

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280830 (3-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283917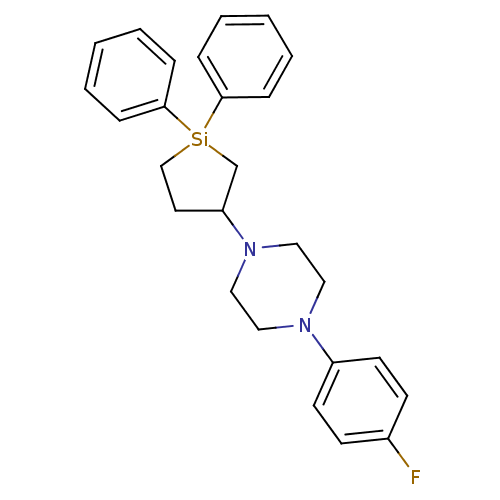 (1-(1,1-Diphenyl-silolan-3-yl)-4-(4-fluoro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283931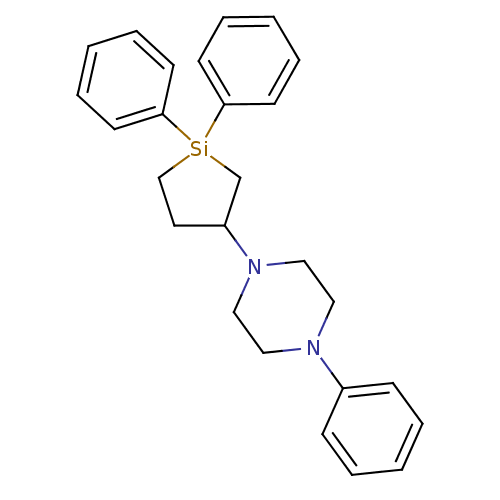 (1-(1,1-Diphenyl-silolan-3-yl)-4-phenyl-piperazine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280826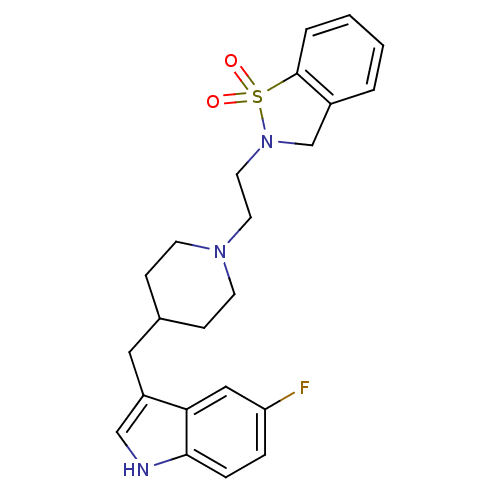 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280822 (4-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50047098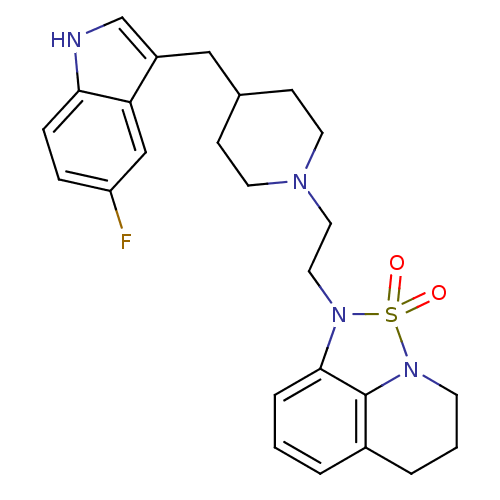 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280819 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280831 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283920 (1-[1,1-Bis-(4-fluoro-phenyl)-silolan-3-yl]-4-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280817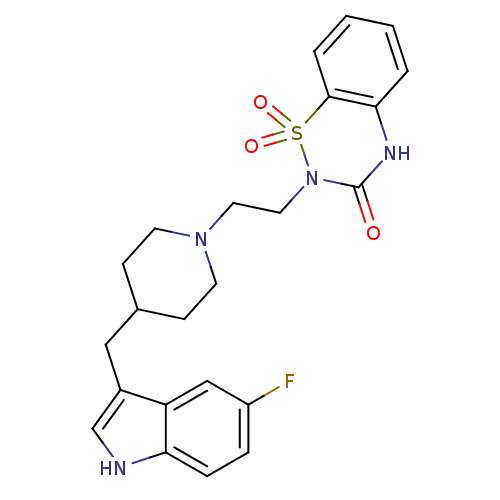 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280828 (3-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283920 (1-[1,1-Bis-(4-fluoro-phenyl)-silolan-3-yl]-4-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280824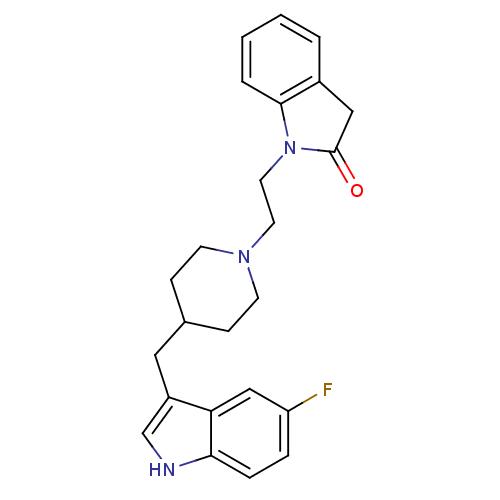 