

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Homo sapiens (Human)) | BDBM155343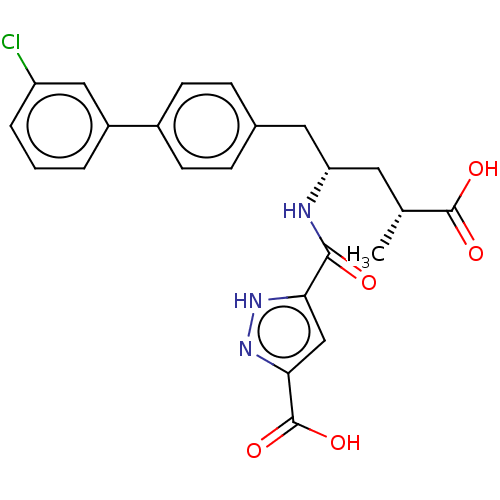 (US9006249, Example 49-2 | US9603819, Example 49-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155343 (US9006249, Example 49-2 | US9603819, Example 49-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM309469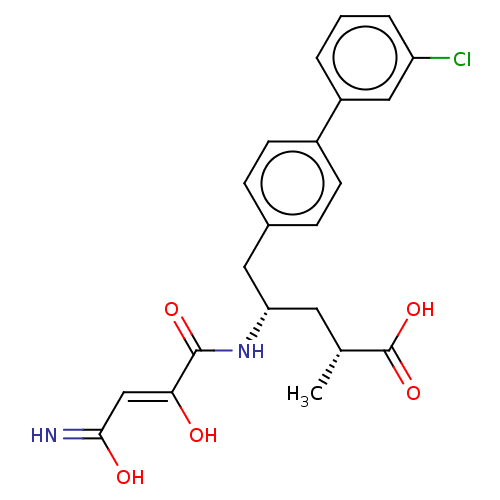 ((2R,4S)-5-(3′-Chloro-biphenyl-4-yl)-4-[(3-hy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153121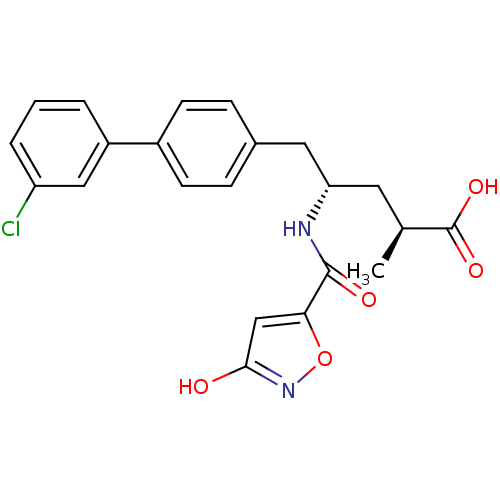 (US8993631, 29-2 | US9006249, Example 49-1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155344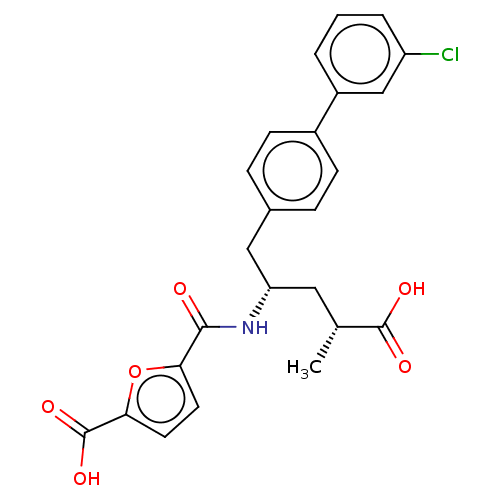 (US9006249, Example 49-3 | US9603819, Example 49-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153128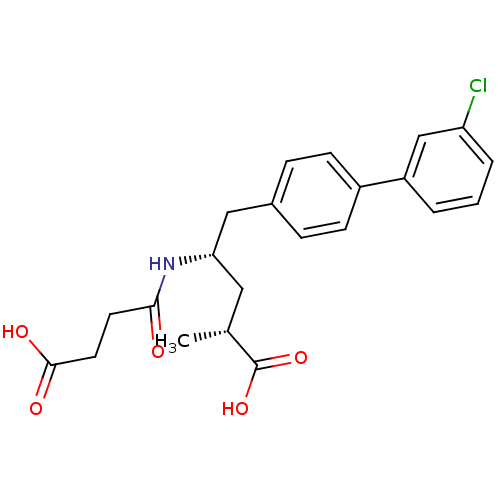 (US8993631, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155344 (US9006249, Example 49-3 | US9603819, Example 49-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130359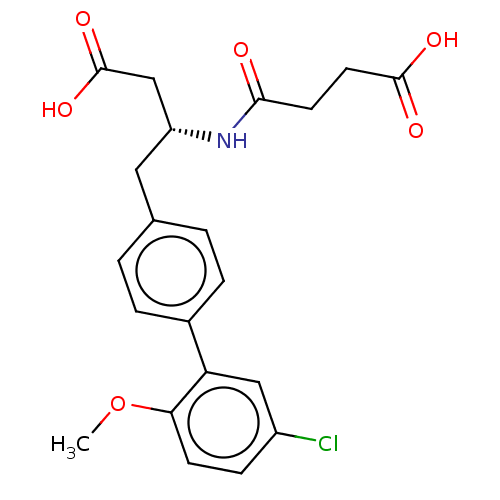 (US8822534, Example 5-39 | US8993631, 5-8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509659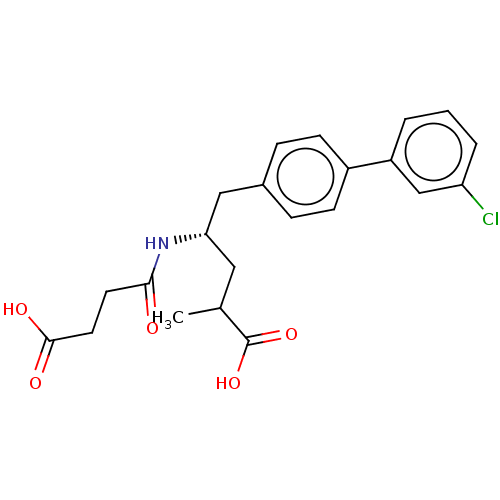 (CHEMBL4443138) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153108 (US8993631, 16-5 | US9006249, Example 3-32 | US9603...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153108 (US8993631, 16-5 | US9006249, Example 3-32 | US9603...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130365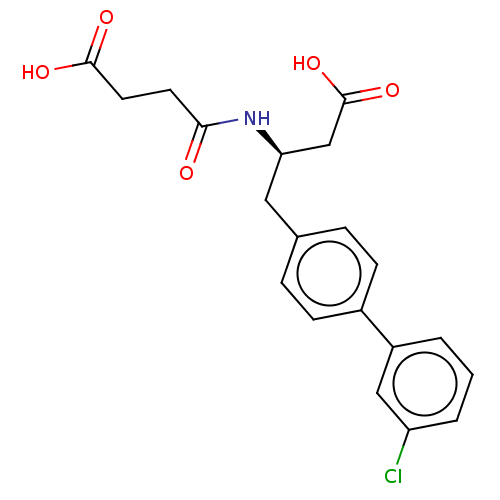 (US8822534, Example 11-1 | US8822534, Example 12-1 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509652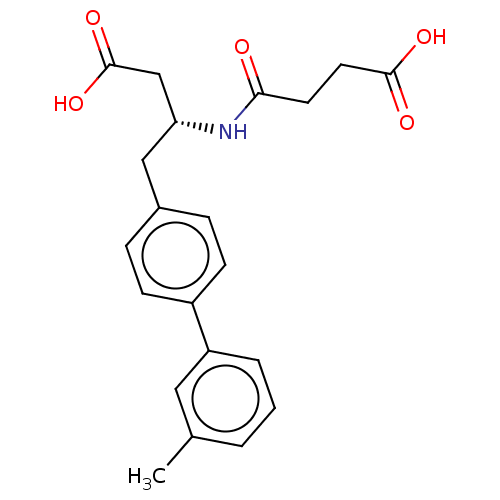 (CHEMBL4557208) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509666 (CHEMBL4442804) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472804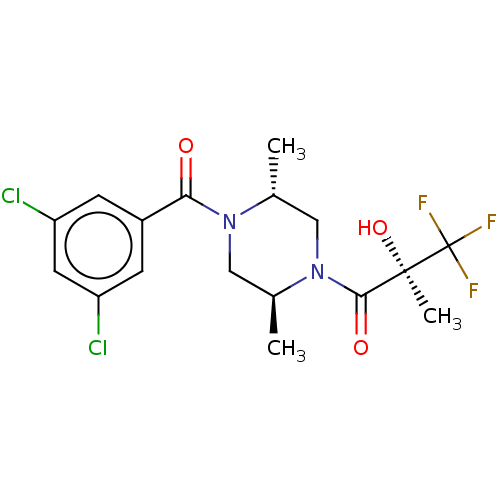 (CHEMBL83273) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50291237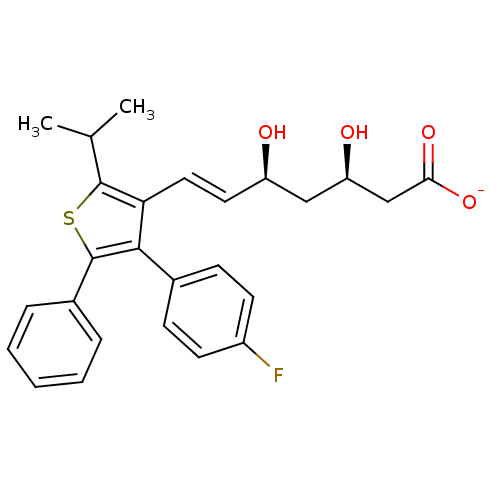 (CHEMBL153492 | Sodium; (E)-(3R,5S)-7-[4-(4-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the HMG-CoA reductase inhibitory activity in a microsomal preparation | Bioorg Med Chem Lett 7: 549-554 (1997) Article DOI: 10.1016/S0960-894X(97)00065-6 BindingDB Entry DOI: 10.7270/Q2RB74NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472836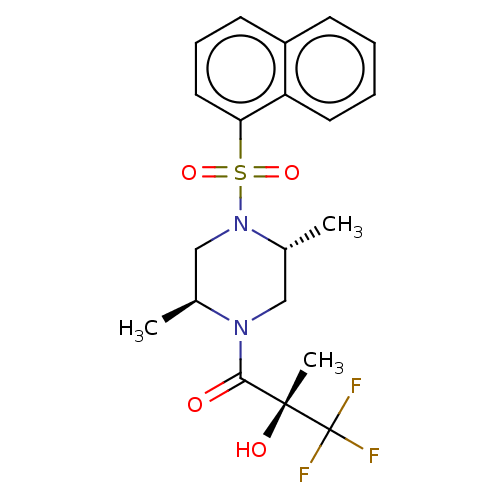 (CHEMBL311125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130355 (US8822534, Example 5-7 | US8993631, 5-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509647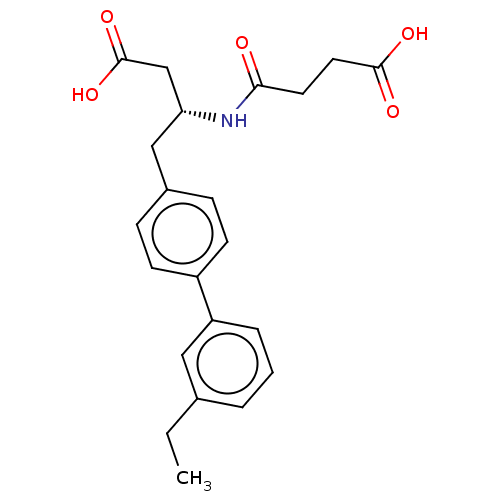 (CHEMBL4518426) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50034842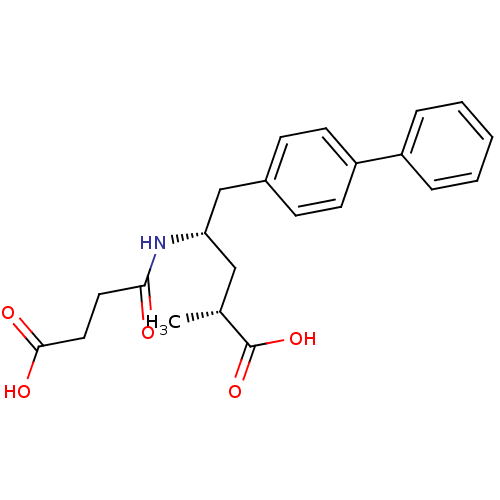 ((2R,4S)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50291241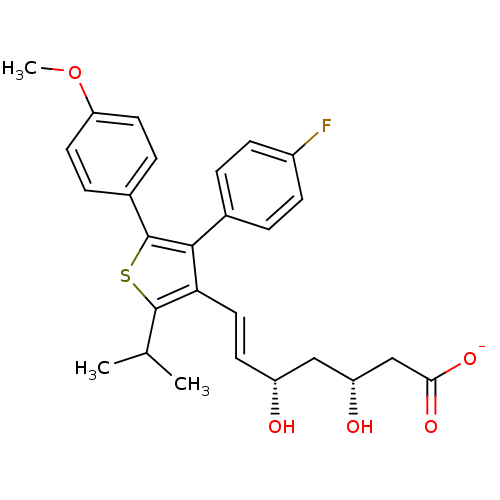 (CHEMBL153021 | Sodium; (E)-(3R,5S)-7-[4-(4-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the HMG-CoA reductase inhibitory activity in a microsomal preparation | Bioorg Med Chem Lett 7: 549-554 (1997) Article