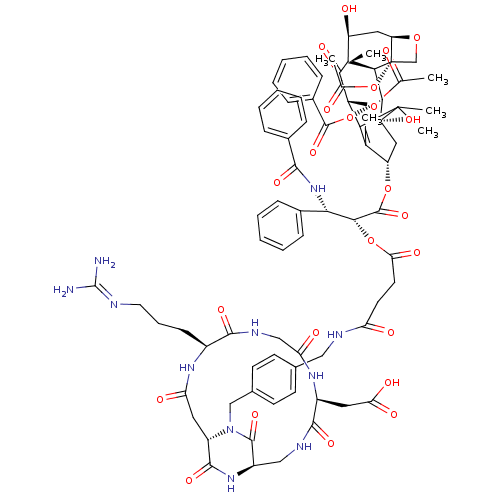
(CHEMBL2180983)Show SMILES CC(=O)O[C@@H]1C2=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c3ccccc3)[C@@H]3[C@@]4(CO[C@@H]4C[C@H](O)[C@@]3(C)C1=O)OC(C)=O)C2(C)C)OC(=O)[C@H](OC(=O)CCC(=O)NCc1ccc(CN2[C@H]3CC(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)NC[C@@H](NC3=O)C2=O)cc1)[C@@H](NC(=O)c1ccccc1)c1ccccc1 |r,wU:65.70,80.85,22.23,26.26,4.3,10.10,30.33,28.30,60.64,wD:97.105,12.12,8.45,23.37,44.49,89.94,c:5,(3.53,-26.48,;3.49,-24.94,;2.14,-24.21,;4.81,-24.14,;6.16,-24.86,;6.22,-27.9,;6.24,-29.43,;4.92,-30.22,;7.59,-30.2,;8.91,-29.4,;8.88,-27.87,;10.23,-28.61,;10.21,-27.08,;11.56,-27.82,;11.57,-29.38,;10.26,-30.14,;12.91,-30.11,;14.22,-29.31,;15.57,-30.05,;15.6,-31.61,;14.28,-32.4,;12.94,-31.65,;10.17,-25.54,;11.49,-24.75,;13.04,-24.72,;13.01,-23.19,;11.46,-23.19,;10.11,-22.45,;8.78,-23.26,;8.73,-21.72,;8.82,-24.8,;9.24,-26.26,;7.47,-24.06,;7.43,-22.52,;11.9,-26.23,;13.25,-26.97,;14.58,-26.18,;13.27,-28.52,;7.54,-27.11,;6.72,-25.81,;8.29,-25.76,;7.6,-31.72,;6.28,-32.51,;4.95,-31.76,;6.3,-34.04,;7.64,-34.8,;8.97,-34.03,;8.96,-32.49,;10.31,-34.79,;11.64,-34.01,;12.98,-34.77,;12.99,-36.31,;14.31,-33.99,;15.65,-34.75,;16.97,-33.97,;18.31,-34.74,;19.64,-33.97,;19.63,-32.42,;20.96,-31.64,;22.3,-32.4,;23.61,-31.62,;23.6,-30.08,;24.92,-29.29,;24.9,-27.75,;26.27,-30.04,;27.59,-29.25,;27.57,-27.71,;26.22,-26.96,;26.2,-25.42,;24.86,-24.67,;24.83,-23.13,;23.49,-22.38,;26.15,-22.34,;28.94,-30,;30.26,-29.21,;28.96,-31.54,;30.3,-32.29,;30.33,-33.83,;31.67,-34.58,;29,-34.62,;29.03,-36.16,;30.37,-36.91,;30.4,-38.45,;29.07,-39.24,;31.74,-39.2,;27.71,-36.95,;27.73,-38.49,;25.04,-36.99,;23.64,-36.24,;23.63,-34.7,;24.96,-33.93,;24.95,-32.39,;26.29,-31.61,;22.3,-33.94,;20.97,-34.72,;18.28,-31.66,;16.96,-32.44,;4.97,-34.83,;3.63,-34.09,;2.32,-34.86,;2.34,-36.42,;.98,-34.13,;.96,-32.57,;-.39,-31.83,;-1.7,-32.63,;-1.69,-34.16,;-.35,-34.92,;5,-36.36,;6.35,-37.1,;6.37,-38.66,;5.04,-39.45,;3.7,-38.69,;3.68,-37.16,)| Show InChI InChI=1S/C78H91N11O24/c1-40-52(34-78(107)66(112-72(105)47-21-14-9-15-22-47)64-76(6,53(92)33-54-77(64,39-108-54)113-42(3)91)65(99)62(109-41(2)90)60(40)75(78,4)5)110-73(106)63(61(45-17-10-7-11-18-45)88-67(100)46-19-12-8-13-20-46)111-59(98)29-28-55(93)82-35-43-24-26-44(27-25-43)38-89-51-32-56(94)85-48(23-16-30-81-74(79)80)68(101)84-37-57(95)86-49(31-58(96)97)69(102)83-36-50(71(89)104)87-70(51)103/h7-15,17-22,24-27,48-54,61-64,66,92,107H,16,23,28-39H2,1-6H3,(H,82,93)(H,83,102)(H,84,101)(H,85,94)(H,86,95)(H,87,103)(H,88,100)(H,96,97)(H4,79,80,81)/t48-,49-,50+,51-,52-,53-,54+,61-,62+,63+,64-,66-,76+,77-,78+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50177879

(CHEMBL200182 | ST-1646)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6@H]-3-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#6](=O)-[#7]-2-3 Show InChI InChI=1S/C22H34N8O7/c23-22(24)25-8-2-5-12-18(34)26-10-16(31)27-14(9-17(32)33)19(35)29-13-4-1-3-11-6-7-15(20(36)28-12)30(11)21(13)37/h11-15H,1-10H2,(H,26,34)(H,27,31)(H,28,36)(H,29,35)(H,32,33)(H4,23,24,25)/t11-,12+,13-,14+,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399918

