 Found 209 hits with Last Name = 'dimarco' and Initial = 'jd'
Found 209 hits with Last Name = 'dimarco' and Initial = 'jd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM50122646

(CHEMBL3623125)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1ccccc1F)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H19F4N3O5S/c1-22-10-17(30-37(34,35)16-6-4-3-5-15(16)25)23(2,36-22)19-18(22)20(32)31(21(19)33)13-8-7-12(11-29)14(9-13)24(26,27)28/h3-9,17-19,30H,10H2,1-2H3/t17-,18-,19+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122650
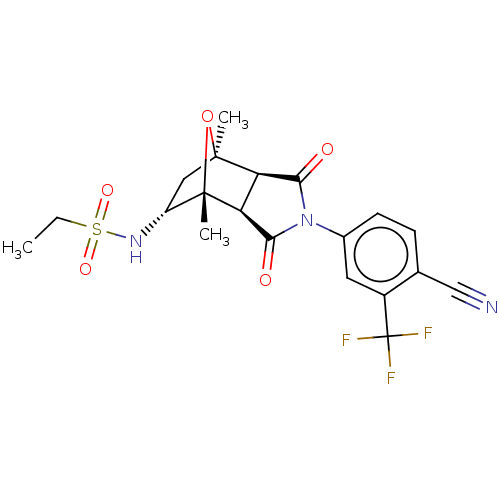
(CHEMBL3623127)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)CC)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C20H20F3N3O5S/c1-4-32(29,30)25-13-8-18(2)14-15(19(13,3)31-18)17(28)26(16(14)27)11-6-5-10(9-24)12(7-11)20(21,22)23/h5-7,13-15,25H,4,8H2,1-3H3/t13-,14-,15+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122647
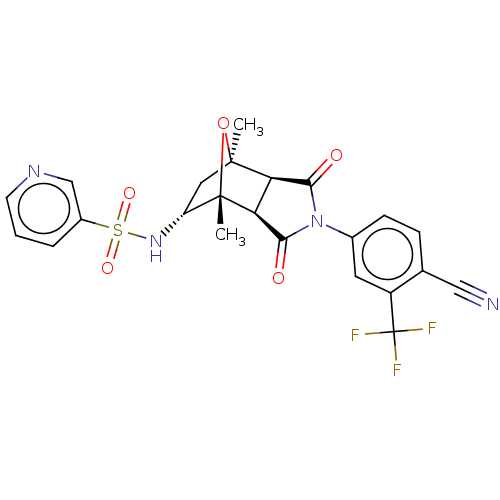
(CHEMBL3623126)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1cccnc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C23H19F3N4O5S/c1-21-9-16(29-36(33,34)14-4-3-7-28-11-14)22(2,35-21)18-17(21)19(31)30(20(18)32)13-6-5-12(10-27)15(8-13)23(24,25)26/h3-8,11,16-18,29H,9H2,1-2H3/t16-,17-,18+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122640

(CHEMBL3623119)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)CC)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C21H20F3N3O4/c1-4-14(28)26-13-8-19(2)15-16(20(13,3)31-19)18(30)27(17(15)29)11-6-5-10(9-25)12(7-11)21(22,23)24/h5-7,13,15-16H,4,8H2,1-3H3,(H,26,28)/t13-,15-,16+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239606

(CHEMBL4080667)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)[C@H]3O)c1ccccc1 |r,wD:17.21,TLB:16:15:8.9.10:12,7:8:14.16.15:10.11.12,7:8:12:14.15.17,THB:16:9:12:14.15.17,18:17:8.9.10:12,17:15:8:10.11.12,17:11:8:14.16.15,19:8:14.16.15:10.11.12,19:8:12:14.15.17,(43.24,-21.55,;43.72,-20.08,;43.03,-18.71,;44.4,-18.01,;45.1,-19.38,;44.88,-16.54,;43.63,-15.64,;46.35,-16.07,;47.49,-17.11,;48.99,-16.68,;48.98,-15.1,;50.02,-13.87,;48.67,-14.34,;48.68,-15.83,;50,-16.32,;51.4,-15.97,;50.4,-17.25,;51.42,-14.44,;52.71,-13.59,;47.48,-18.64,;46.13,-19.39,;46.12,-20.93,;47.44,-21.71,;48.79,-20.95,;48.79,-19.41,)| Show InChI InChI=1S/C21H27NO3/c23-18-11-22(12-18)19(24)10-21(15-4-2-1-3-5-15)16-6-13-7-17(21)9-14(8-16)20(13)25/h1-5,13-14,16-18,20,23,25H,6-12H2/t13?,14?,16?,17?,20-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122643

(CHEMBL3623122)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)ON1CCN(C)CC1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H26F3N5O5/c1-22-11-16(29-21(35)36-31-8-6-30(3)7-9-31)23(2,37-22)18-17(22)19(33)32(20(18)34)14-5-4-13(12-28)15(10-14)24(25,26)27/h4-5,10,16-18H,6-9,11H2,1-3H3,(H,29,35)/t16-,17-,18+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122635
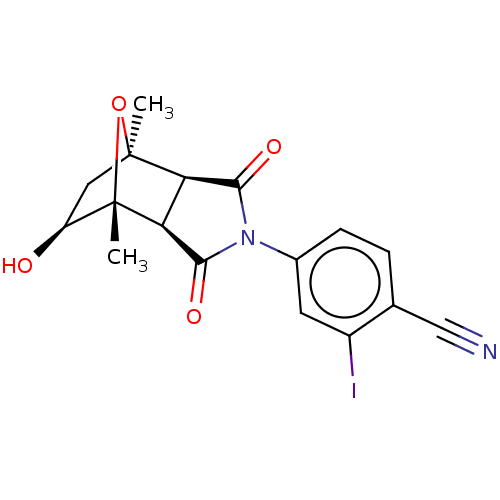
(CHEMBL3623114)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@@H]1O)c1ccc(C#N)c(I)c1 |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C17H15IN2O4/c1-16-6-11(21)17(2,24-16)13-12(16)14(22)20(15(13)23)9-4-3-8(7-19)10(18)5-9/h3-5,11-13,21H,6H2,1-2H3/t11-,12+,13-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122645
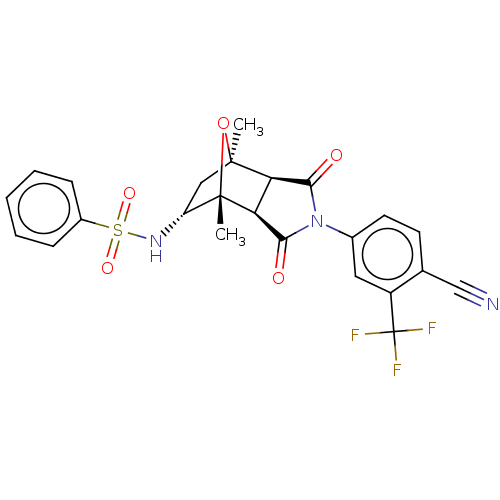
(CHEMBL3623124)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1ccccc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H20F3N3O5S/c1-22-11-17(29-36(33,34)15-6-4-3-5-7-15)23(2,35-22)19-18(22)20(31)30(21(19)32)14-9-8-13(12-28)16(10-14)24(25,26)27/h3-10,17-19,29H,11H2,1-2H3/t17-,18-,19+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122644
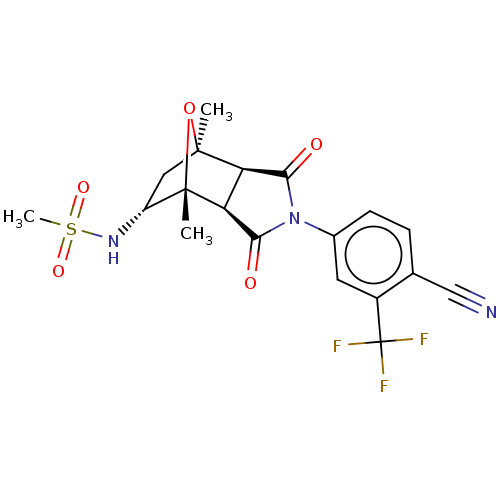
(CHEMBL3623123)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(C)(=O)=O)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C19H18F3N3O5S/c1-17-7-12(24-31(3,28)29)18(2,30-17)14-13(17)15(26)25(16(14)27)10-5-4-9(8-23)11(6-10)19(20,21)22/h4-6,12-14,24H,7H2,1-3H3/t12-,13-,14+,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122637