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283928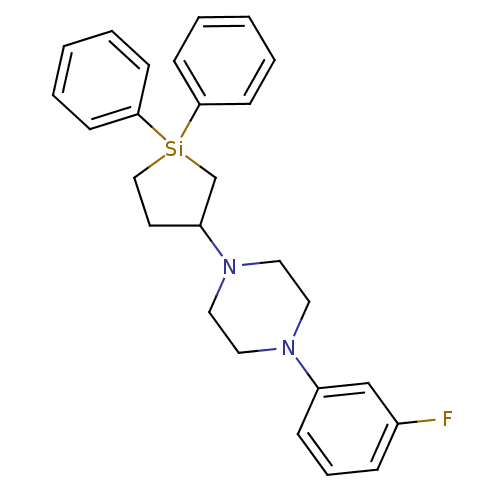 (1-(1,1-Diphenyl-silolan-3-yl)-4-(3-fluoro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280823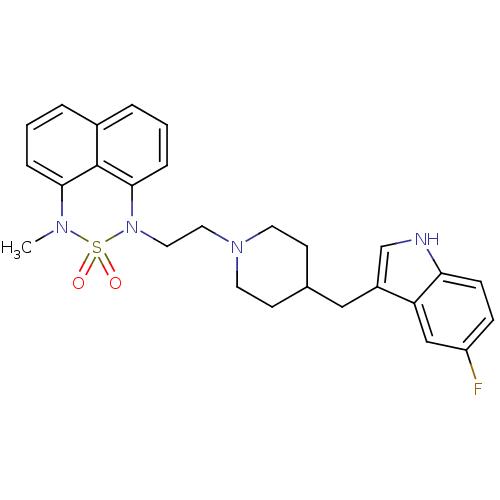 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280832 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280827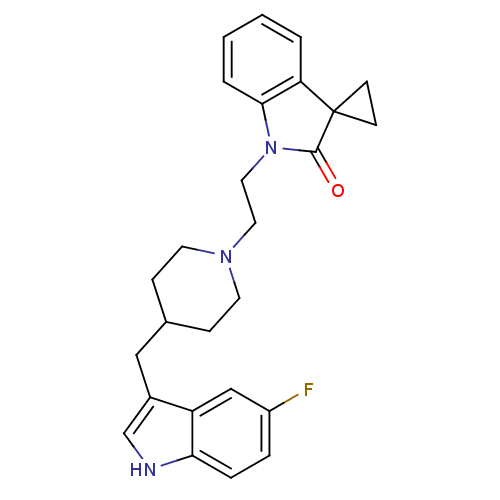 (1'-{2-[4-(5-fluoro-1H-3-indolylmethyl)hexahydro-1-...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280829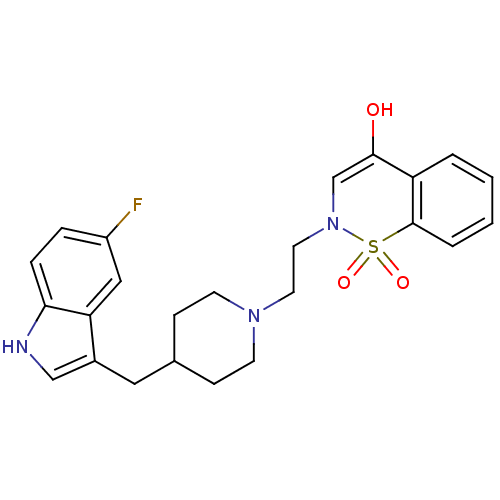 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280825 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280815 (CHEMBL56377 | N-{2-[4-(5-Fluoro-1H-indol-3-ylmethy...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283929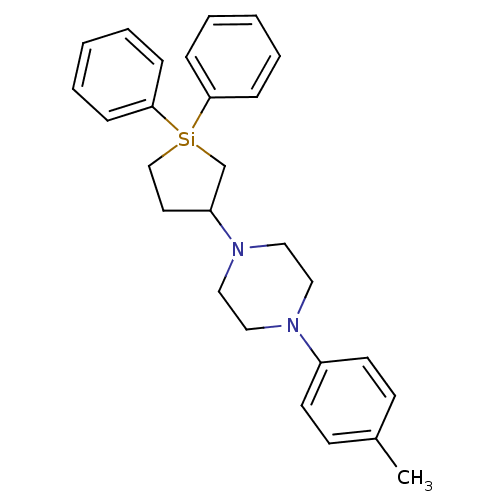 (1-(1,1-Diphenyl-silolan-3-yl)-4-p-tolyl-piperazine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280818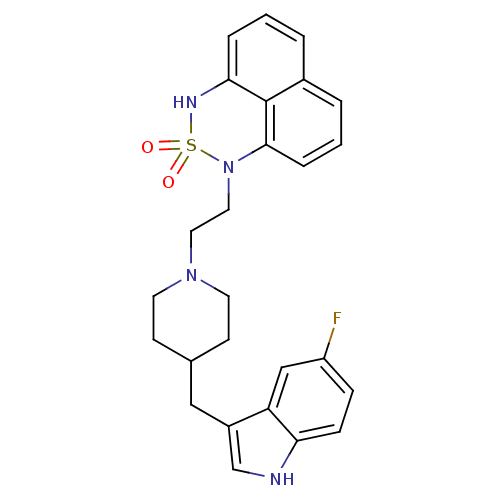 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280821 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283921 (1-(1,1-Diphenyl-silolan-3-yl)-4-(4-fluoro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283919 (1-[1,1-Bis-(4-fluoro-phenyl)-silolan-3-yl]-4-(4-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280820 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283924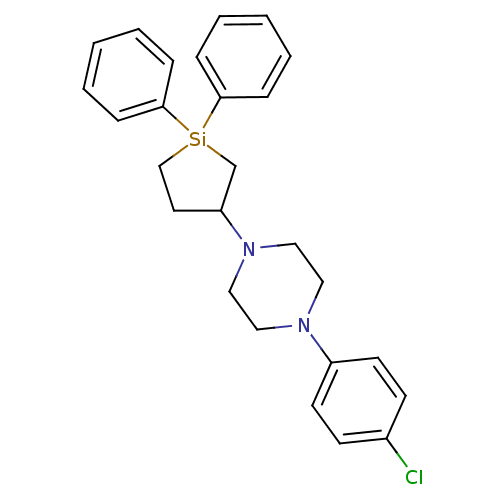 (1-(4-Chloro-phenyl)-4-(1,1-diphenyl-silolan-3-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283918 (1-[1-(4-Fluoro-phenyl)-1-phenyl-silolan-3-yl]-4-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283918 (1-[1-(4-Fluoro-phenyl)-1-phenyl-silolan-3-yl]-4-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283923 (1-(1,1-Diphenyl-silolan-3-yl)-4-pyridin-2-yl-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280816 (CHEMBL293729 | N-{2-[4-(5-Fluoro-1H-indol-3-ylmeth...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283926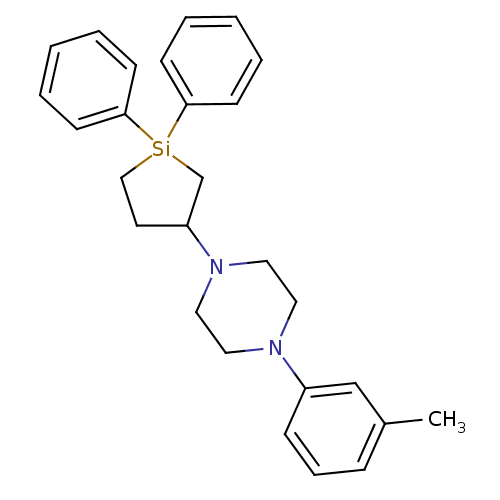 (1-(1,1-Diphenyl-silolan-3-yl)-4-m-tolyl-piperazine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283925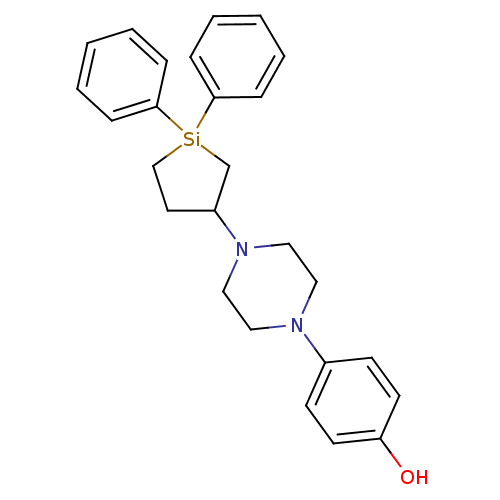 (4-[4-(1,1-Diphenyl-silolan-3-yl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283927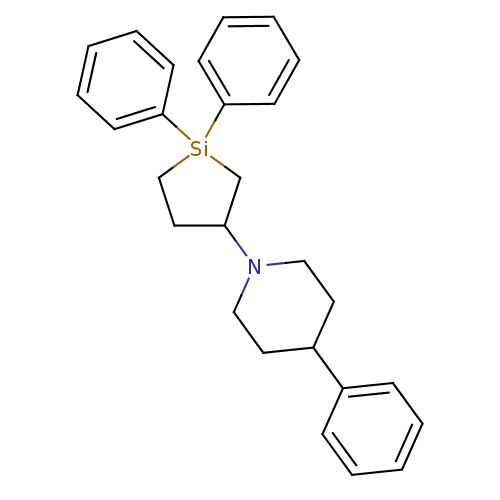 (1-(1,1-Diphenyl-silolan-3-yl)-4-phenyl-piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283930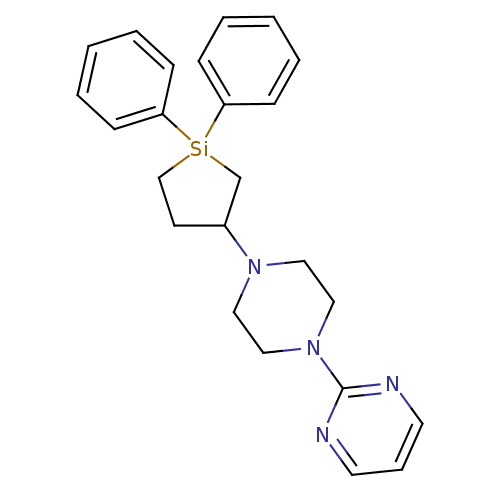 (2-[4-(1,1-Diphenyl-silolan-3-yl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280818 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280826 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280827 (1'-{2-[4-(5-fluoro-1H-3-indolylmethyl)hexahydro-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280824 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280820 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280828 (3-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280822 (4-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280832 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280823 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280821 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280819 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280816 (CHEMBL293729 | N-{2-[4-(5-Fluoro-1H-indol-3-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280831 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 98 total ) | Next | Last >> |