 Found 982 hits with Last Name = 'ducray' and Initial = 'r'
Found 982 hits with Last Name = 'ducray' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50236369
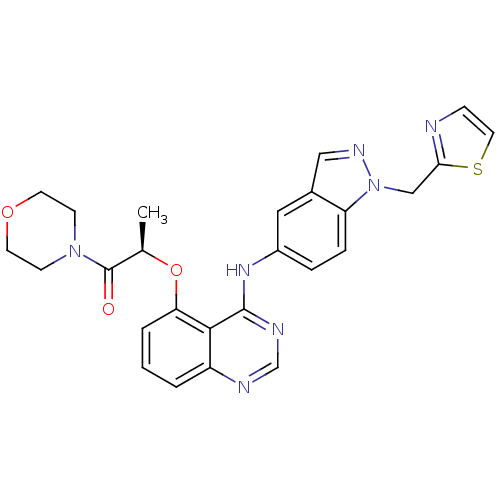
((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50236369

((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245474

(US9428503, 1)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2c1 Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-4-12-34-24-8-6-19(16-28-24)18-5-7-22-21(15-18)25-23(17-27-22)30(3)26(32)31(25)20-9-13-33-14-10-20/h5-8,15-17,20H,4,9-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate preincubated for 30 mins followed by substrate addition and measured after 2 hrs by HTRF as... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
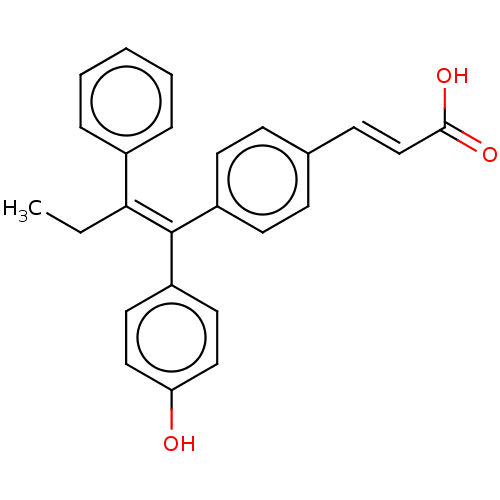
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245500
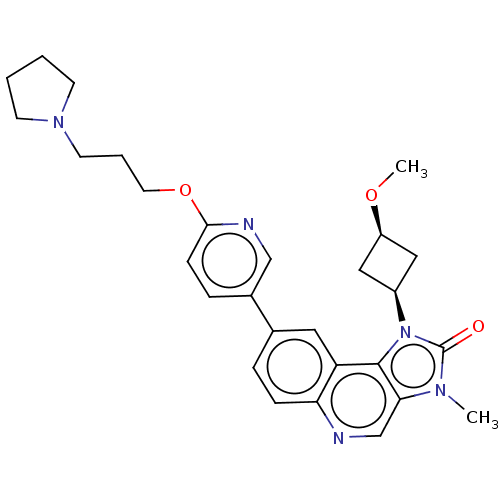
(US9428503, 28)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN3CCCC3)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.62,4.9,;3.22,3.41,;4.31,2.32,;4.31,.78,;5.85,.78,;5.85,2.32,;6.93,-.31,;6.61,-1.82,;7.95,-2.59,;7.95,-4.13,;6.61,-4.9,;5.28,-4.13,;3.95,-4.9,;2.61,-4.13,;2.61,-2.59,;3.95,-1.82,;5.28,-2.59,;1.28,-1.82,;1.28,-.28,;-.05,.49,;-1.39,-.28,;-2.72,.49,;-4.06,-.28,;-5.39,.49,;-6.72,-.28,;-8.06,.49,;-8.06,2.03,;-9.52,2.51,;-10.43,1.26,;-9.52,.02,;-1.39,-1.82,;-.05,-2.59,;9.09,-1.56,;10.43,-2.33,;8.47,-.15,;9.24,1.19,)| Show InChI InChI=1S/C28H33N5O3/c1-31-25-18-29-24-8-6-19(14-23(24)27(25)33(28(31)34)21-15-22(16-21)35-2)20-7-9-26(30-17-20)36-13-5-12-32-10-3-4-11-32/h6-9,14,17-18,21-22H,3-5,10-13,15-16H2,1-2H3/t21-,22+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245505

(US9428503, 33)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN3CCCCC3)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.76,4.9,;3.37,3.41,;4.45,2.32,;4.45,.78,;5.99,.78,;5.99,2.32,;7.08,-.31,;6.76,-1.82,;8.1,-2.59,;8.1,-4.13,;6.76,-4.9,;5.43,-4.13,;4.1,-4.9,;2.76,-4.13,;2.76,-2.59,;4.1,-1.82,;5.43,-2.59,;1.43,-1.82,;1.43,-.28,;.09,.49,;-1.24,-.28,;-2.57,.49,;-3.91,-.28,;-5.24,.49,;-6.57,-.28,;-7.91,.49,;-7.91,2.03,;-9.24,2.8,;-10.57,2.03,;-10.57,.49,;-9.24,-.28,;-1.24,-1.82,;.09,-2.59,;9.24,-1.56,;10.57,-2.33,;8.61,-.15,;9.38,1.19,)| Show InChI InChI=1S/C29H35N5O3/c1-32-26-19-30-25-9-7-20(15-24(25)28(26)34(29(32)35)22-16-23(17-22)36-2)21-8-10-27(31-18-21)37-14-6-13-33-11-4-3-5-12-33/h7-10,15,18-19,22-23H,3-6,11-14,16-17H2,1-2H3/t22-,23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245478
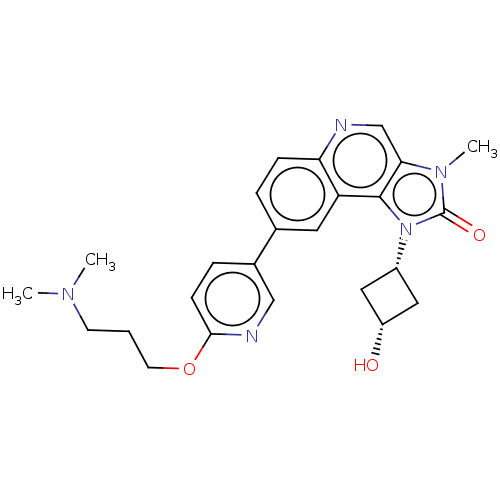
(US9428503, 5)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@@H]4C[C@H](O)C4)c3c2c1 |r,wD:25.25,27.28,(-9.98,.47,;-8.65,1.24,;-8.65,2.78,;-7.32,.47,;-5.98,1.24,;-4.65,.47,;-3.32,1.24,;-1.98,.47,;-.65,1.24,;.68,.47,;.68,-1.07,;-.65,-1.84,;-1.98,-1.07,;2.02,-1.84,;2.02,-3.38,;3.35,-4.15,;4.69,-3.38,;6.02,-4.15,;7.35,-3.38,;7.35,-1.84,;8.5,-.81,;9.98,-1.21,;7.87,.6,;8.64,1.93,;6.34,.43,;5.25,1.52,;3.71,1.52,;3.71,3.06,;2.62,4.15,;5.25,3.06,;6.02,-1.07,;4.69,-1.84,;3.35,-1.07,)| Show InChI InChI=1S/C25H29N5O3/c1-28(2)9-4-10-33-23-8-6-17(14-27-23)16-5-7-21-20(11-16)24-22(15-26-21)29(3)25(32)30(24)18-12-19(31)13-18/h5-8,11,14-15,18-19,31H,4,9-10,12-13H2,1-3H3/t18-,19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245475