(CHEMBL3623116)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1C(=O)OC(C)C)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C22H21F3N2O5/c1-10(2)31-19(30)14-8-20(3)15-16(21(14,4)32-20)18(29)27(17(15)28)12-6-5-11(9-26)13(7-12)22(23,24)25/h5-7,10,14-16H,8H2,1-4H3/t14-,15+,16-,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122639

(CHEMBL3623118)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1N)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C18H16F3N3O3/c1-16-6-11(23)17(2,27-16)13-12(16)14(25)24(15(13)26)9-4-3-8(7-22)10(5-9)18(19,20)21/h3-5,11-13H,6,23H2,1-2H3/t11-,12-,13+,16+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122642

(CHEMBL3623121)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)NC(C)C)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C22H23F3N4O4/c1-10(2)27-19(32)28-14-8-20(3)15-16(21(14,4)33-20)18(31)29(17(15)30)12-6-5-11(9-26)13(7-12)22(23,24)25/h5-7,10,14-16H,8H2,1-4H3,(H2,27,28,32)/t14-,15-,16+,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122641

(CHEMBL3623120)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)NC)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C19H19F3N4O5S/c1-17-7-12(25-32(29,30)24-3)18(2,31-17)14-13(17)15(27)26(16(14)28)10-5-4-9(8-23)11(6-10)19(20,21)22/h4-6,12-14,24-25H,7H2,1-3H3/t12-,13-,14+,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122638
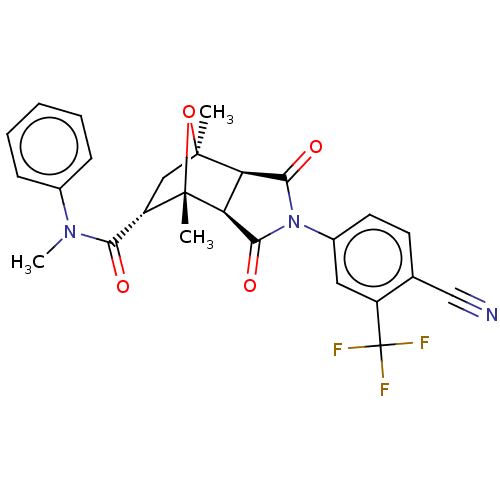
(CHEMBL3623117)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1C(=O)N(C)c1ccccc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C26H22F3N3O4/c1-24-12-18(21(33)31(3)15-7-5-4-6-8-15)25(2,36-24)20-19(24)22(34)32(23(20)35)16-10-9-14(13-30)17(11-16)26(27,28)29/h4-11,18-20H,12H2,1-3H3/t18-,19+,20-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18525
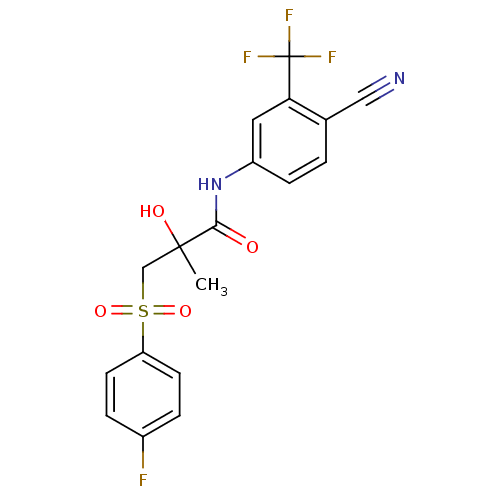
(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122636
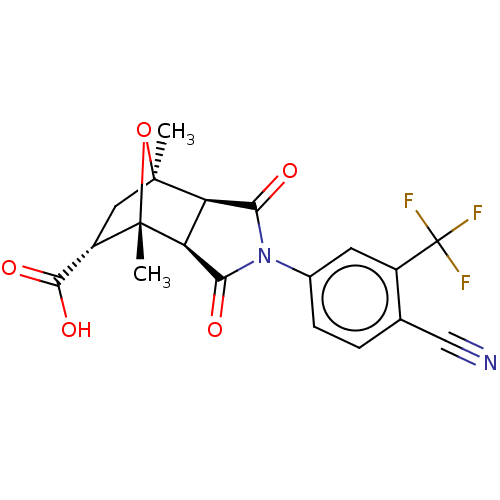
(CHEMBL3623115)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1C(O)=O)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C19H15F3N2O5/c1-17-6-11(16(27)28)18(2,29-17)13-12(17)14(25)24(15(13)26)9-4-3-8(7-23)10(5-9)19(20,21)22/h3-5,11-13H,6H2,1-2H3,(H,27,28)/t11-,12+,13-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239610
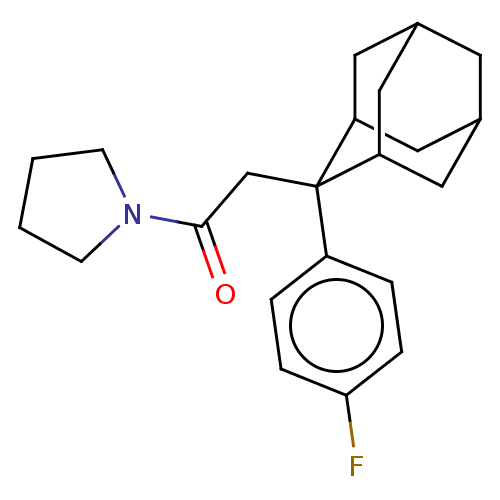
(CHEMBL4073961)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CCCC2)C2CC3CC(C2)CC1C3 |TLB:24:23:21:17.18.19,8:7:17.24.18:22.20.21,4:7:21:17.18.19,THB:24:18:7.23.22:21,19:18:7:22.20.21,19:20:7:17.24.18,8:7:21:17.18.19,4:7:17.24.18:22.20.21,(23.94,-24.95,;23.96,-23.41,;22.63,-22.62,;22.65,-21.09,;23.99,-20.34,;25.31,-21.11,;25.3,-22.64,;24.01,-18.8,;22.86,-17.77,;21.4,-18.25,;20.15,-17.34,;20.92,-19.71,;19.45,-20.18,;19.45,-21.72,;20.91,-22.2,;21.82,-20.95,;25.21,-17.53,;26.53,-18.01,;27.92,-17.67,;27.94,-16.14,;26.54,-15.56,;25.2,-16.04,;25.5,-16.8,;25.5,-18.39,;26.91,-18.95,)| Show InChI InChI=1S/C22H28FNO/c23-20-5-3-17(4-6-20)22(14-21(25)24-7-1-2-8-24)18-10-15-9-16(12-18)13-19(22)11-15/h3-6,15-16,18-19H,1-2,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239632
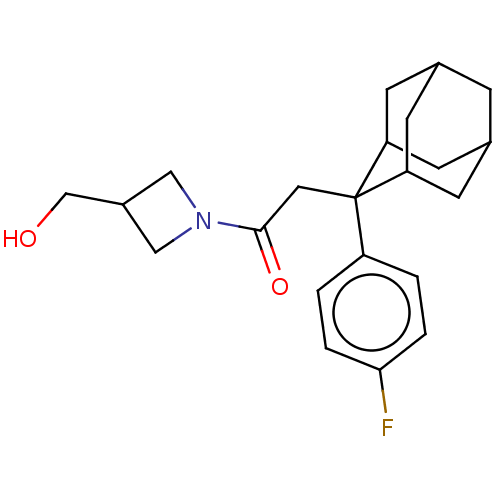
(CHEMBL4071232)Show SMILES OCC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:18:17:15:11.12.13,8:9:11.18.12:16.14.15,19:9:15:11.12.13,THB:18:12:9.17.16:15,13:12:9:16.14.15,13:14:9:11.18.12,8:9:15:11.12.13,19:9:11.18.12:16.14.15,(48.51,-12.14,;50.02,-11.82,;50.49,-10.36,;49.8,-8.98,;51.18,-8.28,;51.87,-9.66,;51.66,-6.82,;50.41,-5.91,;53.13,-6.34,;54.27,-7.37,;55.47,-6.1,;56.79,-6.59,;58.18,-6.24,;58.2,-4.71,;56.8,-4.13,;55.46,-4.61,;55.76,-5.37,;55.77,-6.96,;57.18,-7.52,;54.26,-8.91,;52.91,-9.66,;52.9,-11.19,;54.22,-11.98,;54.21,-13.52,;55.57,-11.21,;55.58,-9.68,)| Show InChI InChI=1S/C22H28FNO2/c23-20-3-1-17(2-4-20)22(10-21(26)24-11-16(12-24)13-25)18-6-14-5-15(8-18)9-19(22)7-14/h1-4,14-16,18-19,25H,5-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239628