DOI: 10.1016/S0960-894X(97)00065-6 BindingDB Entry DOI: 10.7270/Q2RB74NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130354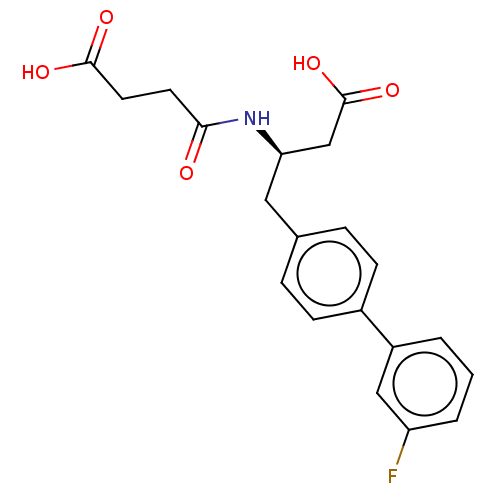 (US8822534, Example 5-4 | US8993631, 5-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509655 (CHEMBL4466321) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153109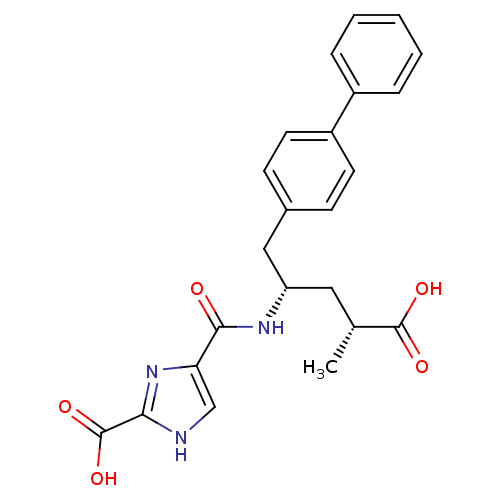 (US8993631, 16-8 | US9006249, Example 3-60) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM309463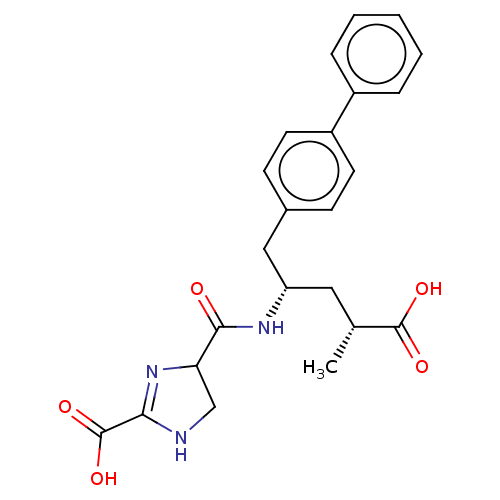 (US9603819, Example 3-60) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509649 (CHEMBL4474653) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472839 (CHEMBL3349325) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50291258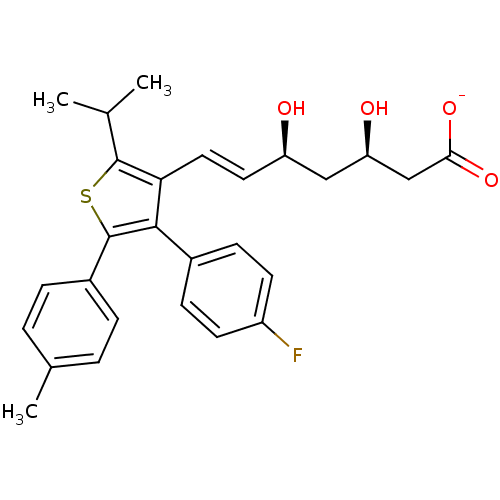 (CHEMBL152366 | Sodium; (E)-(3R,5S)-7-[4-(4-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the HMG-CoA reductase inhibitory activity in a microsomal preparation | Bioorg Med Chem Lett 7: 