(US9428503, 2)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN(C)C)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.02,4.9,;2.62,3.41,;3.71,2.32,;3.71,.78,;5.25,.78,;5.25,2.32,;6.34,-.31,;6.02,-1.82,;7.35,-2.59,;7.35,-4.13,;6.02,-4.9,;4.69,-4.13,;3.35,-4.9,;2.02,-4.13,;2.02,-2.59,;3.35,-1.82,;4.69,-2.59,;.68,-1.82,;.68,-.28,;-.65,.49,;-1.98,-.28,;-3.32,.49,;-4.65,-.28,;-5.98,.49,;-7.32,-.28,;-8.65,.49,;-9.99,-.28,;-8.65,2.03,;-1.98,-1.82,;-.65,-2.59,;8.5,-1.56,;9.99,-1.95,;7.87,-.15,;8.64,1.19,)| Show InChI InChI=1S/C26H31N5O3/c1-29(2)10-5-11-34-24-9-7-18(15-28-24)17-6-8-22-21(12-17)25-23(16-27-22)30(3)26(32)31(25)19-13-20(14-19)33-4/h6-9,12,15-16,19-20H,5,10-11,13-14H2,1-4H3/t19-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245514
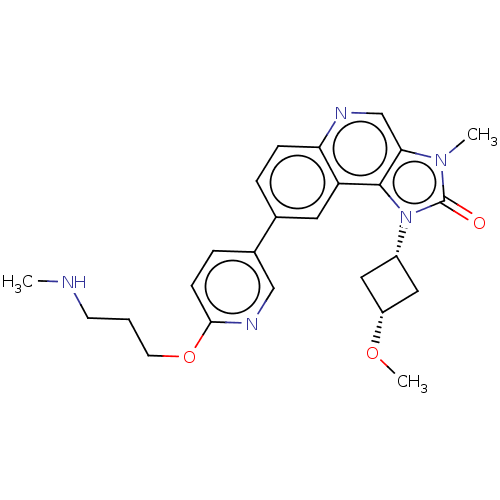
(US9428503, 42)Show SMILES CNCCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@@H]4C[C@@H](C4)OC)c3c2c1 |r,wD:24.24,26.29,(-9.91,.47,;-8.57,1.24,;-7.24,.47,;-5.91,1.24,;-4.57,.47,;-3.24,1.24,;-1.91,.47,;-.57,1.24,;.76,.47,;.76,-1.07,;-.57,-1.84,;-1.91,-1.07,;2.1,-1.84,;2.1,-3.38,;3.43,-4.15,;4.76,-3.38,;6.1,-4.15,;7.43,-3.38,;7.43,-1.84,;8.57,-.81,;9.91,-1.58,;7.95,.6,;8.72,1.93,;6.42,.43,;5.33,1.52,;3.79,1.52,;3.79,3.06,;5.33,3.06,;2.7,4.15,;1.21,3.75,;6.1,-1.07,;4.76,-1.84,;3.43,-1.07,)| Show InChI InChI=1S/C25H29N5O3/c1-26-9-4-10-33-23-8-6-17(14-28-23)16-5-7-21-20(11-16)24-22(15-27-21)29(2)25(31)30(24)18-12-19(13-18)32-3/h5-8,11,14-15,18-19,26H,4,9-10,12-13H2,1-3H3/t18-,19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245510
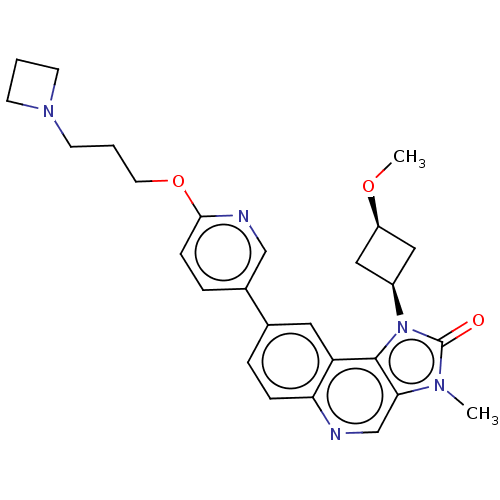
(US9428503, 38)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN3CCC3)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.37,4.9,;2.97,3.41,;4.06,2.32,;4.06,.78,;5.6,.78,;5.6,2.32,;6.69,-.31,;6.37,-1.82,;7.71,-2.59,;7.71,-4.13,;6.37,-4.9,;5.04,-4.13,;3.7,-4.9,;2.37,-4.13,;2.37,-2.59,;3.7,-1.82,;5.04,-2.59,;1.04,-1.82,;1.04,-.28,;-.3,.49,;-1.63,-.28,;-2.96,.49,;-4.3,-.28,;-5.63,.49,;-6.96,-.28,;-8.3,.49,;-8.7,1.98,;-10.18,1.58,;-9.79,.1,;-1.63,-1.82,;-.3,-2.59,;8.85,-1.56,;10.18,-2.33,;8.22,-.15,;8.99,1.19,)| Show InChI InChI=1S/C27H31N5O3/c1-30-24-17-28-23-7-5-18(13-22(23)26(24)32(27(30)33)20-14-21(15-20)34-2)19-6-8-25(29-16-19)35-12-4-11-31-9-3-10-31/h5-8,13,16-17,20-21H,3-4,9-12,14-15H2,1-2H3/t20-,21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245490
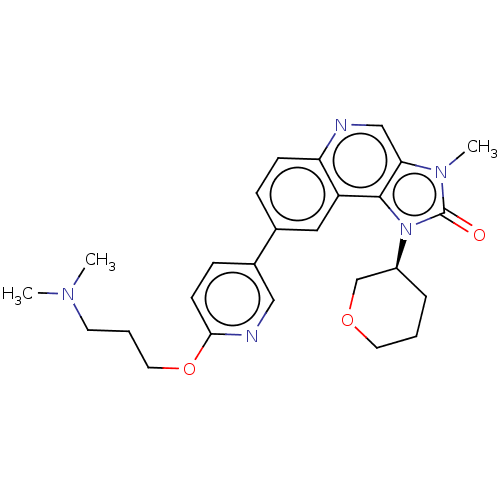
(US9428503, 17)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CCCOC4)c3c2c1 |r| Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-5-13-34-24-10-8-19(15-28-24)18-7-9-22-21(14-18)25-23(16-27-22)30(3)26(32)31(25)20-6-4-12-33-17-20/h7-10,14-16,20H,4-6,11-13,17H2,1-3H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084973