(CHEMBL4102283)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)C3O)c1ccc(cc1)-c1ccc(F)cc1 |TLB:12:11:8.13.14:16,7:8:16:10.11.17,19:8:10.12.11:14.15.16,THB:18:17:8.13.14:16,12:13:16:10.11.17,17:11:8:14.15.16,17:15:8:10.12.11,7:8:10.12.11:14.15.16,19:8:16:10.11.17,(5.77,-1.2,;6.92,-2.24,;8.46,-2.16,;8.53,-3.7,;7,-3.78,;9.68,-4.73,;9.36,-6.24,;11.15,-4.26,;12.29,-5.3,;13.49,-4.02,;14.82,-4.5,;16.22,-4.16,;15.2,-5.44,;13.79,-4.87,;13.78,-3.28,;14.83,-2.05,;13.48,-2.53,;16.23,-2.63,;17.52,-1.78,;12.27,-6.84,;10.94,-7.58,;10.92,-9.12,;12.24,-9.91,;13.59,-9.14,;13.6,-7.61,;12.23,-11.45,;10.89,-12.21,;10.87,-13.75,;12.2,-14.54,;12.19,-16.08,;13.55,-13.77,;13.56,-12.23,)| Show InChI InChI=1S/C27H30FNO3/c28-23-7-3-17(4-8-23)16-1-5-20(6-2-16)27(13-25(31)29-14-24(30)15-29)21-9-18-10-22(27)12-19(11-21)26(18)32/h1-8,18-19,21-22,24,26,30,32H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239619
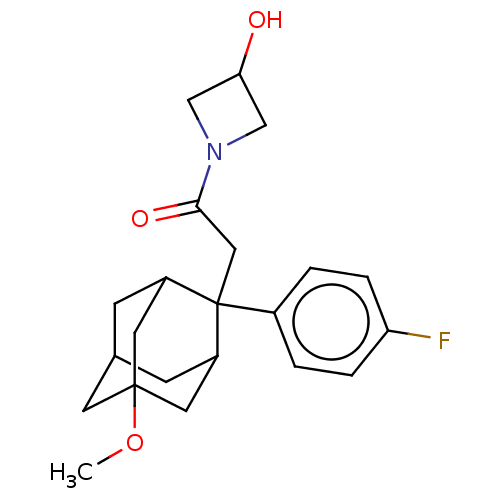
(CHEMBL4087497)Show SMILES COC12CC3CC(C1)C(CC(=O)N1CC(O)C1)(C(C3)C2)c1ccc(F)cc1 |TLB:18:17:7:5.4.3,20:8:5.18.4:19.2.7,20:8:7:5.4.3,THB:18:4:8.17.19:7,3:4:8:19.2.7,3:2:8:5.18.4,1:2:8:5.18.4,9:8:5.18.4:19.2.7,9:8:7:5.4.3,(28.8,-17.02,;27.27,-17.21,;26.66,-18.63,;28.07,-19.21,;28.05,-20.74,;26.65,-21.08,;25.33,-20.59,;25.32,-19.11,;24.13,-21.87,;22.99,-20.84,;21.52,-21.31,;20.27,-20.4,;21.04,-22.78,;19.66,-23.47,;20.36,-24.85,;19.88,-26.32,;21.74,-24.15,;25.63,-21.45,;27.04,-22.02,;25.62,-19.86,;24.12,-23.41,;22.77,-24.16,;22.76,-25.7,;24.08,-26.48,;24.07,-28.02,;25.43,-25.72,;25.44,-24.18,)| Show InChI InChI=1S/C22H28FNO3/c1-27-21-8-14-6-16(9-21)22(17(7-14)10-21,15-2-4-18(23)5-3-15)11-20(26)24-12-19(25)13-24/h2-5,14,16-17,19,25H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239616

(CHEMBL4078671)Show SMILES COC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:18:17:15:11.12.13,8:9:11.18.12:16.14.15,19:9:15:11.12.13,THB:18:12:9.17.16:15,13:12:9:16.14.15,13:14:9:11.18.12,8:9:15:11.12.13,19:9:11.18.12:16.14.15,(63.87,-11.5,;65.38,-11.19,;65.86,-9.72,;65.16,-8.35,;66.55,-7.65,;67.23,-9.02,;67.02,-6.18,;65.77,-5.27,;68.49,-5.7,;69.64,-6.74,;70.83,-5.46,;72.15,-5.95,;73.55,-5.61,;73.57,-4.08,;72.16,-3.5,;70.82,-3.98,;71.13,-4.74,;71.13,-6.32,;72.54,-6.88,;69.62,-8.28,;68.28,-9.02,;68.26,-10.56,;69.59,-11.34,;69.57,-12.89,;70.92,-10.58,;70.93,-9.05,)| Show InChI InChI=1S/C22H28FNO2/c1-26-20-12-24(13-20)21(25)11-22(16-2-4-19(23)5-3-16)17-7-14-6-15(9-17)10-18(22)8-14/h2-5,14-15,17-18,20H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239609

(CHEMBL4093016)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CCC2)C2CC3CC(C2)CC1C3 |TLB:23:22:20:16.17.18,8:7:16.23.17:21.19.20,4:7:20:16.17.18,THB:23:17:7.22.21:20,18:17:7:21.19.20,18:19:7:16.23.17,8:7:20:16.17.18,4:7:16.23.17:21.19.20,(23.9,-12.85,;23.91,-11.3,;22.58,-10.52,;22.6,-8.98,;23.94,-8.23,;25.26,-9,;25.25,-10.54,;23.96,-6.69,;22.81,-5.65,;21.34,-6.13,;20.1,-5.22,;20.86,-7.6,;19.48,-8.3,;20.18,-9.68,;21.56,-8.98,;25.16,-5.41,;26.48,-5.9,;27.88,-5.55,;27.9,-4.02,;26.49,-3.44,;25.15,-3.92,;25.45,-4.68,;25.46,-6.27,;26.87,-6.84,)| Show InChI InChI=1S/C21H26FNO/c22-19-4-2-16(3-5-19)21(13-20(24)23-6-1-7-23)17-9-14-8-15(11-17)12-18(21)10-14/h2-5,14-15,17-18H,1,6-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239603

(CHEMBL4101370)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)C3O)c1ccc(cc1)-c1ccncc1 |TLB:12:11:8.13.14:16,7:8:16:10.11.17,19:8:10.12.11:14.15.16,THB:18:17:8.13.14:16,12:13:16:10.11.17,17:11:8:14.15.16,17:15:8:10.12.11,7:8:10.12.11:14.15.16,19:8:16:10.11.17,(5.99,-16.91,;7.14,-17.94,;8.68,-17.86,;8.76,-19.41,;7.22,-19.48,;9.9,-20.44,;9.58,-21.95,;11.37,-19.97,;12.51,-21,;13.72,-19.72,;15.04,-20.22,;16.44,-19.87,;15.43,-21.14,;14.01,-20.58,;14.01,-18.99,;15.05,-17.75,;13.71,-18.23,;16.46,-18.34,;17.75,-17.49,;12.5,-22.54,;11.16,-23.3,;11.14,-24.83,;12.46,-25.61,;13.81,-24.85,;13.82,-23.32,;12.45,-27.16,;11.11,-27.92,;11.09,-29.46,;12.42,-30.24,;13.77,-29.48,;13.78,-27.94,)| Show InChI InChI=1S/C26H30N2O3/c29-23-14-28(15-23)24(30)13-26(21-9-18-10-22(26)12-19(11-21)25(18)31)20-3-1-16(2-4-20)17-5-7-27-8-6-17/h1-8,18-19,21-23,25,29,31H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]PIA from adenosine A1 receptor of rat brain membranes |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239614