(CHEMBL2180982)Show SMILES CC(=O)O[C@@H]1C2=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c3ccccc3)[C@@H]3[C@@]4(CO[C@@H]4C[C@H](O)[C@@]3(C)C1=O)OC(C)=O)C2(C)C)OC(=O)[C@H](OC(=O)CCC(=O)NCc1ccc(CN2[C@@H]3CC(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)NC[C@H](NC3=O)C2=O)cc1)[C@@H](NC(=O)c1ccccc1)c1ccccc1 |r,wU:65.70,80.85,22.23,26.26,4.3,10.10,30.33,28.30,89.94,wD:97.105,12.12,8.45,23.37,44.49,60.64,c:5,(43.79,-26.11,;43.75,-24.57,;42.4,-23.84,;45.06,-23.78,;46.41,-24.49,;46.47,-27.53,;46.5,-29.07,;45.17,-29.85,;47.85,-29.83,;49.16,-29.04,;49.14,-27.51,;50.49,-28.25,;50.46,-26.71,;51.81,-27.46,;51.83,-29.01,;50.51,-29.78,;53.17,-29.74,;54.48,-28.95,;55.83,-29.69,;55.85,-31.24,;54.53,-32.04,;53.2,-31.28,;50.43,-25.18,;51.74,-24.38,;53.29,-24.36,;53.26,-22.82,;51.72,-22.83,;50.37,-22.09,;49.04,-22.9,;48.99,-21.35,;49.08,-24.44,;49.5,-25.89,;47.73,-23.7,;47.69,-22.16,;52.16,-25.86,;53.51,-26.6,;54.83,-25.81,;53.53,-28.16,;47.8,-26.75,;46.98,-25.44,;48.54,-25.4,;47.86,-31.36,;46.54,-32.15,;45.21,-31.39,;46.56,-33.68,;47.9,-34.44,;49.23,-33.66,;49.22,-32.12,;50.57,-34.42,;51.9,-33.64,;53.23,-34.4,;53.24,-35.94,;54.56,-33.62,;55.9,-34.39,;57.23,-33.61,;58.57,-34.38,;59.89,-33.6,;59.88,-32.06,;61.21,-31.28,;62.55,-32.04,;63.87,-31.25,;63.86,-29.71,;65.18,-28.92,;65.16,-27.38,;66.53,-29.67,;67.85,-28.88,;67.82,-27.34,;66.48,-26.59,;66.46,-25.05,;65.11,-24.3,;65.09,-22.76,;63.74,-22.01,;66.41,-21.97,;69.19,-29.63,;70.51,-28.84,;69.22,-31.17,;70.56,-31.92,;70.58,-33.46,;71.93,-34.21,;69.26,-34.25,;69.28,-35.79,;70.63,-36.54,;70.65,-38.08,;69.33,-38.87,;72,-38.83,;67.96,-36.58,;67.98,-38.12,;65.3,-36.62,;63.89,-35.88,;63.89,-34.34,;65.22,-33.56,;65.21,-32.02,;66.54,-31.25,;62.56,-33.58,;61.22,-34.35,;58.54,-31.3,;57.21,-32.08,;45.23,-34.46,;43.89,-33.73,;42.57,-34.49,;42.6,-36.05,;41.24,-33.76,;41.22,-32.21,;39.87,-31.47,;38.55,-32.26,;38.58,-33.8,;39.92,-34.56,;45.26,-36,;46.61,-36.74,;46.63,-38.29,;45.3,-39.08,;43.96,-38.32,;43.94,-36.79,)| Show InChI InChI=1S/C78H91N11O24/c1-40-52(34-78(107)66(112-72(105)47-21-14-9-15-22-47)64-76(6,53(92)33-54-77(64,39-108-54)113-42(3)91)65(99)62(109-41(2)90)60(40)75(78,4)5)110-73(106)63(61(45-17-10-7-11-18-45)88-67(100)46-19-12-8-13-20-46)111-59(98)29-28-55(93)82-35-43-24-26-44(27-25-43)38-89-51-32-56(94)85-48(23-16-30-81-74(79)80)68(101)84-37-57(95)86-49(31-58(96)97)69(102)83-36-50(71(89)104)87-70(51)103/h7-15,17-22,24-27,48-54,61-64,66,92,107H,16,23,28-39H2,1-6H3,(H,82,93)(H,83,102)(H,84,101)(H,85,94)(H,86,95)(H,87,103)(H,88,100)(H,96,97)(H4,79,80,81)/t48-,49-,50-,51+,52-,53-,54+,61-,62+,63+,64-,66-,76+,77-,78+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50177879

(CHEMBL200182 | ST-1646)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6@H]-3-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#6](=O)-[#7]-2-3 Show InChI InChI=1S/C22H34N8O7/c23-22(24)25-8-2-5-12-18(34)26-10-16(31)27-14(9-17(32)33)19(35)29-13-4-1-3-11-6-7-15(20(36)28-12)30(11)21(13)37/h11-15H,1-10H2,(H,26,34)(H,27,31)(H,28,36)(H,29,35)(H,32,33)(H4,23,24,25)/t11-,12+,13-,14+,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta5 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31532
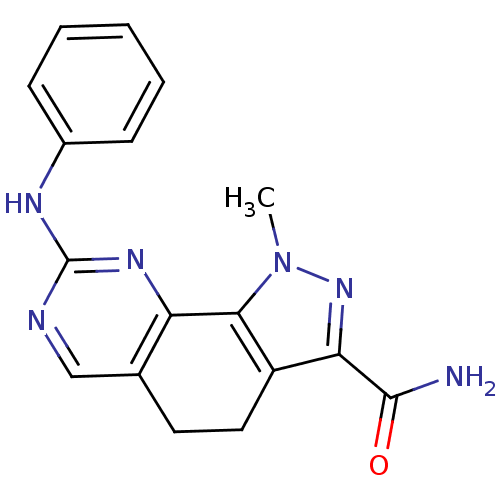
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399912