549-554 (1997) Article DOI: 10.1016/S0960-894X(97)00065-6 BindingDB Entry DOI: 10.7270/Q2RB74NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239481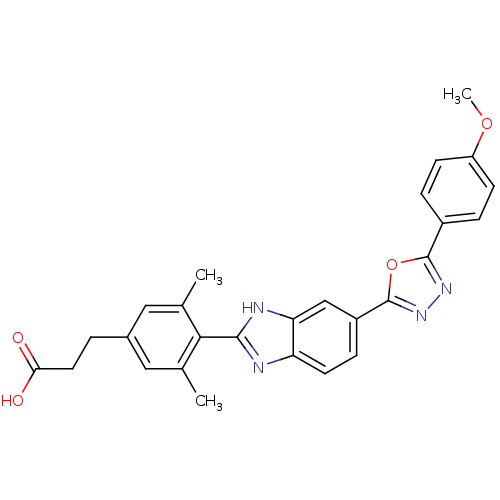 (CHEMBL4061308) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472841 (CHEMBL443012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239482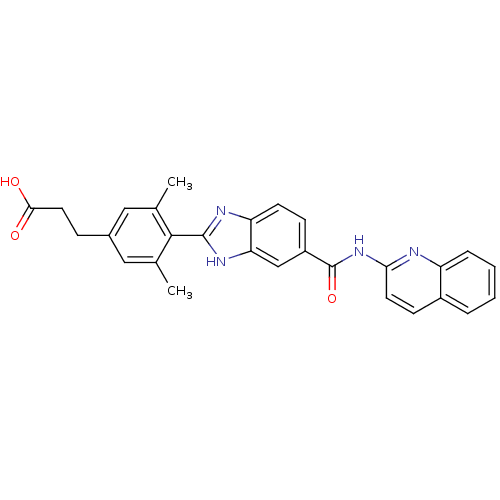 (CHEMBL1683001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472793 (CHEMBL313878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155342 (US9006249, Example 13-1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM309468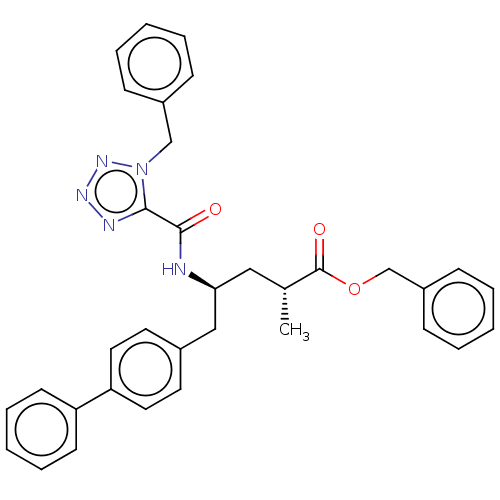 ((2R,4S)-5-biphenyl-4-yl-2-methyl-4-[(1H-tetrazole-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50291255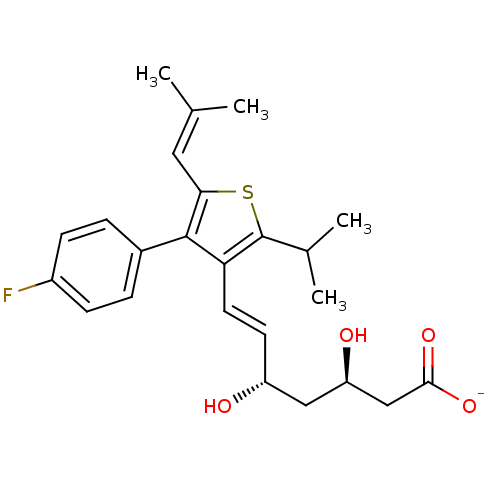 (CHEMBL358623 | Sodium; (E)-(3R,5S)-7-[4-(4-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the HMG-CoA reductase inhibitory activity in a microsomal preparation | Bioorg Med Chem Lett 7: 549-554 (1997) Article DOI: 10.1016/S0960-894X(97)00065-6 BindingDB Entry DOI: 10.7270/Q2RB74NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50236533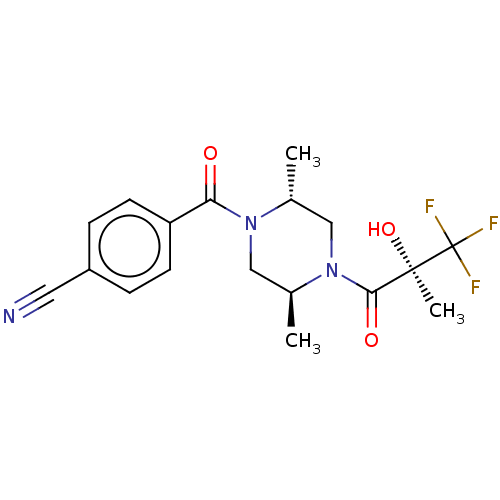 (CHEMBL316388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1,/2,/3,/4, mitochondrial (Homo sapiens (Human)) | BDBM50236533 (CHEMBL316388) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Pyruvate dehydrogenase kinase by primary enzymatic assay | J Med Chem 42: 2741-6 (1999) Article DOI: 10.1021/jm9902584 BindingDB Entry DOI: 10.7270/Q2G73HG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239480 (CHEMBL4074410) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472825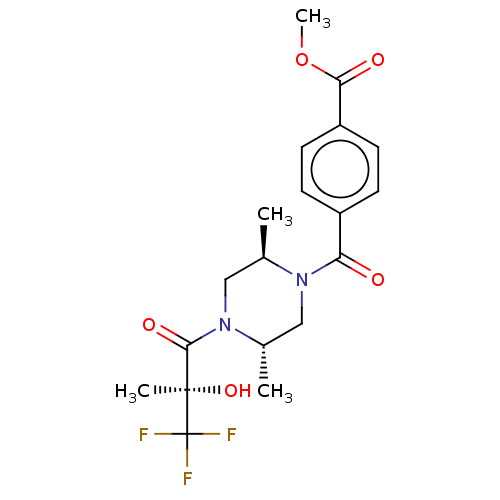 (CHEMBL81574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509648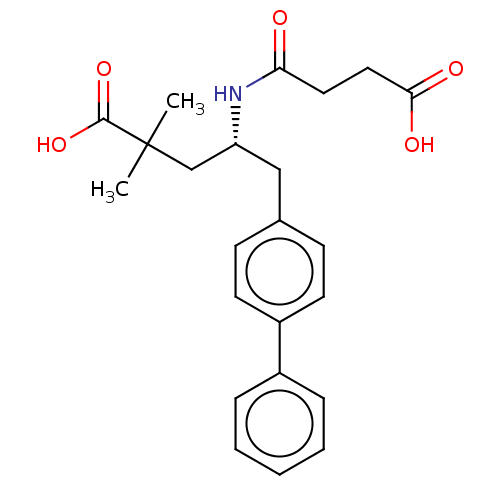 (CHEMBL4439270) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472811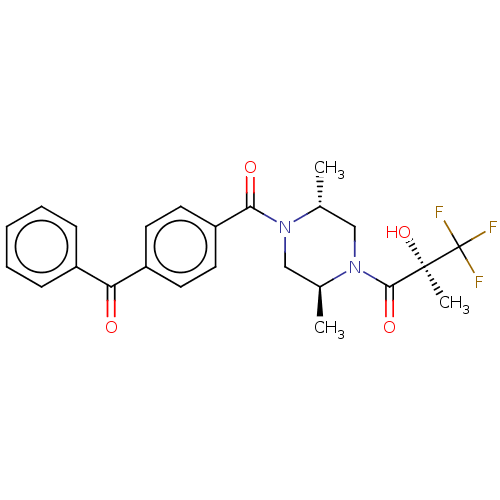 (CHEMBL310879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509650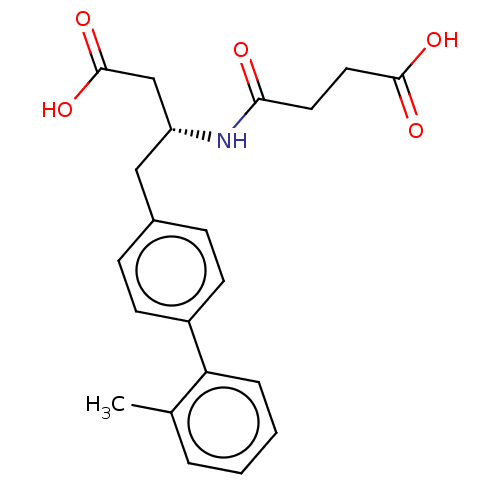 (CHEMBL4592467) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1,/2,/3,/4, mitochondrial (Homo sapiens (Human)) | BDBM50472310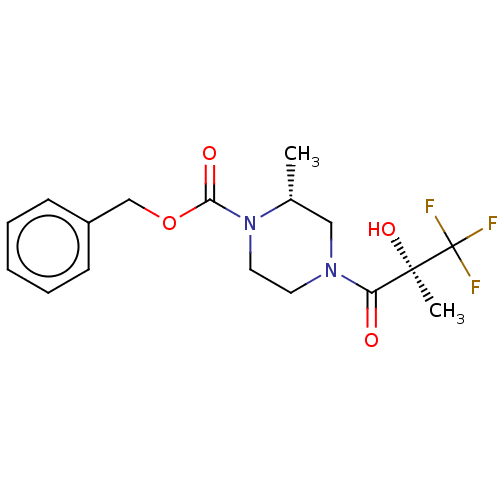 (CHEMBL84231) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Pyruvate dehydrogenase kinase by primary enzymatic assay | J Med Chem 42: 2741-6 (1999) Article DOI: 10.1021/jm9902584 BindingDB Entry DOI: 10.7270/Q2G73HG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50291256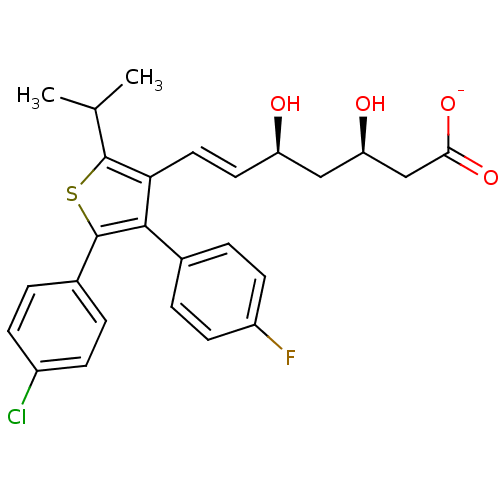 (CHEMBL152129 | Sodium; (E)-(3R,5S)-7-[5-(4-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the HMG-CoA reductase inhibitory activity in a microsomal preparation | Bioorg Med Chem Lett 7: 549-554 (1997) Article DOI: 10.1016/S0960-894X(97)00065-6 BindingDB Entry DOI: 10.7270/Q2RB74NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472310 (CHEMBL84231) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239484 (CHEMBL4103025) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509660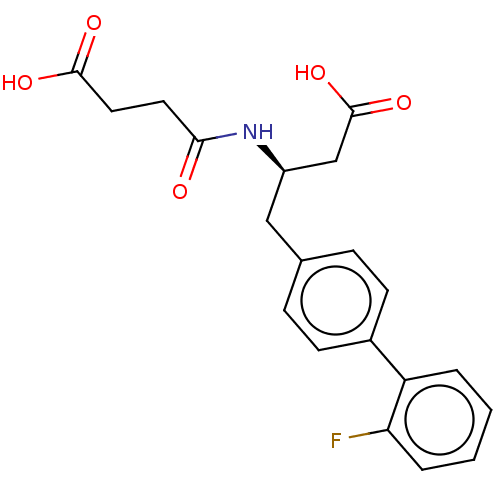 (CHEMBL4434901) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial (Rattus norvegicus) | BDBM50472831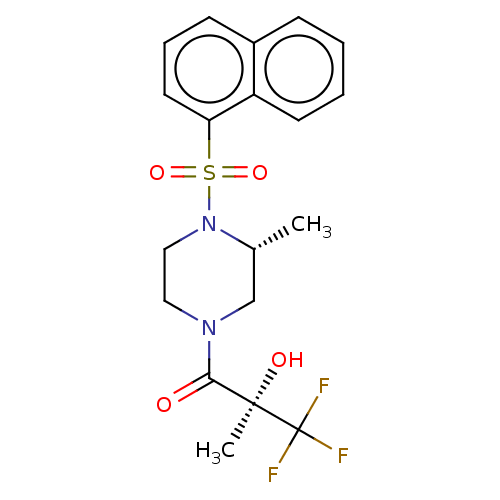 (CHEMBL84271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. | J Med Chem 43: 236-49 (2000) Article DOI: 10.1021/jm990358+ BindingDB Entry DOI: 10.7270/Q2B85BVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM7494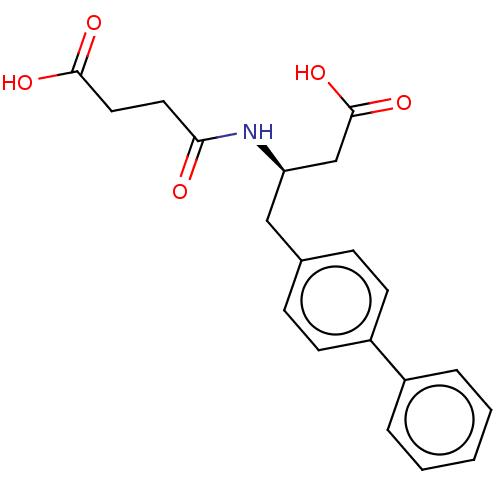 (US8822534, 5-36 | US8822534, Example 5-1 | US89936...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50291252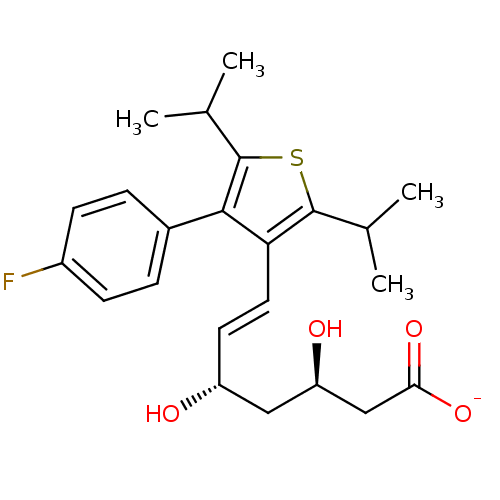 (CHEMBL152551 | Sodium; (E)-(3R,5S)-7-[4-(4-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the HMG-CoA reductase inhibitory activity in a microsomal preparation | Bioorg Med Chem Lett 7: 549-554 (1997) Article DOI: 10.1016/S0960-894X(97)00065-6 BindingDB Entry DOI: 10.7270/Q2RB74NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 242 total ) | Next | Last >> |