(CHEMBL3427401)Show SMILES Cc1cc(F)ccc1-c1c(Oc2ccc(\C=C\C(O)=O)cc2)c2ccc(O)cc2oc1=O |(-5.36,-.45,;-5.35,.78,;-6.68,1.56,;-6.67,3.1,;-7.73,3.72,;-5.33,3.86,;-4,3.08,;-4.01,1.54,;-2.68,.77,;-1.33,1.54,;-1.33,3.08,;.01,3.84,;1.34,3.07,;2.68,3.83,;2.69,5.37,;4.02,6.14,;4.03,7.68,;5.37,8.44,;6.43,7.82,;5.37,9.68,;1.36,6.15,;.02,5.38,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C25H17FO6/c1-14-12-16(26)5-9-19(14)23-24(20-10-6-17(27)13-21(20)32-25(23)30)31-18-7-2-15(3-8-18)4-11-22(28)29/h2-13,27H,1H3,(H,28,29)/b11-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084974

(CHEMBL3427402)Show SMILES Cc1cc(OC(F)(F)F)ccc1-c1c(Oc2ccc(\C=C\C(O)=O)cc2)c2ccc(O)cc2oc1=O |(5.33,.26,;5.33,-.77,;6.66,-1.54,;6.66,-3.08,;8,-3.85,;9.33,-3.08,;9.33,-2.06,;10.4,-2.47,;10.22,-3.6,;5.33,-3.85,;3.99,-3.08,;4,-1.54,;2.66,-.77,;1.33,-1.54,;1.33,-3.08,;0,-3.85,;0,-5.39,;-1.33,-6.16,;-2.66,-5.39,;-4,-6.16,;-5.33,-5.39,;-6.67,-6.16,;-6.67,-7.39,;-7.74,-5.54,;-2.67,-3.85,;-1.33,-3.08,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-3.75,1.39,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.73,1.38,)| Show InChI InChI=1S/C26H17F3O7/c1-14-12-18(36-26(27,28)29)8-10-19(14)23-24(20-9-5-16(30)13-21(20)35-25(23)33)34-17-6-2-15(3-7-17)4-11-22(31)32/h2-13,30H,1H3,(H,31,32)/b11-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245474

(US9428503, 1)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2c1 Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-4-12-34-24-8-6-19(16-28-24)18-5-7-22-21(15-18)25-23(17-27-22)30(3)26(32)31(25)20-9-13-33-14-10-20/h5-8,15-17,20H,4,9-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459018

(CHEMBL4166405)Show SMILES COCc1ccc(cn1)-c1cc2c(N[C@@H](C)c3ccn(C)n3)c(cnc2cc1F)C(N)=O |r| Show InChI InChI=1S/C23H23FN6O2/c1-13(20-6-7-30(2)29-20)28-22-17-8-16(14-4-5-15(12-32-3)26-10-14)19(24)9-21(17)27-11-18(22)23(25)31/h4-11,13H,12H2,1-3H3,(H2,25,31)(H,27,28)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... |
J Med Chem 59: 6281-92 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00519
BindingDB Entry DOI: 10.7270/Q2NV9NR8 |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245491
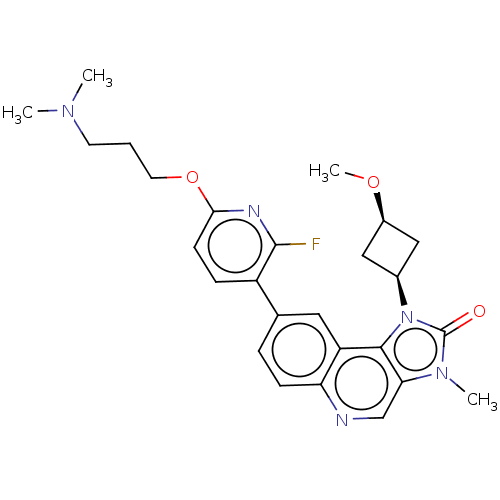
(US9428503, 18)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN(C)C)nc2F)n(C)c1=O |r,wD:4.6,2.1,(3.02,4.9,;2.62,3.41,;3.71,2.32,;3.71,.78,;5.25,.78,;5.25,2.32,;6.34,-.31,;6.02,-1.82,;7.35,-2.59,;7.35,-4.13,;6.02,-4.9,;4.69,-4.13,;3.35,-4.9,;2.02,-4.13,;2.02,-2.59,;3.35,-1.82,;4.69,-2.59,;.68,-1.82,;.68,-.28,;-.65,.49,;-1.98,-.28,;-3.32,.49,;-4.65,-.28,;-5.98,.49,;-7.32,-.28,;-8.65,.49,;-9.98,-.28,;-8.65,2.03,;-1.98,-1.82,;-.65,-2.59,;-.65,-4.13,;8.5,-1.56,;9.98,-1.95,;7.87,-.15,;8.64,1.19,)| Show InChI InChI=1S/C26H30FN5O3/c1-30(2)10-5-11-35-23-9-7-19(25(27)29-23)16-6-8-21-20(12-16)24-22(15-28-21)31(3)26(33)32(24)17-13-18(14-17)34-4/h6-9,12,15,17-18H,5,10-11,13-14H2,1-4H3/t17-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245481