(CHEMBL2180973)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@H]-2-[#7](-[#6]-c3ccccc3)-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7]-[#6]-2=O |r| Show InChI InChI=1S/C26H35N9O8/c27-26(28)29-8-4-7-15-22(40)31-12-20(37)33-16(9-21(38)39)23(41)30-11-17-25(43)35(13-14-5-2-1-3-6-14)18(24(42)34-17)10-19(36)32-15/h1-3,5-6,15-18H,4,7-13H2,(H,30,41)(H,31,40)(H,32,36)(H,33,37)(H,34,42)(H,38,39)(H4,27,28,29)/t15-,16-,17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318085
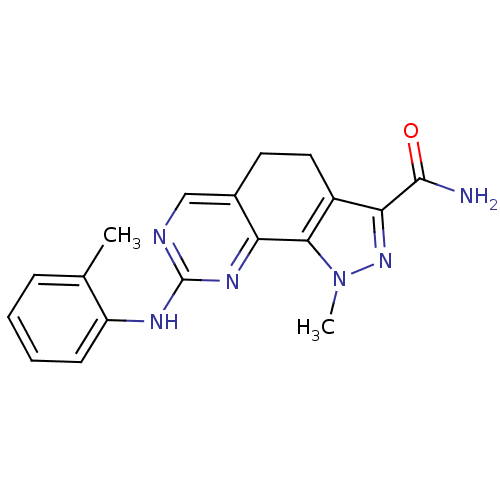
(1-Methyl-8-[(2-methylphenyl)amino]-4,5-dihydro-1H-...)Show InChI InChI=1S/C18H18N6O/c1-10-5-3-4-6-13(10)21-18-20-9-11-7-8-12-15(17(19)25)23-24(2)16(12)14(11)22-18/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50237601
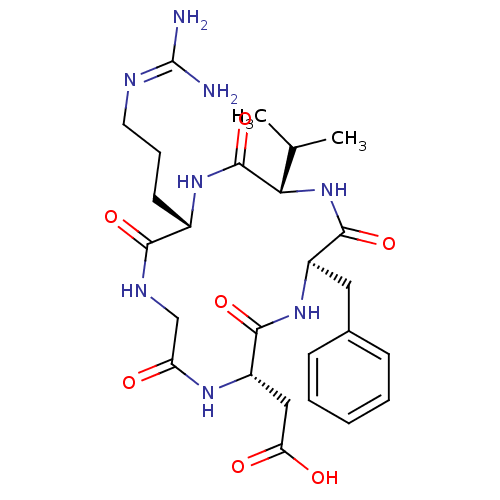
(CHEMBL411941 | CycloRGDfV | [(2S,5R,8S,11S)-5-Benz...)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C26H38N8O7/c1-14(2)21-25(41)32-16(9-6-10-29-26(27)28)22(38)30-13-19(35)31-18(12-20(36)37)23(39)33-17(24(40)34-21)11-15-7-4-3-5-8-15/h3-5,7-8,14,16-18,21H,6,9-13H2,1-2H3,(H,30,38)(H,31,35)(H,32,41)(H,33,39)(H,34,40)(H,36,37)(H4,27,28,29)/t16-,17+,18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399915

(CHEMBL2180976)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6@@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7](-[#6]-c1ccccc1)-[#6]-2=O |r| Show InChI InChI=1S/C26H35N9O8/c27-26(28)29-8-4-7-15-22(40)31-12-20(37)33-16(10-21(38)39)23(41)30-11-18-24(42)34-17(9-19(36)32-15)25(43)35(18)13-14-5-2-1-3-6-14/h1-3,5-6,15-18H,4,7-13H2,(H,30,41)(H,31,40)(H,32,36)(H,33,37)(H,34,42)(H,38,39)(H4,27,28,29)/t15-,16-,17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399924

(CHEMBL2180975)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7](-[#6]-c1ccccc1)-[#6]-2=O |r| Show InChI InChI=1S/C26H35N9O8/c27-26(28)29-8-4-7-15-22(40)31-12-20(37)33-16(10-21(38)39)23(41)30-11-18-24(42)34-17(9-19(36)32-15)25(43)35(18)13-14-5-2-1-3-6-14/h1-3,5-6,15-18H,4,7-13H2,(H,30,41)(H,31,40)(H,32,36)(H,33,37)(H,34,42)(H,38,39)(H4,27,28,29)/t15-,16-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399916
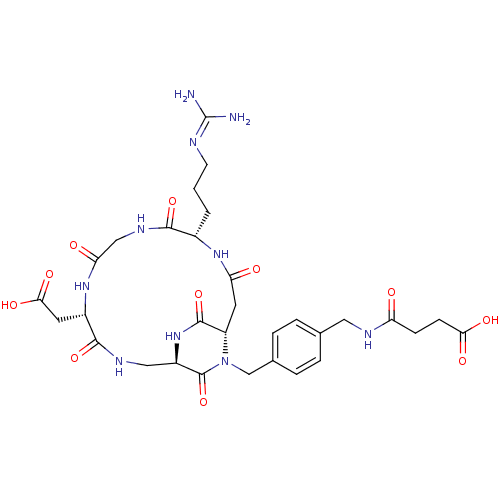
(CHEMBL2180980)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@@H]-2-[#7](-[#6]-c3ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](-[#8])=O)cc3)-[#6](=O)-[#6@@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7]-[#6]-2=O |r| Show InChI InChI=1S/C31H42N10O11/c32-31(33)34-9-1-2-18-27(49)37-14-24(44)39-19(10-26(47)48)28(50)36-13-20-30(52)41(21(29(51)40-20)11-23(43)38-18)15-17-5-3-16(4-6-17)12-35-22(42)7-8-25(45)46/h3-6,18-21H,1-2,7-15H2,(H,35,42)(H,36,50)(H,37,49)(H,38,43)(H,39,44)(H,40,51)(H,45,46)(H,47,48)(H4,32,33,34)/t18-,19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399914