(CHEMBL4067777)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:17:16:14:10.11.12,7:8:10.17.11:15.13.14,18:8:14:10.11.12,THB:17:11:8.16.15:14,12:11:8:15.13.14,12:13:8:10.17.11,7:8:14:10.11.12,18:8:10.17.11:15.13.14,(49.46,-25.67,;49.94,-24.2,;49.24,-22.82,;50.62,-22.13,;51.32,-23.5,;51.1,-20.66,;49.85,-19.75,;52.57,-20.18,;53.71,-21.22,;54.91,-19.94,;56.23,-20.43,;57.63,-20.08,;57.65,-18.55,;56.24,-17.97,;54.9,-18.45,;55.2,-19.21,;55.21,-20.8,;56.62,-21.36,;53.69,-22.76,;52.35,-23.51,;52.33,-25.04,;53.66,-25.83,;53.65,-27.37,;55.01,-25.06,;55.01,-23.53,)| Show InChI InChI=1S/C21H26FNO2/c22-18-3-1-15(2-4-18)21(10-20(25)23-11-19(24)12-23)16-6-13-5-14(8-16)9-17(21)7-13/h1-4,13-14,16-17,19,24H,5-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122645

(CHEMBL3623124)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1ccccc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H20F3N3O5S/c1-22-11-17(29-36(33,34)15-6-4-3-5-7-15)23(2,35-22)19-18(22)20(31)30(21(19)32)14-9-8-13(12-28)16(10-14)24(25,26)27/h3-10,17-19,29H,11H2,1-2H3/t17-,18-,19+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human MDA-MB-453 cells assessed as inhibition of DHT-induced PSA expression by alkaline phosphatase repor... |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239633

(CHEMBL4102950)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(F)(C3)C2)c1ccc(F)cc1 |TLB:12:13:18:10.11.17,12:11:8.13.14:18,19:8:10.12.11:14.15.18,19:8:18:10.11.17,THB:17:11:8:14.15.18,17:15:8:10.12.11,16:15:8:10.12.11,7:8:10.12.11:14.15.18,7:8:18:10.11.17,(64.54,-24.64,;65.02,-23.17,;64.31,-21.79,;65.69,-21.1,;66.39,-22.47,;66.18,-19.63,;64.92,-18.71,;67.65,-19.16,;68.79,-20.18,;69.99,-18.91,;71.31,-19.4,;72.71,-19.06,;71.7,-20.34,;70.29,-19.77,;70.28,-18.18,;71.32,-16.94,;71.93,-15.52,;72.73,-17.52,;69.98,-17.42,;68.77,-21.72,;67.43,-22.48,;67.41,-24.02,;68.74,-24.8,;68.72,-26.34,;70.08,-24.04,;70.09,-22.5,)| Show InChI InChI=1S/C21H25F2NO2/c22-17-3-1-14(2-4-17)21(10-19(26)24-11-18(25)12-24)15-5-13-6-16(21)9-20(23,7-13)8-15/h1-4,13,15-16,18,25H,5-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239621
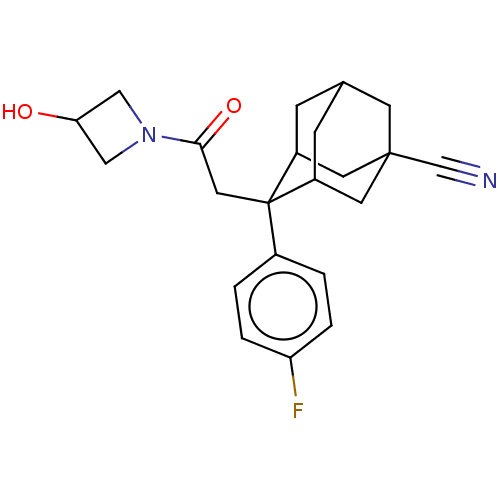
(CHEMBL4060170)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C3)(C2)C#N)c1ccc(F)cc1 |TLB:17:15:8.9.10:12,7:8:14.17.15:10.11.12,7:8:12:14.15.16,THB:17:9:12:14.15.16,16:15:8:10.11.12,16:11:8:14.17.15,18:15:8:10.11.12,18:15:8.9.10:12,20:8:14.17.15:10.11.12,20:8:12:14.15.16,(34.25,-39.18,;34.73,-37.71,;34.04,-36.34,;35.41,-35.64,;36.11,-37.02,;35.89,-34.17,;34.64,-33.26,;37.36,-33.7,;38.51,-34.73,;40,-34.31,;40,-32.72,;41.04,-31.49,;39.7,-31.97,;39.71,-33.45,;41.03,-33.94,;42.43,-33.6,;42.45,-32.07,;41.42,-34.88,;43.96,-33.54,;45.5,-33.47,;38.49,-36.27,;37.15,-37.02,;37.13,-38.56,;38.46,-39.34,;38.44,-40.89,;39.8,-38.58,;39.81,-37.05,)| Show InChI InChI=1S/C22H25FN2O2/c23-18-3-1-15(2-4-18)22(10-20(27)25-11-19(26)12-25)16-5-14-6-17(22)9-21(7-14,8-16)13-24/h1-4,14,16-17,19,26H,5-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239615
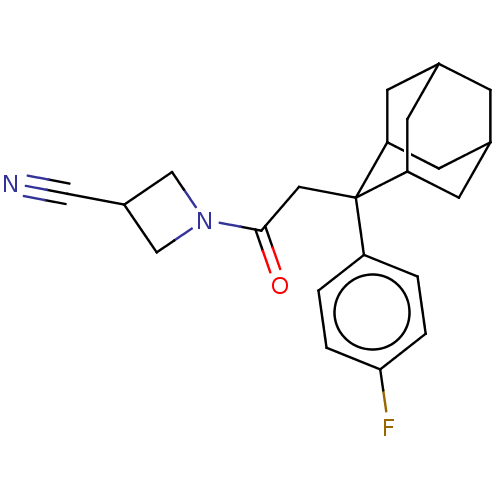
(CHEMBL4095204)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CC(C2)C#N)C2CC3CC(C2)CC1C3 |TLB:25:24:22:18.19.20,8:7:18.25.19:23.21.22,4:7:22:18.19.20,THB:25:19:7.24.23:22,20:19:7:23.21.22,20:21:7:18.25.19,8:7:22:18.19.20,4:7:18.25.19:23.21.22,(54.09,-42.35,;54.11,-40.81,;52.78,-40.02,;52.8,-38.49,;54.14,-37.74,;55.46,-38.51,;55.45,-40.04,;54.16,-36.2,;53.02,-35.16,;51.55,-35.64,;50.3,-34.73,;51.06,-37.11,;49.69,-37.8,;50.39,-39.18,;51.77,-38.48,;49.91,-40.65,;49.43,-42.11,;55.36,-34.92,;56.68,-35.41,;58.08,-35.06,;58.1,-33.53,;56.69,-32.96,;55.35,-33.43,;55.65,-34.19,;55.65,-35.78,;57.07,-36.34,)| Show InChI InChI=1S/C22H25FN2O/c23-20-3-1-17(2-4-20)22(10-21(26)25-12-16(11-24)13-25)18-6-14-5-15(8-18)9-19(22)7-14/h1-4,14-16,18-19H,5-10,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239631

(CHEMBL4100730)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CC(F)(F)C2)C2CC3CC(C2)CC1C3 |TLB:8:7:18.25.19:23.21.22,4:7:22:18.19.20,25:24:22:18.19.20,THB:8:7:22:18.19.20,4:7:18.25.19:23.21.22,25:19:7.24.23:22,20:19:7:23.21.22,20:21:7:18.25.19,(23.41,-40.89,;23.42,-39.35,;22.1,-38.56,;22.11,-37.02,;23.46,-36.27,;24.78,-37.04,;24.77,-38.58,;23.47,-34.73,;22.33,-33.7,;20.87,-34.18,;19.61,-33.26,;20.38,-35.64,;19,-36.34,;19.7,-37.71,;20.09,-39.2,;18.61,-38.81,;21.08,-37.01,;24.67,-33.45,;26,-33.94,;27.4,-33.6,;27.42,-32.07,;26.01,-31.49,;24.67,-31.97,;24.97,-32.73,;24.97,-34.32,;26.39,-34.88,)| Show InChI InChI=1S/C21H24F3NO/c22-18-3-1-15(2-4-18)21(10-19(26)25-11-20(23,24)12-25)16-6-13-5-14(8-16)9-17(21)7-13/h1-4,13-14,16-17H,5-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239604