(US9428503, 8)Show SMILES CN(C)CCCOc1ccc(cn1)-c1cc2c3n(C4CCOCC4)c(=O)n(C)c3cnc2cc1F Show InChI InChI=1S/C26H30FN5O3/c1-30(2)9-4-10-35-24-6-5-17(15-29-24)19-13-20-22(14-21(19)27)28-16-23-25(20)32(26(33)31(23)3)18-7-11-34-12-8-18/h5-6,13-16,18H,4,7-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50535970
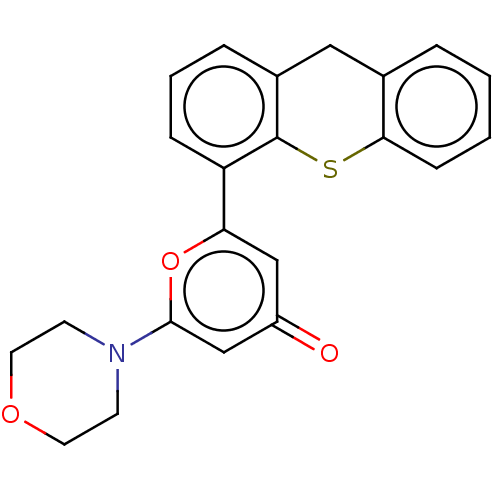
(CHEMBL4569967)Show SMILES O=c1cc(oc(c1)-c1cccc2Cc3ccccc3Sc12)N1CCOCC1 Show InChI InChI=1S/C22H19NO3S/c24-17-13-19(26-21(14-17)23-8-10-25-11-9-23)18-6-3-5-16-12-15-4-1-2-7-20(15)27-22(16)18/h1-7,13-14H,8-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... |
J Med Chem 59: 6281-92 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00519
BindingDB Entry DOI: 10.7270/Q2NV9NR8 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079
(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245489

(US9428503, 16)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@@H]4CCCOC4)c3c2c1 |r| Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-5-13-34-24-10-8-19(15-28-24)18-7-9-22-21(14-18)25-23(16-27-22)30(3)26(32)31(25)20-6-4-12-33-17-20/h7-10,14-16,20H,4-6,11-13,17H2,1-3H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373853
(CHEMBL258282)Show SMILES CN1CCN(CCOc2[nH]nc3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C24H27ClN8O2/c1-32-8-10-33(11-9-32)12-13-34-24-21-22(27-16-28-23(21)30-31-24)29-17-5-6-20(19(25)14-17)35-15-18-4-2-3-7-26-18/h2-7,14,16H,8-13,15H2,1H3,(H2,27,28,29,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084971
(CHEMBL3427399)Show SMILES Cc1cc(OC(F)(F)F)ccc1-c1c(Cc2ccc(\C=C\C(O)=O)cc2)c2ccc(O)cc2oc1=O |(2.93,3.7,;4,3.08,;5.33,3.85,;6.67,3.07,;8,3.84,;9.34,3.07,;10.4,3.68,;9.33,1.83,;10.4,2.45,;6.66,1.53,;5.33,.77,;4,1.54,;2.66,.77,;1.33,1.54,;1.33,3.08,;0,3.85,;0,5.39,;-1.33,6.16,;-2.66,5.39,;-4,6.16,;-5.33,5.39,;-6.67,6.16,;-6.67,7.39,;-7.74,5.54,;-2.67,3.85,;-1.33,3.08,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-3.75,-1.39,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C27H19F3O6/c1-15-12-19(36-27(28,29)30)8-10-20(15)25-22(21-9-7-18(31)14-23(21)35-26(25)34)13-17-4-2-16(3-5-17)6-11-24(32)33/h2-12,14,31H,13H2,1H3,(H,32,33)/b11-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50373856

(CHEMBL271705)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCOCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H24ClN7O3/c24-18-13-16(4-5-19(18)34-14-17-3-1-2-6-25-17)28-21-20-22(27-15-26-21)29-30-23(20)33-12-9-31-7-10-32-11-8-31/h1-6,13,15H,7-12,14H2,(H2,26,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7657
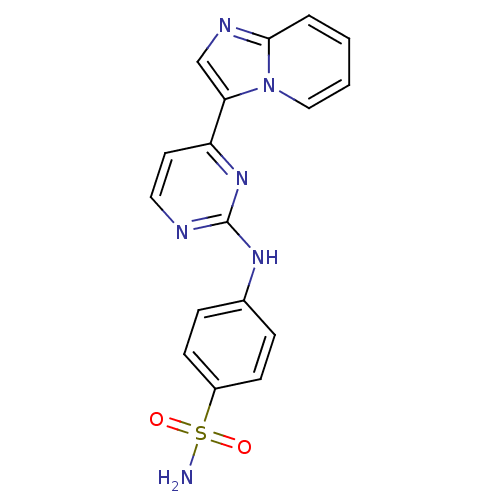
(4-[(4-{imidazo[1,2-a]pyridin-3-yl}pyrimidin-2-yl)a...)Show SMILES NS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C17H14N6O2S/c18-26(24,25)13-6-4-12(5-7-13)21-17-19-9-8-14(22-17)15-11-20-16-3-1-2-10-23(15)16/h1-11H,(H2,18,24,25)(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 21: 4698-701 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.093
BindingDB Entry DOI: 10.7270/Q2GF0TVK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245479
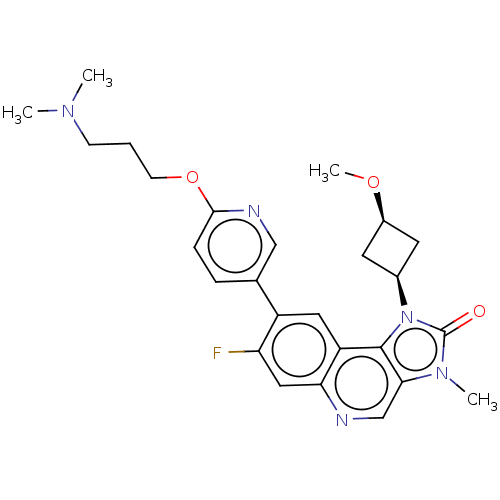
(US9428503, 6)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3cc(F)c(cc23)-c2ccc(OCCCN(C)C)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.02,4.9,;2.62,3.41,;3.71,2.32,;3.71,.78,;5.25,.78,;5.25,2.32,;6.34,-.31,;6.02,-1.82,;7.35,-2.59,;7.35,-4.13,;6.02,-4.9,;4.69,-4.13,;3.35,-4.9,;2.02,-4.13,;.68,-4.9,;2.02,-2.59,;3.35,-1.82,;4.69,-2.59,;.68,-1.82,;.68,-.28,;-.65,.49,;-1.98,-.28,;-3.32,.49,;-4.65,-.28,;-5.98,.49,;-7.32,-.28,;-8.65,.49,;-9.98,-.28,;-8.65,2.03,;-1.98,-1.82,;-.65,-2.59,;8.5,-1.56,;9.98,-1.95,;7.87,-.15,;8.64,1.19,)| Show InChI InChI=1S/C26H30FN5O3/c1-30(2)8-5-9-35-24-7-6-16(14-29-24)19-12-20-22(13-21(19)27)28-15-23-25(20)32(26(33)31(23)3)17-10-18(11-17)34-4/h6-7,12-15,17-18H,5,8-11H2,1-4H3/t17-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245519