(CHEMBL2180977)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7](-[#6]-c1ccccc1)-[#6]-2=O |r| Show InChI InChI=1S/C26H35N9O8/c27-26(28)29-8-4-7-15-22(40)31-12-20(37)33-16(10-21(38)39)23(41)30-11-18-24(42)34-17(9-19(36)32-15)25(43)35(18)13-14-5-2-1-3-6-14/h1-3,5-6,15-18H,4,7-13H2,(H,30,41)(H,31,40)(H,32,36)(H,33,37)(H,34,42)(H,38,39)(H4,27,28,29)/t15-,16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399920
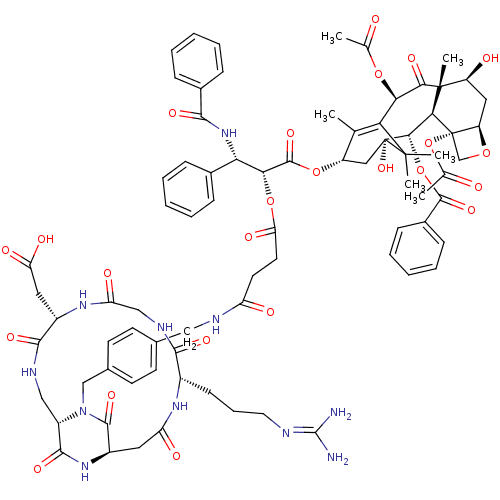
(CHEMBL2180984)Show SMILES CC(=O)O[C@@H]1C2=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c3ccccc3)[C@@H]3[C@@]4(CO[C@@H]4C[C@H](O)[C@@]3(C)C1=O)OC(C)=O)C2(C)C)OC(=O)[C@H](OC(=O)CCC(=O)NCc1ccc(CN2[C@H]3CNC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CCCN=C(N)N)NC(=O)C[C@@H](NC3=O)C2=O)cc1)[C@@H](NC(=O)c1ccccc1)c1ccccc1 |r,wU:22.23,26.26,4.3,10.10,30.33,28.30,89.94,77.82,65.70,wD:97.105,12.12,8.45,23.37,44.49,60.64,c:5,(45,-3.57,;44.96,-2.03,;43.6,-1.29,;46.27,-1.23,;47.62,-1.94,;47.68,-4.99,;47.71,-6.52,;46.38,-7.31,;49.05,-7.28,;50.37,-6.49,;50.35,-4.96,;51.7,-5.7,;51.67,-4.17,;53.02,-4.91,;53.03,-6.46,;51.72,-7.23,;54.37,-7.2,;55.69,-6.4,;57.04,-7.14,;57.06,-8.7,;55.74,-9.49,;54.41,-8.73,;51.63,-2.63,;52.95,-1.84,;54.5,-1.81,;54.47,-.28,;52.92,-.28,;51.57,.47,;50.24,-.35,;50.19,1.2,;50.28,-1.89,;50.71,-3.35,;48.93,-1.15,;48.89,.4,;53.36,-3.31,;54.71,-4.05,;56.04,-3.27,;54.73,-5.61,;49.01,-4.2,;48.19,-2.9,;49.75,-2.85,;49.07,-8.81,;47.75,-9.6,;46.41,-8.84,;47.77,-11.13,;49.11,-11.89,;50.43,-11.11,;50.42,-9.57,;51.77,-11.87,;53.1,-11.09,;54.44,-11.86,;54.45,-13.4,;55.77,-11.08,;57.11,-11.84,;58.44,-11.06,;59.77,-11.83,;61.1,-11.05,;61.09,-9.51,;62.42,-8.73,;63.76,-9.49,;63.76,-11.03,;62.43,-11.81,;62.44,-13.35,;63.78,-15.65,;62.45,-16.42,;65.11,-16.41,;65.12,-17.95,;63.79,-18.73,;62.45,-17.96,;63.8,-20.27,;66.44,-15.63,;67.78,-16.4,;67.79,-17.94,;69.11,-15.62,;69.1,-14.08,;70.43,-13.31,;71.77,-14.07,;70.43,-11.77,;71.76,-10.99,;71.75,-9.45,;73.08,-8.67,;73.07,-7.13,;74.41,-6.36,;74.4,-4.82,;75.74,-7.12,;69.09,-11,;69.08,-9.46,;70.41,-8.69,;67.75,-8.7,;66.42,-9.47,;66.43,-11.02,;65.09,-11.79,;65.1,-13.33,;65.08,-8.71,;65.06,-7.17,;59.74,-8.75,;58.42,-9.53,;46.43,-11.91,;45.1,-11.18,;43.78,-11.95,;43.8,-13.5,;42.44,-11.21,;42.42,-9.66,;41.07,-8.92,;39.76,-9.72,;39.79,-11.25,;41.13,-12.01,;46.47,-13.45,;47.82,-14.19,;47.83,-15.74,;46.51,-16.53,;45.16,-15.78,;45.14,-14.24,)| Show InChI InChI=1S/C78H91N11O24/c1-40-52(34-78(107)66(112-72(105)47-21-14-9-15-22-47)64-76(6,53(92)33-54-77(64,39-108-54)113-42(3)91)65(99)62(109-41(2)90)60(40)75(78,4)5)110-73(106)63(61(45-17-10-7-11-18-45)88-67(100)46-19-12-8-13-20-46)111-59(98)29-28-55(93)82-35-43-24-26-44(27-25-43)38-89-51-36-83-69(102)49(32-58(96)97)86-57(95)37-84-68(101)48(23-16-30-81-74(79)80)85-56(94)31-50(71(89)104)87-70(51)103/h7-15,17-22,24-27,48-54,61-64,66,92,107H,16,23,28-39H2,1-6H3,(H,82,93)(H,83,102)(H,84,101)(H,85,94)(H,86,95)(H,87,103)(H,88,100)(H,96,97)(H4,79,80,81)/t48-,49-,50+,51-,52-,53-,54+,61-,62+,63+,64-,66-,76+,77-,78+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50237601