(CHEMBL4098342)Show SMILES OC1CN(C1)C(=O)CC1(Cc2ccccc2)C2CC3CC1CC(C2)C3O |TLB:19:18:8.20.21:23,7:8:23:17.18.24,9:8:17.19.18:21.22.23,THB:19:20:23:17.18.24,24:18:8:21.22.23,24:22:8:17.19.18,7:8:17.19.18:21.22.23,9:8:23:17.18.24,25:24:8.20.21:23,(6.47,-33.11,;7.62,-34.16,;9.17,-34.08,;9.24,-35.62,;7.7,-35.7,;10.39,-36.65,;10.07,-38.17,;11.87,-36.18,;13.01,-37.22,;12.99,-38.76,;14.32,-39.54,;14.3,-41.07,;15.62,-41.85,;16.96,-41.09,;16.97,-39.55,;15.64,-38.78,;14.21,-35.94,;15.54,-36.43,;16.95,-36.08,;15.93,-37.37,;14.51,-36.8,;14.51,-35.21,;15.55,-33.96,;14.21,-34.44,;16.97,-34.54,;18.26,-33.7,)| Show InChI InChI=1S/C22H29NO3/c24-19-12-23(13-19)20(25)11-22(10-14-4-2-1-3-5-14)17-6-15-7-18(22)9-16(8-17)21(15)26/h1-5,15-19,21,24,26H,6-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239596

(CHEMBL4083748)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)C3O)c1ccc(Cl)cc1 |TLB:12:11:8.13.14:16,7:8:16:10.11.17,19:8:10.12.11:14.15.16,THB:18:17:8.13.14:16,12:13:16:10.11.17,17:11:8:14.15.16,17:15:8:10.12.11,7:8:10.12.11:14.15.16,19:8:16:10.11.17,(5.9,-31.02,;7.04,-32.05,;8.58,-31.97,;8.65,-33.51,;7.12,-33.59,;9.8,-34.55,;9.48,-36.06,;11.27,-34.07,;12.41,-35.11,;13.61,-33.83,;14.93,-34.32,;16.33,-33.97,;15.32,-35.25,;13.91,-34.69,;13.9,-33.1,;14.94,-31.86,;13.6,-32.34,;16.35,-32.44,;17.64,-31.6,;12.4,-36.64,;11.05,-37.4,;11.04,-38.93,;12.36,-39.72,;12.35,-41.26,;13.71,-38.95,;13.72,-37.42,)| Show InChI InChI=1S/C21H26ClNO3/c22-17-3-1-14(2-4-17)21(9-19(25)23-10-18(24)11-23)15-5-12-6-16(21)8-13(7-15)20(12)26/h1-4,12-13,15-16,18,20,24,26H,5-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]PIA from adenosine A1 receptor of rat brain membranes |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199036
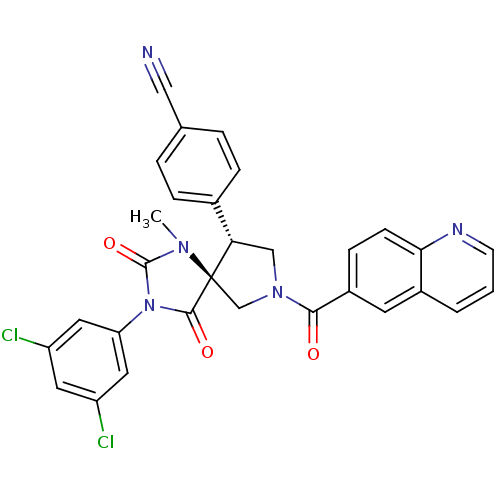
(4-[(5S,9R)-3-(3,5-dichloro-phenyl)-1-methyl-2,4-di...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(C[C@H]1c1ccc(cc1)C#N)C(=O)c1ccc2ncccc2c1)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C30H21Cl2N5O3/c1-35-29(40)37(24-13-22(31)12-23(32)14-24)28(39)30(35)17-36(16-25(30)19-6-4-18(15-33)5-7-19)27(38)21-8-9-26-20(11-21)3-2-10-34-26/h2-14,25H,16-17H2,1H3/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50104843
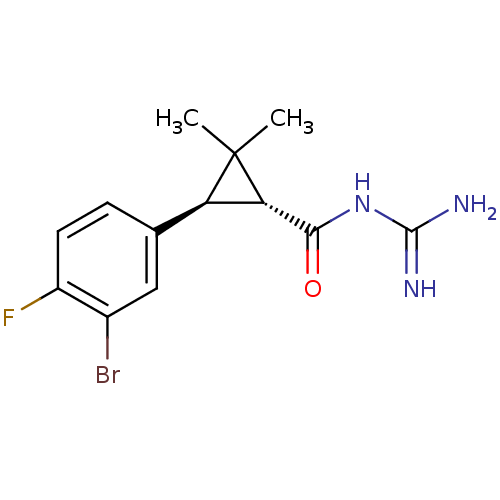
(CHEMBL419578 | N-[3-(3-Bromo-4-fluoro-phenyl)-2,2-...)Show SMILES CC1(C)[C@@H]([C@H]1c1ccc(F)c(Br)c1)C(=O)NC(N)=N Show InChI InChI=1S/C13H15BrFN3O/c1-13(2)9(10(13)11(19)18-12(16)17)6-3-4-8(15)7(14)5-6/h3-5,9-10H,1-2H3,(H4,16,17,18,19)/t9-,10+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Screened in AP1 cells expressing human NHE-1 for sodium hydrogen exchange activity |
J Med Chem 44: 3302-10 (2001)
BindingDB Entry DOI: 10.7270/Q2H41QQ4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239625
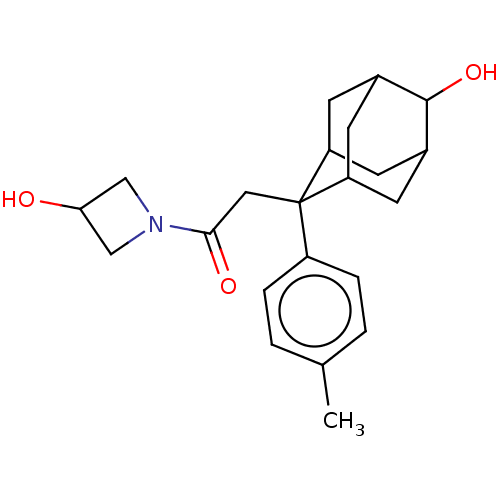
(CHEMBL4080314)Show SMILES Cc1ccc(cc1)C1(CC(=O)N2CC(O)C2)C2CC3CC1CC(C2)C3O |TLB:19:18:7.20.21:23,8:7:23:17.18.24,4:7:17.19.18:21.22.23,THB:25:24:7.20.21:23,19:20:23:17.18.24,24:18:7:21.22.23,24:22:7:17.19.18,8:7:17.19.18:21.22.23,4:7:23:17.18.24,(11.65,-14.63,;11.66,-13.08,;10.33,-12.3,;10.35,-10.76,;11.69,-10.01,;13.01,-10.79,;13,-12.32,;11.71,-8.47,;10.56,-7.44,;9.1,-7.91,;8.77,-9.42,;7.95,-6.88,;7.88,-5.34,;6.34,-5.42,;5.19,-4.39,;6.42,-6.96,;12.91,-7.19,;14.23,-7.69,;15.63,-7.34,;14.62,-8.62,;13.2,-8.05,;13.2,-6.47,;14.24,-5.23,;12.9,-5.71,;15.65,-5.81,;16.93,-4.97,)| Show InChI InChI=1S/C22H29NO3/c1-13-2-4-16(5-3-13)22(10-20(25)23-11-19(24)12-23)17-6-14-7-18(22)9-15(8-17)21(14)26/h2-5,14-15,17-19,21,24,26H,6-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239606