(US9428503, 47)Show SMILES Cn1c2cnc3cc(F)c(cc3c2n([C@H]2CCCOC2)c1=O)-c1ccc(OCCCN2CCCCC2)nc1 |r| Show InChI InChI=1S/C29H34FN5O3/c1-33-26-18-31-25-16-24(30)22(15-23(25)28(26)35(29(33)36)21-7-5-13-37-19-21)20-8-9-27(32-17-20)38-14-6-12-34-10-3-2-4-11-34/h8-9,15-18,21H,2-7,10-14,19H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459007
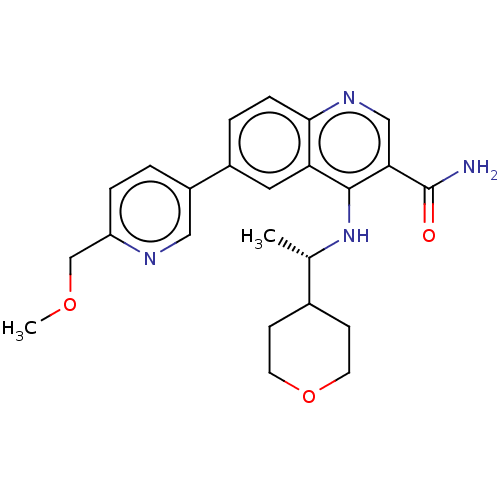
(CHEMBL4218734)Show SMILES COCc1ccc(cn1)-c1ccc2ncc(C(N)=O)c(N[C@@H](C)C3CCOCC3)c2c1 |r| Show InChI InChI=1S/C24H28N4O3/c1-15(16-7-9-31-10-8-16)28-23-20-11-17(18-3-5-19(14-30-2)26-12-18)4-6-22(20)27-13-21(23)24(25)29/h3-6,11-13,15-16H,7-10,14H2,1-2H3,(H2,25,29)(H,27,28)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... |
J Med Chem 59: 6281-92 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00519
BindingDB Entry DOI: 10.7270/Q2NV9NR8 |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245525
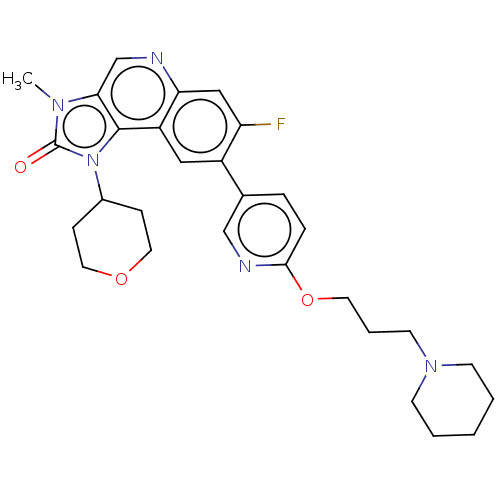
(US9428503, 53)Show SMILES Cn1c2cnc3cc(F)c(cc3c2n(C2CCOCC2)c1=O)-c1ccc(OCCCN2CCCCC2)nc1 Show InChI InChI=1S/C29H34FN5O3/c1-33-26-19-31-25-17-24(30)22(16-23(25)28(26)35(29(33)36)21-8-14-37-15-9-21)20-6-7-27(32-18-20)38-13-5-12-34-10-3-2-4-11-34/h6-7,16-19,21H,2-5,8-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070
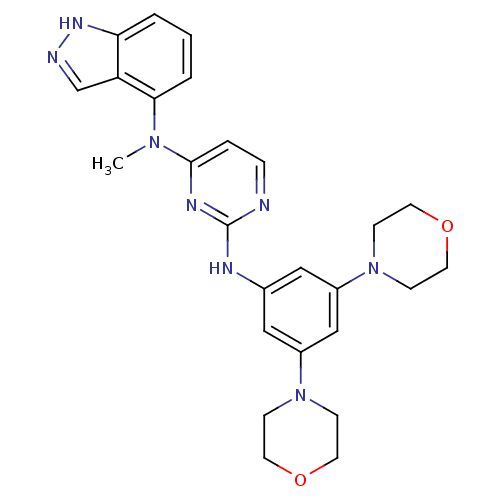
(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084986
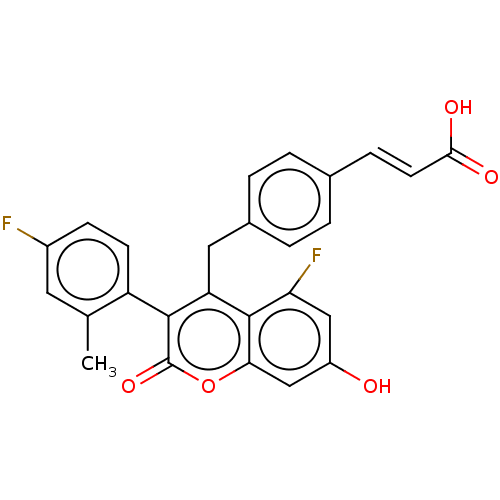
(CHEMBL3427405)Show SMILES Cc1cc(F)ccc1-c1c(Cc2ccc(\C=C\C(O)=O)cc2)c2c(F)cc(O)cc2oc1=O |(5.33,.46,;5.33,-.77,;6.66,-1.54,;6.66,-3.08,;7.73,-3.7,;5.33,-3.85,;3.99,-3.08,;4,-1.54,;2.66,-.77,;1.33,-1.54,;1.86,-2.98,;.87,-4.17,;1.4,-5.61,;.41,-6.79,;-1.1,-6.53,;-2.1,-7.71,;-3.61,-7.44,;-4.61,-8.62,;-4.19,-9.78,;-5.82,-8.4,;-1.63,-5.08,;-.64,-3.9,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-3.75,1.39,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.73,1.38,)| Show InChI InChI=1S/C26H18F2O5/c1-14-10-17(27)7-8-19(14)24-20(11-16-4-2-15(3-5-16)6-9-23(30)31)25-21(28)12-18(29)13-22(25)33-26(24)32/h2-10,12-13,29H,11H2,1H3,(H,30,31)/b9-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084970