(CHEMBL411941 | CycloRGDfV | [(2S,5R,8S,11S)-5-Benz...)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C26H38N8O7/c1-14(2)21-25(41)32-16(9-6-10-29-26(27)28)22(38)30-13-19(35)31-18(12-20(36)37)23(39)33-17(24(40)34-21)11-15-7-4-3-5-8-15/h3-5,7-8,14,16-18,21H,6,9-13H2,1-2H3,(H,30,38)(H,31,35)(H,32,41)(H,33,39)(H,34,40)(H,36,37)(H4,27,28,29)/t16-,17+,18-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta5 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399913
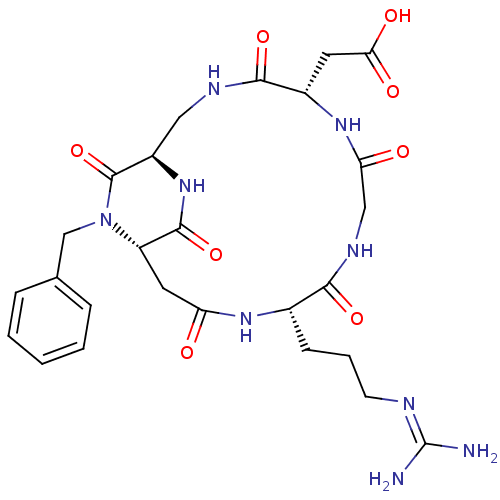
(CHEMBL2180978)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6]-[#6@@H]-2-[#7](-[#6]-c3ccccc3)-[#6](=O)-[#6@@H](-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6]-1=O)-[#7]-[#6]-2=O |r| Show InChI InChI=1S/C26H35N9O8/c27-26(28)29-8-4-7-15-22(40)31-12-20(37)33-16(9-21(38)39)23(41)30-11-17-25(43)35(13-14-5-2-1-3-6-14)18(24(42)34-17)10-19(36)32-15/h1-3,5-6,15-18H,4,7-13H2,(H,30,41)(H,31,40)(H,32,36)(H,33,37)(H,34,42)(H,38,39)(H4,27,28,29)/t15-,16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50399921
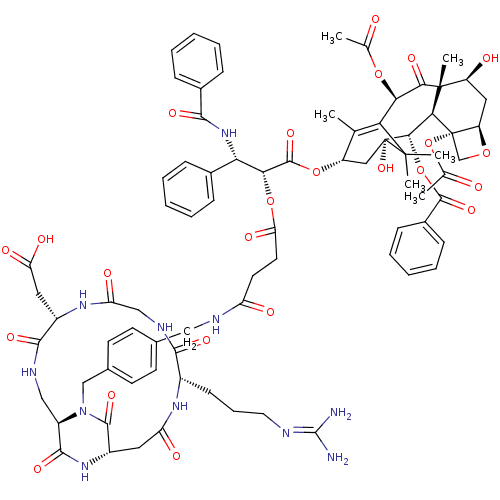
(CHEMBL2180985)Show SMILES CC(=O)O[C@@H]1C2=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c3ccccc3)[C@@H]3[C@@]4(CO[C@@H]4C[C@H](O)[C@@]3(C)C1=O)OC(C)=O)C2(C)C)OC(=O)[C@H](OC(=O)CCC(=O)NCc1ccc(CN2[C@@H]3CNC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CCCN=C(N)N)NC(=O)C[C@H](NC3=O)C2=O)cc1)[C@@H](NC(=O)c1ccccc1)c1ccccc1 |r,wU:22.23,26.26,4.3,10.10,30.33,28.30,60.64,77.82,65.70,wD:97.105,12.12,8.45,23.37,44.49,89.94,c:5,(5.81,-4.11,;5.77,-2.57,;4.42,-1.84,;7.09,-1.78,;8.43,-2.49,;8.5,-5.53,;8.52,-7.06,;7.19,-7.85,;9.87,-7.83,;11.18,-7.03,;11.16,-5.5,;12.51,-6.24,;12.48,-4.71,;13.83,-5.45,;13.85,-7.01,;12.53,-7.77,;15.19,-7.74,;16.5,-6.94,;17.85,-7.69,;17.88,-9.24,;16.55,-10.03,;15.22,-9.28,;12.45,-3.18,;13.76,-2.38,;15.31,-2.35,;15.29,-.82,;13.74,-.82,;12.39,-.08,;11.06,-.89,;11.01,.66,;11.1,-2.43,;11.52,-3.89,;9.75,-1.69,;9.71,-.15,;14.18,-3.86,;15.53,-4.6,;16.86,-3.81,;15.55,-6.16,;9.82,-4.74,;9,-3.44,;10.56,-3.39,;9.88,-9.35,;8.56,-10.14,;7.23,-9.39,;8.58,-11.67,;9.92,-12.44,;11.25,-11.66,;11.24,-10.12,;12.59,-12.42,;13.92,-11.64,;15.26,-12.4,;15.27,-13.94,;16.59,-11.62,;17.92,-12.38,;19.25,-11.6,;20.59,-12.37,;21.92,-11.6,;21.91,-10.05,;23.23,-9.27,;24.57,-10.03,;24.58,-11.57,;23.25,-12.35,;23.25,-13.89,;24.59,-16.19,;23.26,-16.97,;25.93,-16.95,;25.94,-18.49,;24.61,-19.27,;23.27,-18.51,;24.61,-20.81,;27.26,-16.18,;28.6,-16.94,;28.6,-18.48,;29.93,-16.17,;29.92,-14.63,;31.25,-13.85,;32.59,-14.62,;31.24,-12.31,;32.57,-11.54,;32.57,-10,;33.9,-9.22,;33.89,-7.68,;35.22,-6.9,;35.21,-5.36,;36.56,-7.67,;29.91,-11.55,;29.9,-10.01,;31.23,-9.23,;28.56,-9.24,;27.23,-10.02,;27.24,-11.56,;25.91,-12.33,;25.92,-13.87,;25.89,-9.25,;25.88,-7.71,;20.56,-9.29,;19.24,-10.08,;7.25,-12.46,;5.91,-11.72,;4.59,-12.49,;4.62,-14.05,;3.26,-11.76,;3.24,-10.21,;1.89,-9.46,;.58,-10.26,;.6,-11.79,;1.94,-12.55,;7.28,-13.99,;8.63,-14.74,;8.65,-16.29,;7.32,-17.08,;5.98,-16.32,;5.96,-14.79,)| Show InChI InChI=1S/C78H91N11O24/c1-40-52(34-78(107)66(112-72(105)47-21-14-9-15-22-47)64-76(6,53(92)33-54-77(64,39-108-54)113-42(3)91)65(99)62(109-41(2)90)60(40)75(78,4)5)110-73(106)63(61(45-17-10-7-11-18-45)88-67(100)46-19-12-8-13-20-46)111-59(98)29-28-55(93)82-35-43-24-26-44(27-25-43)38-89-51-36-83-69(102)49(32-58(96)97)86-57(95)37-84-68(101)48(23-16-30-81-74(79)80)85-56(94)31-50(71(89)104)87-70(51)103/h7-15,17-22,24-27,48-54,61-64,66,92,107H,16,23,28-39H2,1-6H3,(H,82,93)(H,83,102)(H,84,101)(H,85,94)(H,86,95)(H,87,103)(H,88,100)(H,96,97)(H4,79,80,81)/t48-,49-,50-,51+,52-,53-,54+,61-,62+,63+,64-,66-,76+,77-,78+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM536747

(US11247985, Table 3.51)Show SMILES CC(C)N1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(ccc3[nH]2)C#N)CCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50468106