(CHEMBL4080667)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)[C@H]3O)c1ccccc1 |r,wD:17.21,TLB:16:15:8.9.10:12,7:8:14.16.15:10.11.12,7:8:12:14.15.17,THB:16:9:12:14.15.17,18:17:8.9.10:12,17:15:8:10.11.12,17:11:8:14.16.15,19:8:14.16.15:10.11.12,19:8:12:14.15.17,(43.24,-21.55,;43.72,-20.08,;43.03,-18.71,;44.4,-18.01,;45.1,-19.38,;44.88,-16.54,;43.63,-15.64,;46.35,-16.07,;47.49,-17.11,;48.99,-16.68,;48.98,-15.1,;50.02,-13.87,;48.67,-14.34,;48.68,-15.83,;50,-16.32,;51.4,-15.97,;50.4,-17.25,;51.42,-14.44,;52.71,-13.59,;47.48,-18.64,;46.13,-19.39,;46.12,-20.93,;47.44,-21.71,;48.79,-20.95,;48.79,-19.41,)| Show InChI InChI=1S/C21H27NO3/c23-18-11-22(12-18)19(24)10-21(15-4-2-1-3-5-15)16-6-13-7-17(21)9-14(8-16)20(13)25/h1-5,13-14,16-18,20,23,25H,6-12H2/t13?,14?,16?,17?,20-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239626
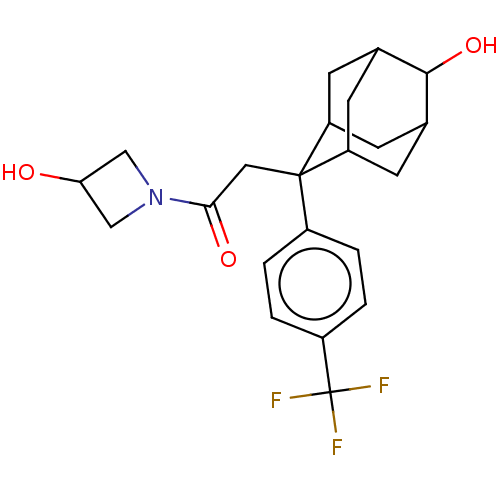
(CHEMBL4076403)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)C3O)c1ccc(cc1)C(F)(F)F |TLB:12:11:8.13.14:16,7:8:16:10.11.17,19:8:10.12.11:14.15.16,THB:18:17:8.13.14:16,12:13:16:10.11.17,17:11:8:14.15.16,17:15:8:10.12.11,7:8:10.12.11:14.15.16,19:8:16:10.11.17,(24.84,-17.39,;25.99,-18.42,;27.53,-18.34,;27.6,-19.88,;26.06,-19.96,;28.74,-20.92,;28.42,-22.42,;30.21,-20.44,;31.36,-21.48,;32.56,-20.2,;33.88,-20.69,;35.28,-20.34,;34.27,-21.62,;32.85,-21.05,;32.85,-19.47,;33.89,-18.23,;32.55,-18.71,;35.3,-18.81,;36.58,-17.97,;31.34,-23.01,;30,-23.77,;29.98,-25.3,;31.31,-26.09,;32.65,-25.32,;32.66,-23.79,;31.29,-27.63,;29.95,-28.39,;32.62,-28.41,;31.28,-29.17,)| Show InChI InChI=1S/C22H26F3NO3/c23-22(24,25)15-3-1-14(2-4-15)21(9-19(28)26-10-18(27)11-26)16-5-12-6-17(21)8-13(7-16)20(12)29/h1-4,12-13,16-18,20,27,29H,5-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50104848
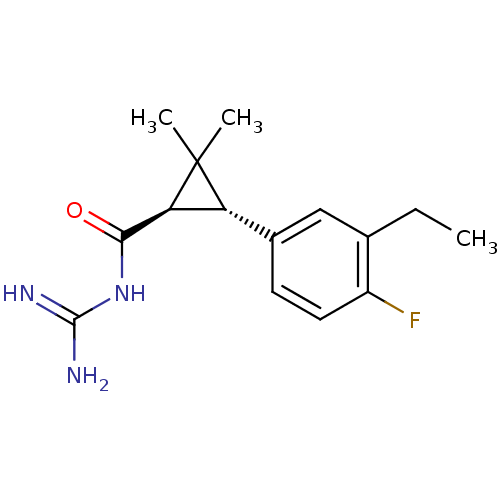
(CHEMBL112903 | N-[3-(3-Ethyl-4-fluoro-phenyl)-2,2-...)Show SMILES CCc1cc(ccc1F)[C@@H]1[C@@H](C(=O)NC(N)=N)C1(C)C Show InChI InChI=1S/C15H20FN3O/c1-4-8-7-9(5-6-10(8)16)11-12(15(11,2)3)13(20)19-14(17)18/h5-7,11-12H,4H2,1-3H3,(H4,17,18,19,20)/t11-,12+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Screened in AP1 cells expressing human NHE-1 for sodium hydrogen exchange activity |
J Med Chem 44: 3302-10 (2001)
BindingDB Entry DOI: 10.7270/Q2H41QQ4 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50104846
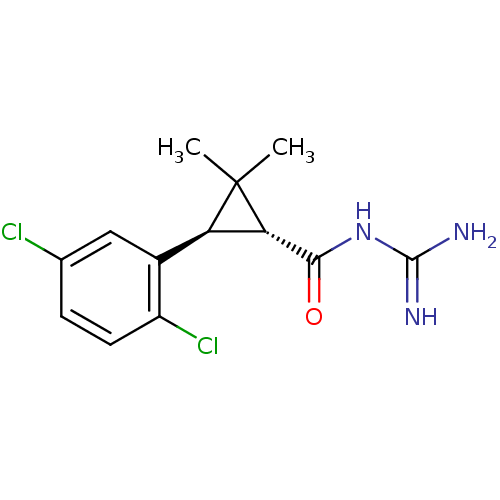
(CHEMBL113443 | N-[3-(2,5-Dichloro-phenyl)-2,2-dime...)Show SMILES CC1(C)[C@@H]([C@H]1c1cc(Cl)ccc1Cl)C(=O)NC(N)=N Show InChI InChI=1S/C13H15Cl2N3O/c1-13(2)9(10(13)11(19)18-12(16)17)7-5-6(14)3-4-8(7)15/h3-5,9-10H,1-2H3,(H4,16,17,18,19)/t9-,10+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Screened in AP1 cells expressing human NHE-1 for sodium hydrogen exchange activity |
J Med Chem 44: 3302-10 (2001)
BindingDB Entry DOI: 10.7270/Q2H41QQ4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239635
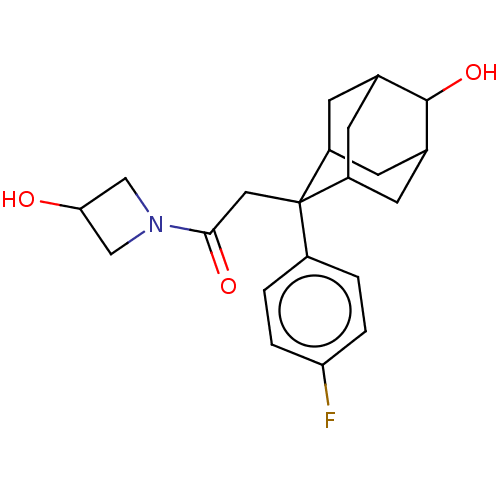
(CHEMBL4069598)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)C3O)c1ccc(F)cc1 |TLB:12:11:8.13.14:16,7:8:16:10.11.17,19:8:10.12.11:14.15.16,THB:12:13:16:10.11.17,17:11:8:14.15.16,17:15:8:10.12.11,18:17:8.13.14:16,7:8:10.12.11:14.15.16,19:8:16:10.11.17,(51.57,-9.93,;52.05,-8.46,;51.35,-7.08,;52.73,-6.39,;53.43,-7.76,;53.21,-4.92,;51.96,-4.01,;54.68,-4.45,;55.82,-5.48,;57.02,-4.2,;58.34,-4.69,;59.74,-4.35,;58.73,-5.63,;57.32,-5.06,;57.31,-3.47,;58.35,-2.24,;57.01,-2.72,;59.76,-2.82,;61.05,-1.97,;55.81,-7.02,;54.46,-7.77,;54.45,-9.31,;55.77,-10.09,;55.76,-11.63,;57.12,-9.33,;57.12,-7.79,)| Show InChI InChI=1S/C21H26FNO3/c22-17-3-1-14(2-4-17)21(9-19(25)23-10-18(24)11-23)15-5-12-6-16(21)8-13(7-15)20(12)26/h1-4,12-13,15-16,18,20,24,26H,5-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50104856
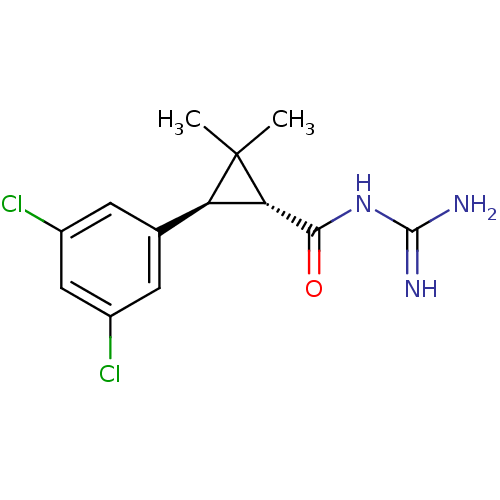
(CHEMBL51363 | N-[(1R,3S)-3-(3,5-Dichloro-phenyl)-2...)Show SMILES CC1(C)[C@@H]([C@H]1c1cc(Cl)cc(Cl)c1)C(=O)NC(N)=N Show InChI InChI=1S/C13H15Cl2N3O/c1-13(2)9(10(13)11(19)18-12(16)17)6-3-7(14)5-8(15)4-6/h3-5,9-10H,1-2H3,(H4,16,17,18,19)/t9-,10+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Screened in AP1 cells expressing human NHE-1 for sodium hydrogen exchange activity |
J Med Chem 44: 3302-10 (2001)
BindingDB Entry DOI: 10.7270/Q2H41QQ4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50239632