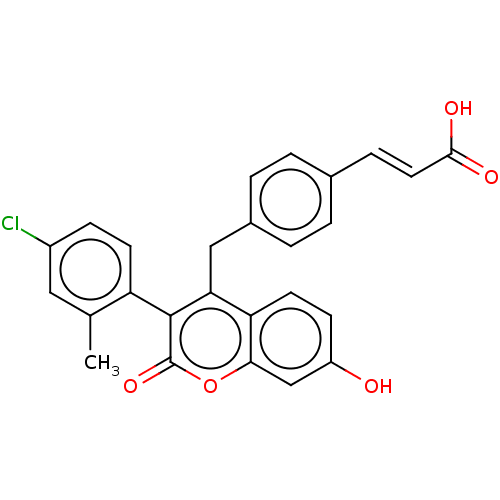
(CHEMBL3427398)Show SMILES Cc1cc(Cl)ccc1-c1c(Cc2ccc(\C=C\C(O)=O)cc2)c2ccc(O)cc2oc1=O |(5.33,-.46,;5.33,.77,;6.66,1.54,;6.66,3.08,;7.73,3.7,;5.33,3.85,;3.99,3.08,;4,1.54,;2.66,.77,;1.33,1.54,;1.33,3.08,;0,3.85,;0,5.39,;-1.33,6.16,;-2.66,5.39,;-4,6.16,;-5.33,5.39,;-6.67,6.16,;-6.67,7.39,;-7.74,5.54,;-2.67,3.85,;-1.33,3.08,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-3.75,-1.39,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C26H19ClO5/c1-15-12-18(27)7-9-20(15)25-22(21-10-8-19(28)14-23(21)32-26(25)31)13-17-4-2-16(3-5-17)6-11-24(29)30/h2-12,14,28H,13H2,1H3,(H,29,30)/b11-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245477
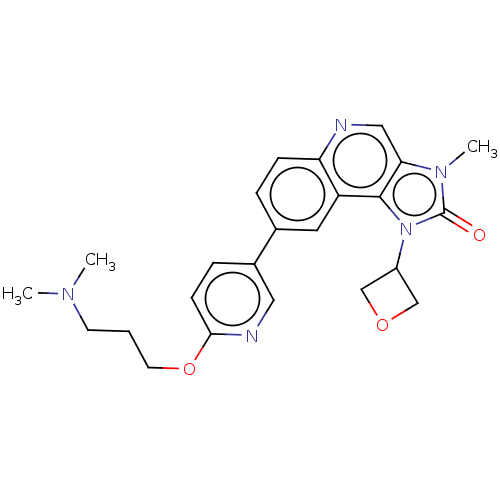
(US9428503, 4)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4COC4)c3c2c1 Show InChI InChI=1S/C24H27N5O3/c1-27(2)9-4-10-32-22-8-6-17(12-26-22)16-5-7-20-19(11-16)23-21(13-25-20)28(3)24(30)29(23)18-14-31-15-18/h5-8,11-13,18H,4,9-10,14-15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084965
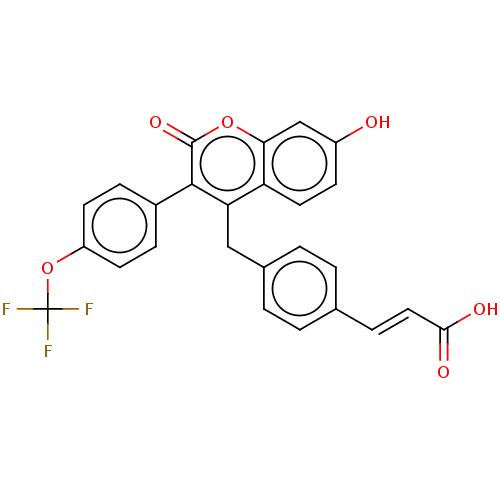
(CHEMBL3427393)Show SMILES OC(=O)\C=C\c1ccc(Cc2c(-c3ccc(OC(F)(F)F)cc3)c(=O)oc3cc(O)ccc23)cc1 Show InChI InChI=1S/C26H17F3O6/c27-26(28,29)35-19-9-6-17(7-10-19)24-21(20-11-8-18(30)14-22(20)34-25(24)33)13-16-3-1-15(2-4-16)5-12-23(31)32/h1-12,14,30H,13H2,(H,31,32)/b12-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50378199
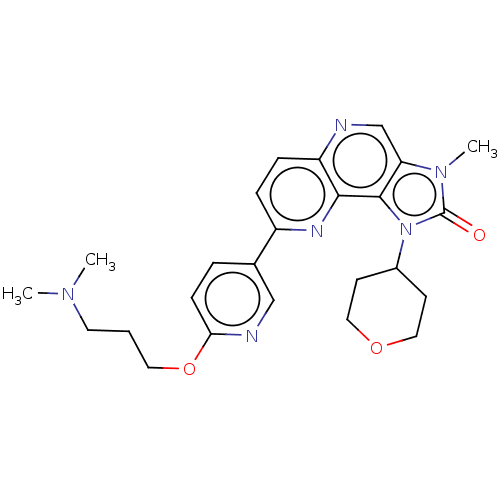
(CHEMBL4163629)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2n1 Show InChI InChI=1S/C25H30N6O3/c1-29(2)11-4-12-34-22-8-5-17(15-27-22)19-6-7-20-23(28-19)24-21(16-26-20)30(3)25(32)31(24)18-9-13-33-14-10-18/h5-8,15-16,18H,4,9-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340572
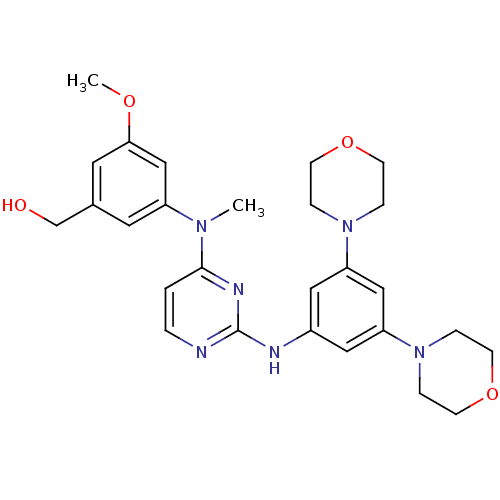
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES COc1cc(CO)cc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C27H34N6O4/c1-31(22-13-20(19-34)14-25(18-22)35-2)26-3-4-28-27(30-26)29-21-15-23(32-5-9-36-10-6-32)17-24(16-21)33-7-11-37-12-8-33/h3-4,13-18,34H,5-12,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371358