(CHEMBL4280801 | US11247985, Table 3.49)Show SMILES CN1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(F)ccc3[nH]2)CCC1 Show InChI InChI=1S/C29H38FN5/c1-33-14-16-35(17-15-33)29(10-3-11-29)21-31-25-8-12-34(13-9-25)26-5-2-4-22(19-26)28-20-23-18-24(30)6-7-27(23)32-28/h2,4-7,18-20,25,31-32H,3,8-17,21H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50318085

(1-Methyl-8-[(2-methylphenyl)amino]-4,5-dihydro-1H-...)Show InChI InChI=1S/C18H18N6O/c1-10-5-3-4-6-13(10)21-18-20-9-11-7-8-12-15(17(19)25)23-24(2)16(12)14(11)22-18/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
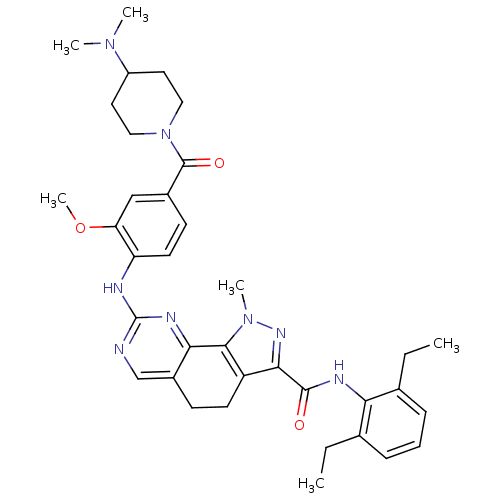
(Homo sapiens (Human)) | BDBM50349092
(CHEMBL1807303)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)N3CCC(CC3)N(C)C)nc-21 Show InChI InChI=1S/C36H44N8O3/c1-7-22-10-9-11-23(8-2)30(22)39-34(45)32-27-14-12-25-21-37-36(40-31(25)33(27)43(5)41-32)38-28-15-13-24(20-29(28)47-6)35(46)44-18-16-26(17-19-44)42(3)4/h9-11,13,15,20-21,26H,7-8,12,14,16-19H2,1-6H3,(H,39,45)(H,37,38,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31533

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 16)Show InChI InChI=1S/C18H18N6O/c1-19-17(25)15-13-9-8-11-10-20-18(21-12-6-4-3-5-7-12)22-14(11)16(13)24(2)23-15/h3-7,10H,8-9H2,1-2H3,(H,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50468106

(CHEMBL4280801 | US11247985, Table 3.49)Show SMILES CN1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(F)ccc3[nH]2)CCC1 Show InChI InChI=1S/C29H38FN5/c1-33-14-16-35(17-15-33)29(10-3-11-29)21-31-25-8-12-34(13-9-25)26-5-2-4-22(19-26)28-20-23-18-24(30)6-7-27(23)32-28/h2,4-7,18-20,25,31-32H,3,8-17,21H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2 (DE3) using 100 uM ATP as substrate after... |
ACS Med Chem Lett 9: 1075-1081 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00372
BindingDB Entry DOI: 10.7270/Q23F4SBJ |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349094

(CHEMBL1807305)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)N3CCCN(C)CC3)nc-21 Show InChI InChI=1S/C35H42N8O3/c1-6-22-10-8-11-23(7-2)29(22)38-33(44)31-26-14-12-25-21-36-35(39-30(25)32(26)42(4)40-31)37-27-15-13-24(20-28(27)46-5)34(45)43-17-9-16-41(3)18-19-43/h8,10-11,13,15,20-21H,6-7,9,12,14,16-19H2,1-5H3,(H,38,44)(H,36,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50399918