(CHEMBL4071232)Show SMILES OCC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:18:17:15:11.12.13,8:9:11.18.12:16.14.15,19:9:15:11.12.13,THB:18:12:9.17.16:15,13:12:9:16.14.15,13:14:9:11.18.12,8:9:15:11.12.13,19:9:11.18.12:16.14.15,(48.51,-12.14,;50.02,-11.82,;50.49,-10.36,;49.8,-8.98,;51.18,-8.28,;51.87,-9.66,;51.66,-6.82,;50.41,-5.91,;53.13,-6.34,;54.27,-7.37,;55.47,-6.1,;56.79,-6.59,;58.18,-6.24,;58.2,-4.71,;56.8,-4.13,;55.46,-4.61,;55.76,-5.37,;55.77,-6.96,;57.18,-7.52,;54.26,-8.91,;52.91,-9.66,;52.9,-11.19,;54.22,-11.98,;54.21,-13.52,;55.57,-11.21,;55.58,-9.68,)| Show InChI InChI=1S/C22H28FNO2/c23-20-3-1-17(2-4-20)22(10-21(26)24-11-16(12-24)13-25)18-6-14-5-15(8-18)9-19(22)7-14/h1-4,14-16,18-19,25H,5-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199031
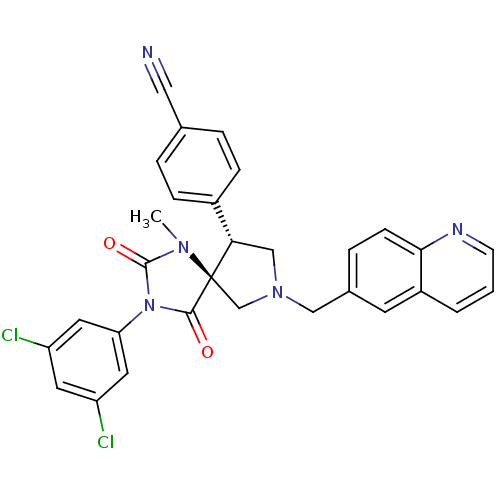
(4-[(5S,9R)-3-(3,5-dichloro-phenyl)-1-methyl-2,4-di...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(Cc2ccc3ncccc3c2)C[C@H]1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C30H23Cl2N5O2/c1-35-29(39)37(25-13-23(31)12-24(32)14-25)28(38)30(35)18-36(17-26(30)21-7-4-19(15-33)5-8-21)16-20-6-9-27-22(11-20)3-2-10-34-27/h2-14,26H,16-18H2,1H3/t26-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122643

(CHEMBL3623122)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)ON1CCN(C)CC1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H26F3N5O5/c1-22-11-16(29-21(35)36-31-8-6-30(3)7-9-31)23(2,37-22)18-17(22)19(33)32(20(18)34)14-5-4-13(12-28)15(10-14)24(25,26)27/h4-5,10,16-18H,6-9,11H2,1-3H3,(H,29,35)/t16-,17-,18+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human MDA-MB-453 cells assessed as inhibition of DHT-induced PSA expression by alkaline phosphatase repor... |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122647

(CHEMBL3623126)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1cccnc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C23H19F3N4O5S/c1-21-9-16(29-36(33,34)14-4-3-7-28-11-14)22(2,35-21)18-17(21)19(31)30(20(18)32)13-6-5-12(10-27)15(8-13)23(24,25)26/h3-8,11,16-18,29H,9H2,1-2H3/t16-,17-,18+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human MDA-MB-453 cells assessed as inhibition of DHT-induced PSA expression by alkaline phosphatase repor... |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239620
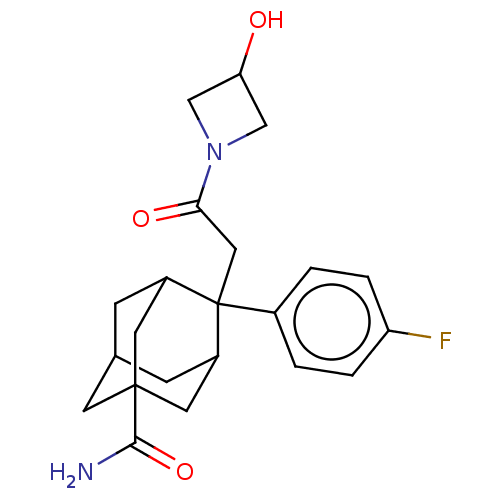
(CHEMBL4082055)Show SMILES NC(=O)C12CC3CC(C1)C(CC(=O)N1CC(O)C1)(C(C3)C2)c1ccc(F)cc1 |TLB:19:18:8:6.5.4,21:9:6.19.5:20.3.8,21:9:8:6.5.4,THB:19:5:9.18.20:8,4:5:9:20.3.8,4:3:9:6.19.5,1:3:9:6.19.5,10:9:6.19.5:20.3.8,10:9:8:6.5.4,(44.31,-2.3,;42.78,-2.49,;41.85,-1.26,;42.17,-3.91,;43.58,-4.49,;43.56,-6.02,;42.16,-6.36,;40.84,-5.87,;40.83,-4.39,;39.65,-7.15,;38.5,-6.12,;37.03,-6.59,;35.78,-5.68,;36.55,-8.06,;35.18,-8.75,;35.88,-10.13,;35.4,-11.59,;37.25,-9.43,;41.14,-6.73,;42.55,-7.3,;41.14,-5.15,;39.63,-8.69,;38.29,-9.44,;38.27,-10.97,;39.6,-11.76,;39.58,-13.3,;40.94,-10.99,;40.95,-9.46,)| Show InChI InChI=1S/C22H27FN2O3/c23-17-3-1-14(2-4-17)22(10-19(27)25-11-18(26)12-25)15-5-13-6-16(22)9-21(7-13,8-15)20(24)28/h1-4,13,15-16,18,26H,5-12H2,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199037
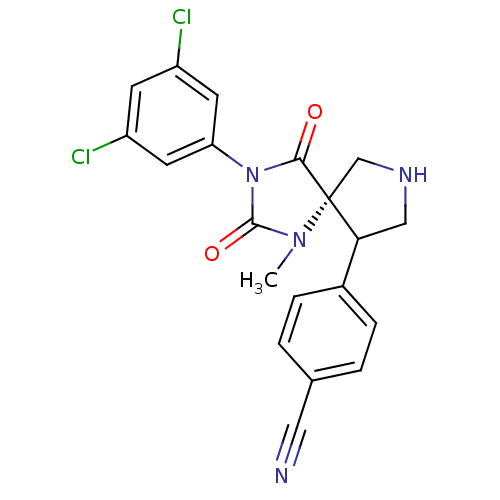
(4-[(5S,9R)-3-(3,5-dichloro-phenyl)-1-methyl-2,4-di...)Show SMILES CN1C(=O)N(C(=O)[C@]11CNCC1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-19(28)26(16-7-14(21)6-15(22)8-16)18(27)20(25)11-24-10-17(20)13-4-2-12(9-23)3-5-13/h2-8,17,24H,10-11H2,1H3/t17?,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50239616