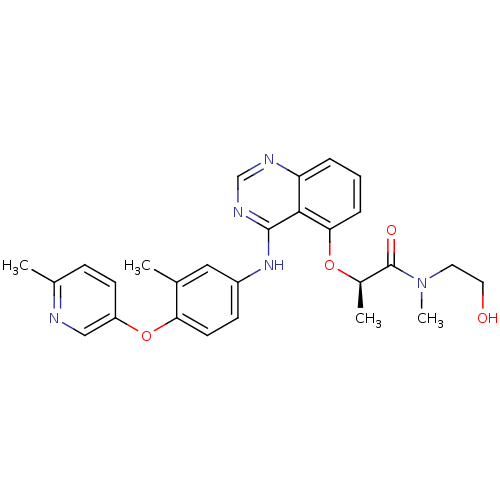
(CHEMBL257478)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N(C)CCO Show InChI InChI=1S/C27H29N5O4/c1-17-14-20(9-11-23(17)36-21-10-8-18(2)28-15-21)31-26-25-22(29-16-30-26)6-5-7-24(25)35-19(3)27(34)32(4)12-13-33/h5-11,14-16,19,33H,12-13H2,1-4H3,(H,29,30,31)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50208517
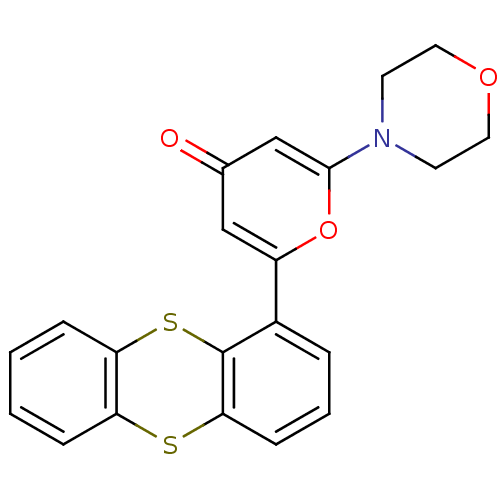
(2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...)Show SMILES O=c1cc(oc(c1)-c1cccc2Sc3ccccc3Sc12)N1CCOCC1 Show InChI InChI=1S/C21H17NO3S2/c23-14-12-16(25-20(13-14)22-8-10-24-11-9-22)15-4-3-7-19-21(15)27-18-6-2-1-5-17(18)26-19/h1-7,12-13H,8-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... |
J Med Chem 59: 6281-92 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00519
BindingDB Entry DOI: 10.7270/Q2NV9NR8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50535972
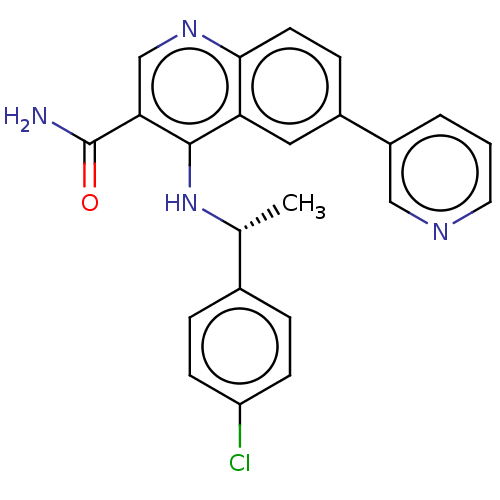
(CHEMBL4565988)Show SMILES C[C@@H](Nc1c(cnc2ccc(cc12)-c1cccnc1)C(N)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H19ClN4O/c1-14(15-4-7-18(24)8-5-15)28-22-19-11-16(17-3-2-10-26-12-17)6-9-21(19)27-13-20(22)23(25)29/h2-14H,1H3,(H2,25,29)(H,27,28)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... |
J Med Chem 59: 6281-92 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00519
BindingDB Entry DOI: 10.7270/Q2NV9NR8 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
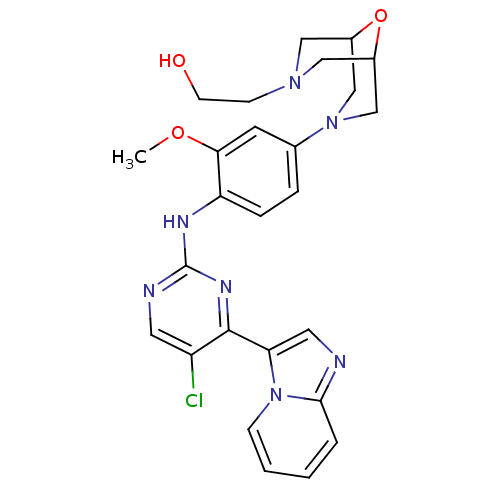
(Homo sapiens (Human)) | BDBM50349716
(CHEMBL1809059)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)N1CC2CN(CCO)CC(C1)O2 Show InChI InChI=1S/C26H28ClN7O3/c1-36-23-10-17(33-15-18-13-32(8-9-35)14-19(16-33)37-18)5-6-21(23)30-26-29-11-20(27)25(31-26)22-12-28-24-4-2-3-7-34(22)24/h2-7,10-12,18-19,35H,8-9,13-16H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of IGFR1 by cell based assay |
Bioorg Med Chem Lett 21: 4702-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.090
BindingDB Entry DOI: 10.7270/Q2QZ2B9Q |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340566