(CHEMBL2180982)Show SMILES CC(=O)O[C@@H]1C2=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c3ccccc3)[C@@H]3[C@@]4(CO[C@@H]4C[C@H](O)[C@@]3(C)C1=O)OC(C)=O)C2(C)C)OC(=O)[C@H](OC(=O)CCC(=O)NCc1ccc(CN2[C@@H]3CC(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)NC[C@H](NC3=O)C2=O)cc1)[C@@H](NC(=O)c1ccccc1)c1ccccc1 |r,wU:65.70,80.85,22.23,26.26,4.3,10.10,30.33,28.30,89.94,wD:97.105,12.12,8.45,23.37,44.49,60.64,c:5,(43.79,-26.11,;43.75,-24.57,;42.4,-23.84,;45.06,-23.78,;46.41,-24.49,;46.47,-27.53,;46.5,-29.07,;45.17,-29.85,;47.85,-29.83,;49.16,-29.04,;49.14,-27.51,;50.49,-28.25,;50.46,-26.71,;51.81,-27.46,;51.83,-29.01,;50.51,-29.78,;53.17,-29.74,;54.48,-28.95,;55.83,-29.69,;55.85,-31.24,;54.53,-32.04,;53.2,-31.28,;50.43,-25.18,;51.74,-24.38,;53.29,-24.36,;53.26,-22.82,;51.72,-22.83,;50.37,-22.09,;49.04,-22.9,;48.99,-21.35,;49.08,-24.44,;49.5,-25.89,;47.73,-23.7,;47.69,-22.16,;52.16,-25.86,;53.51,-26.6,;54.83,-25.81,;53.53,-28.16,;47.8,-26.75,;46.98,-25.44,;48.54,-25.4,;47.86,-31.36,;46.54,-32.15,;45.21,-31.39,;46.56,-33.68,;47.9,-34.44,;49.23,-33.66,;49.22,-32.12,;50.57,-34.42,;51.9,-33.64,;53.23,-34.4,;53.24,-35.94,;54.56,-33.62,;55.9,-34.39,;57.23,-33.61,;58.57,-34.38,;59.89,-33.6,;59.88,-32.06,;61.21,-31.28,;62.55,-32.04,;63.87,-31.25,;63.86,-29.71,;65.18,-28.92,;65.16,-27.38,;66.53,-29.67,;67.85,-28.88,;67.82,-27.34,;66.48,-26.59,;66.46,-25.05,;65.11,-24.3,;65.09,-22.76,;63.74,-22.01,;66.41,-21.97,;69.19,-29.63,;70.51,-28.84,;69.22,-31.17,;70.56,-31.92,;70.58,-33.46,;71.93,-34.21,;69.26,-34.25,;69.28,-35.79,;70.63,-36.54,;70.65,-38.08,;69.33,-38.87,;72,-38.83,;67.96,-36.58,;67.98,-38.12,;65.3,-36.62,;63.89,-35.88,;63.89,-34.34,;65.22,-33.56,;65.21,-32.02,;66.54,-31.25,;62.56,-33.58,;61.22,-34.35,;58.54,-31.3,;57.21,-32.08,;45.23,-34.46,;43.89,-33.73,;42.57,-34.49,;42.6,-36.05,;41.24,-33.76,;41.22,-32.21,;39.87,-31.47,;38.55,-32.26,;38.58,-33.8,;39.92,-34.56,;45.26,-36,;46.61,-36.74,;46.63,-38.29,;45.3,-39.08,;43.96,-38.32,;43.94,-36.79,)| Show InChI InChI=1S/C78H91N11O24/c1-40-52(34-78(107)66(112-72(105)47-21-14-9-15-22-47)64-76(6,53(92)33-54-77(64,39-108-54)113-42(3)91)65(99)62(109-41(2)90)60(40)75(78,4)5)110-73(106)63(61(45-17-10-7-11-18-45)88-67(100)46-19-12-8-13-20-46)111-59(98)29-28-55(93)82-35-43-24-26-44(27-25-43)38-89-51-32-56(94)85-48(23-16-30-81-74(79)80)68(101)84-37-57(95)86-49(31-58(96)97)69(102)83-36-50(71(89)104)87-70(51)103/h7-15,17-22,24-27,48-54,61-64,66,92,107H,16,23,28-39H2,1-6H3,(H,82,93)(H,83,102)(H,84,101)(H,85,94)(H,86,95)(H,87,103)(H,88,100)(H,96,97)(H4,79,80,81)/t48-,49-,50-,51+,52-,53-,54+,61-,62+,63+,64-,66-,76+,77-,78+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta5 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM536749
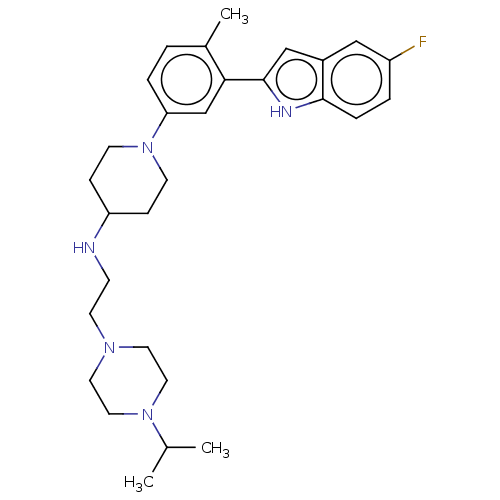
(US11247985, Table 3.53)Show SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2ccc(C)c(c2)-c2cc3cc(F)ccc3[nH]2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM536753