(CHEMBL4078671)Show SMILES COC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:18:17:15:11.12.13,8:9:11.18.12:16.14.15,19:9:15:11.12.13,THB:18:12:9.17.16:15,13:12:9:16.14.15,13:14:9:11.18.12,8:9:15:11.12.13,19:9:11.18.12:16.14.15,(63.87,-11.5,;65.38,-11.19,;65.86,-9.72,;65.16,-8.35,;66.55,-7.65,;67.23,-9.02,;67.02,-6.18,;65.77,-5.27,;68.49,-5.7,;69.64,-6.74,;70.83,-5.46,;72.15,-5.95,;73.55,-5.61,;73.57,-4.08,;72.16,-3.5,;70.82,-3.98,;71.13,-4.74,;71.13,-6.32,;72.54,-6.88,;69.62,-8.28,;68.28,-9.02,;68.26,-10.56,;69.59,-11.34,;69.57,-12.89,;70.92,-10.58,;70.93,-9.05,)| Show InChI InChI=1S/C22H28FNO2/c1-26-20-12-24(13-20)21(25)11-22(16-2-4-19(23)5-3-16)17-7-14-6-15(9-17)10-18(22)8-14/h2-5,14-15,17-18,20H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239622
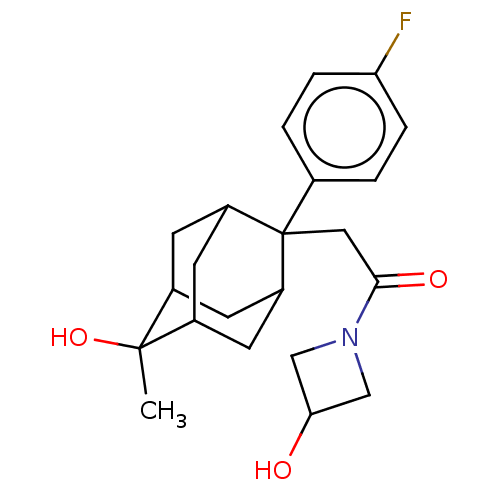
(CHEMBL4090613)Show SMILES CC1(O)C2CC3CC1CC(C2)C3(CC(=O)N1CC(O)C1)c1ccc(F)cc1 |TLB:10:9:6:4.3.1,1:3:11:8.7.6,1:7:11:4.10.3,2:1:11.9.8:6,0:1:11.9.8:6,12:11:4.10.3:8.7.6,20:11:6:4.3.1,THB:10:3:11.9.8:6,12:11:6:4.3.1,20:11:4.10.3:8.7.6,(60.85,-17.76,;59.31,-17.76,;59.71,-16.26,;59.3,-19.29,;57.9,-19.64,;56.57,-19.15,;56.57,-17.66,;57.91,-17.18,;56.87,-18.42,;56.87,-20.01,;58.29,-20.57,;55.38,-20.43,;54.23,-19.39,;52.76,-19.87,;51.51,-18.96,;52.28,-21.33,;50.91,-22.03,;51.6,-23.41,;51.12,-24.87,;52.98,-22.71,;55.36,-21.96,;54.02,-22.72,;54,-24.25,;55.33,-25.04,;55.31,-26.58,;56.67,-24.27,;56.68,-22.74,)| Show InChI InChI=1S/C22H28FNO3/c1-21(27)14-6-16-8-15(21)9-17(7-14)22(16,13-2-4-18(23)5-3-13)10-20(26)24-11-19(25)12-24/h2-5,14-17,19,25,27H,6-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239605
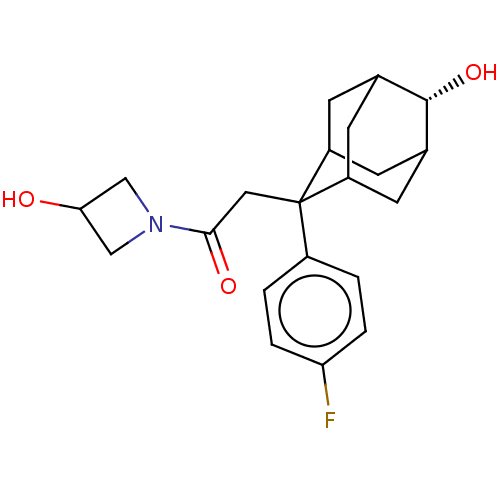
(CHEMBL4073762)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)[C@H]3O)c1ccc(F)cc1 |r,wD:17.21,TLB:16:15:8.9.10:12,7:8:14.16.15:10.11.12,7:8:12:14.15.17,THB:16:9:12:14.15.17,17:15:8:10.11.12,17:11:8:14.16.15,18:17:8.9.10:12,19:8:14.16.15:10.11.12,19:8:12:14.15.17,(27.3,-38.92,;27.77,-37.45,;27.08,-36.08,;28.46,-35.39,;29.15,-36.76,;28.93,-33.92,;27.68,-33.01,;30.4,-33.44,;31.54,-34.48,;33.04,-34.06,;33.03,-32.47,;34.07,-31.24,;32.73,-31.71,;32.74,-33.2,;34.06,-33.69,;35.46,-33.34,;34.45,-34.62,;35.47,-31.81,;36.76,-30.97,;31.53,-36.01,;30.19,-36.77,;30.17,-38.3,;31.49,-39.08,;31.48,-40.62,;32.84,-38.32,;32.85,-36.79,)| Show InChI InChI=1S/C21H26FNO3/c22-17-3-1-14(2-4-17)21(9-19(25)23-10-18(24)11-23)15-5-12-6-16(21)8-13(7-15)20(12)26/h1-4,12-13,15-16,18,20,24,26H,5-11H2/t12?,13?,15?,16?,20-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239630
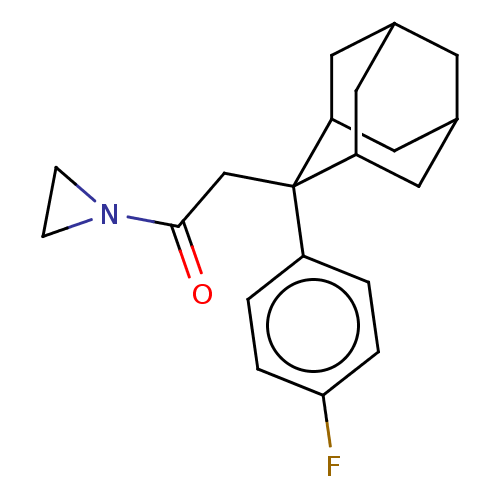
(CHEMBL4065347)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CC2)C2CC3CC(C2)CC1C3 |TLB:22:21:19:15.16.17,8:7:15.22.16:20.18.19,4:7:19:15.16.17,THB:22:16:7.21.20:19,17:16:7:20.18.19,17:18:7:15.22.16,8:7:19:15.16.17,4:7:15.22.16:20.18.19,(8.85,-39.12,;8.86,-37.58,;7.53,-36.79,;7.55,-35.25,;8.9,-34.51,;10.22,-35.28,;10.21,-36.81,;8.91,-32.97,;7.76,-31.93,;6.29,-32.41,;5.05,-31.5,;5.82,-33.88,;4.67,-34.9,;6.13,-35.39,;10.11,-31.69,;11.44,-32.18,;12.84,-31.83,;12.85,-30.3,;11.45,-29.72,;10.1,-30.2,;10.4,-30.96,;10.41,-32.55,;11.83,-33.11,)| Show InChI InChI=1S/C20H24FNO/c21-18-3-1-15(2-4-18)20(12-19(23)22-5-6-22)16-8-13-7-14(10-16)11-17(20)9-13/h1-4,13-14,16-17H,5-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data