(US11247985, Table 3.57)Show SMILES CN1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(ccc3[nH]2)C#N)CCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
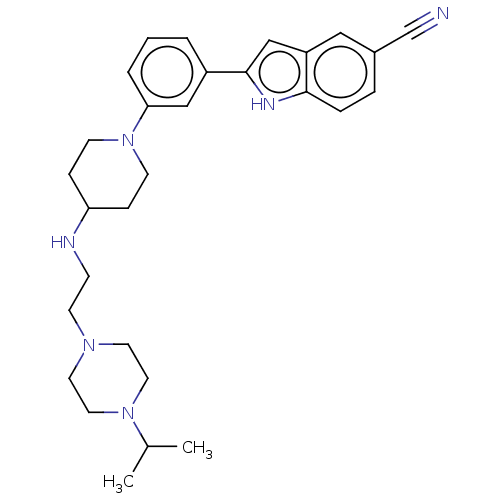
(Homo sapiens (Human)) | BDBM50514013
(CHEMBL4445673 | US11247985, Table 3.55)Show SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2cccc(c2)-c2cc3cc(ccc3[nH]2)C#N)CC1 Show InChI InChI=1S/C29H38N6/c1-22(2)34-16-14-33(15-17-34)13-10-31-26-8-11-35(12-9-26)27-5-3-4-24(19-27)29-20-25-18-23(21-30)6-7-28(25)32-29/h3-7,18-20,22,26,31-32H,8-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
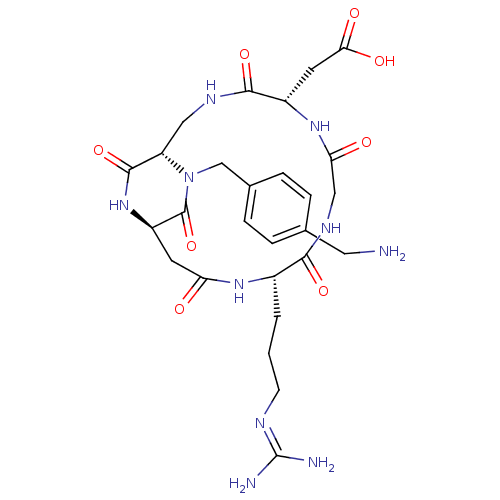
(Homo sapiens (Human)) | BDBM50399917
(CHEMBL2180981)Show SMILES [#7]-[#6]-c1ccc(-[#6]-[#7]-2-[#6@H]-3-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6@@H](-[#7]-[#6]-3=O)-[#6]-2=O)cc1 |r| Show InChI InChI=1S/C27H38N10O8/c28-10-14-3-5-15(6-4-14)13-37-19-11-32-24(43)17(9-22(40)41)35-21(39)12-33-23(42)16(2-1-7-31-27(29)30)34-20(38)8-18(26(37)45)36-25(19)44/h3-6,16-19H,1-2,7-13,28H2,(H,32,43)(H,33,42)(H,34,38)(H,35,39)(H,36,44)(H,40,41)(H4,29,30,31)/t16-,17-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis |
J Med Chem 55: 10460-74 (2012)
Article DOI: 10.1021/jm301058f
BindingDB Entry DOI: 10.7270/Q2WD41Q5 |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
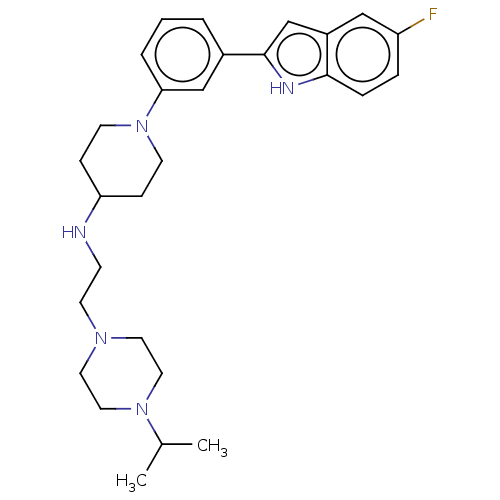
(Homo sapiens (Human)) | BDBM50468108
(CHEMBL4280125 | US11247985, Table 3.59)Show SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2cccc(c2)-c2cc3cc(F)ccc3[nH]2)CC1 Show InChI InChI=1S/C28H38FN5/c1-21(2)33-16-14-32(15-17-33)13-10-30-25-8-11-34(12-9-25)26-5-3-4-22(19-26)28-20-23-18-24(29)6-7-27(23)31-28/h3-7,18-21,25,30-31H,8-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase TTK
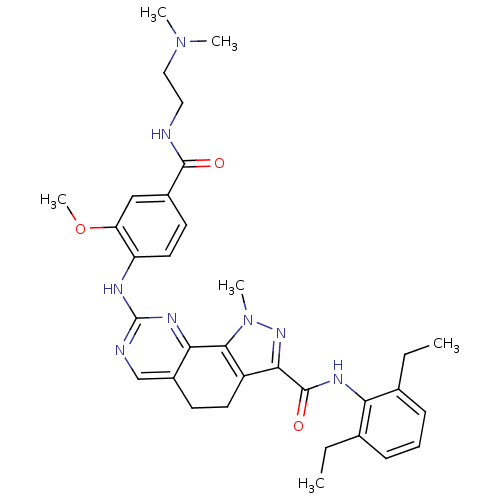
(Homo sapiens (Human)) | BDBM50349103
(CHEMBL1808341)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)NCCN(C)C)nc-21 Show InChI InChI=1S/C33H40N8O3/c1-7-20-10-9-11-21(8-2)27(20)37-32(43)29-24-14-12-23-19-35-33(38-28(23)30(24)41(5)39-29)36-25-15-13-22(18-26(25)44-6)31(42)34-16-17-40(3)4/h9-11,13,15,18-19H,7-8,12,14,16-17H2,1-6H3,(H,34,42)(H,37,43)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50158905
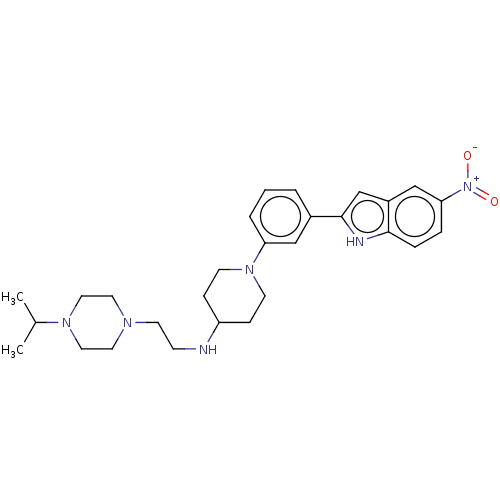
(CHEMBL3787674 | US11247985, Table 3.61)Show SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2cccc(c2)-c2cc3cc(ccc3[nH]2)[N+]([O-])=O)CC1 Show InChI InChI=1S/C28H38N6O2/c1-21(2)32-16-14-31(15-17-32)13-10-29-24-8-11-33(12-9-24)25-5-3-4-22(18-25)28-20-23-19-26(34(35)36)6-7-27(23)30-28/h3-7,18-21,24,29-30H,8-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349099
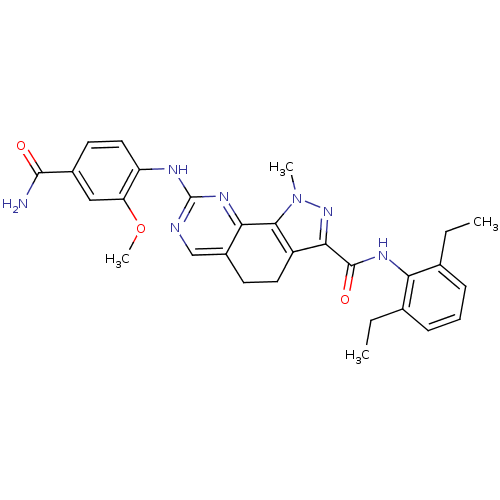
(CHEMBL1808338)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(N)=O)nc-21 Show InChI InChI=1S/C29H31N7O3/c1-5-16-8-7-9-17(6-2)23(16)33-28(38)25-20-12-10-19-15-31-29(34-24(19)26(20)36(3)35-25)32-21-13-11-18(27(30)37)14-22(21)39-4/h7-9,11,13-15H,5-6,10,12H2,1-4H3,(H2,30,37)(H,33,38)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50468112
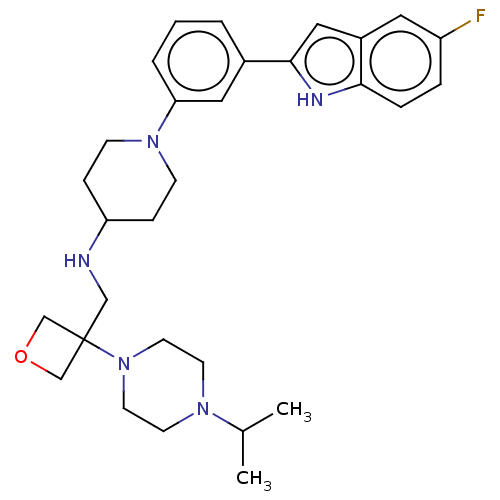
(CHEMBL4277404 | US11247985, Table 3.63)Show SMILES CC(C)N1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(F)ccc3[nH]2)COC1 Show InChI InChI=1S/C30H40FN5O/c1-22(2)34-12-14-36(15-13-34)30(20-37-21-30)19-32-26-8-10-35(11-9-26)27-5-3-4-23(17-27)29-18-24-16-25(31)6-7-28(24)33-29/h3-7,16-18,22,26,32-33H,8-15,19-21H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data