(CHEMBL1762535 | N2-(3,5-dimorpholinophenyl)-N4-(5-...)Show SMILES COc1cncc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H31N7O3/c1-30(22-16-23(33-2)18-26-17-22)24-3-4-27-25(29-24)28-19-13-20(31-5-9-34-10-6-31)15-21(14-19)32-7-11-35-12-8-32/h3-4,13-18H,5-12H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340570

((2-chloro-5-((2-(3,5-dimorpholinophenylamino)pyrim...)Show SMILES CN(c1ccc(Cl)c(CO)c1)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H31ClN6O3/c1-31(21-2-3-24(27)19(14-21)18-34)25-4-5-28-26(30-25)29-20-15-22(32-6-10-35-11-7-32)17-23(16-20)33-8-12-36-13-9-33/h2-5,14-17,34H,6-13,18H2,1H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373862

(CHEMBL402553)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H24ClN7O2/c24-18-13-16(6-7-19(18)33-14-17-5-1-2-8-25-17)28-21-20-22(27-15-26-21)29-30-23(20)32-12-11-31-9-3-4-10-31/h1-2,5-8,13,15H,3-4,9-12,14H2,(H2,26,27,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340576

(CHEMBL1762525 | N2-(3,5-dimorpholinophenyl)-N4-(3-...)Show SMILES COc1cccc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H32N6O3/c1-30(21-4-3-5-24(19-21)33-2)25-6-7-27-26(29-25)28-20-16-22(31-8-12-34-13-9-31)18-23(17-20)32-10-14-35-15-11-32/h3-7,16-19H,8-15H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
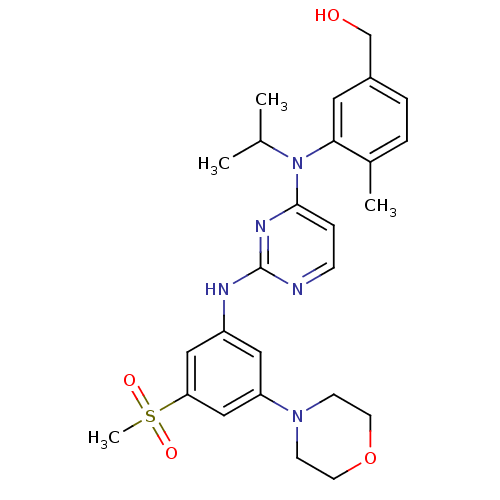
(Homo sapiens (Human)) | BDBM50340551
((3-(isopropyl(2-(3-(methylsulfonyl)-5-morpholinoph...)Show SMILES CC(C)N(c1ccnc(Nc2cc(cc(c2)S(C)(=O)=O)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C26H33N5O4S/c1-18(2)31(24-13-20(17-32)6-5-19(24)3)25-7-8-27-26(29-25)28-21-14-22(30-9-11-35-12-10-30)16-23(15-21)36(4,33)34/h5-8,13-16,18,32H,9-12,17H2,1-4H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084967
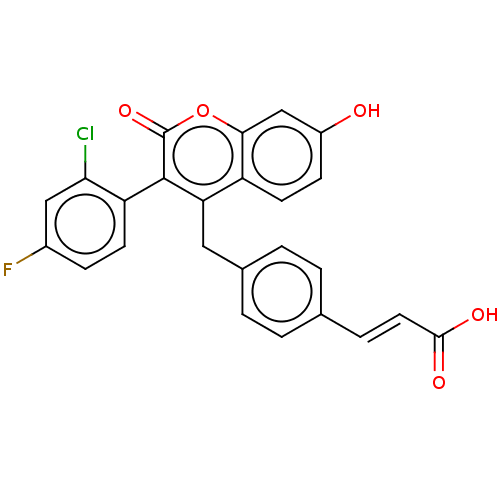
(CHEMBL3427395)Show SMILES OC(=O)\C=C\c1ccc(Cc2c(-c3ccc(F)cc3Cl)c(=O)oc3cc(O)ccc23)cc1 |(-6.67,7.39,;-6.67,6.16,;-7.74,5.54,;-5.33,5.39,;-4,6.16,;-2.66,5.39,;-1.33,6.16,;0,5.39,;0,3.85,;1.33,3.08,;1.33,1.54,;2.66,.77,;4,1.54,;3.99,3.08,;5.33,3.85,;6.66,3.08,;7.73,3.7,;6.66,1.54,;5.33,.77,;5.33,-.46,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-3.75,-1.39,;-2.68,.77,;-1.33,1.54,;,.77,;-1.33,3.08,;-2.67,3.85,)| Show InChI InChI=1S/C25H16ClFO5/c26-21-12-16(27)6-8-19(21)24-20(18-9-7-17(28)13-22(18)32-25(24)31)11-15-3-1-14(2-4-15)5-10-23(29)30/h1-10,12-13,28H,11H2,(H,29,30)/b10-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245492
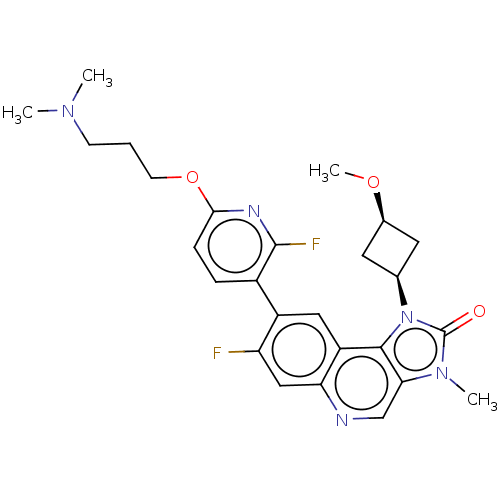
(US9428503, 19)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3cc(F)c(cc23)-c2ccc(OCCCN(C)C)nc2F)n(C)c1=O |r,wD:4.6,2.1,(3.02,4.9,;2.62,3.41,;3.71,2.32,;3.71,.78,;5.25,.78,;5.25,2.32,;6.34,-.31,;6.02,-1.82,;7.35,-2.59,;7.35,-4.13,;6.02,-4.9,;4.69,-4.13,;3.35,-4.9,;2.02,-4.13,;.68,-4.9,;2.02,-2.59,;3.35,-1.82,;4.69,-2.59,;.68,-1.82,;.68,-.28,;-.65,.49,;-1.98,-.28,;-3.32,.49,;-4.65,-.28,;-5.98,.49,;-7.32,-.28,;-8.65,.49,;-9.98,-.28,;-8.65,2.03,;-1.98,-1.82,;-.65,-2.59,;-.65,-4.13,;8.5,-1.56,;9.98,-1.95,;7.87,-.15,;8.64,1.19,)| Show InChI InChI=1S/C26H29F2N5O3/c1-31(2)8-5-9-36-23-7-6-17(25(28)30-23)18-12-19-21(13-20(18)27)29-14-22-24(19)33(26(34)32(22)3)15-10-16(11-15)35-4/h6-7,12-16H,5,8-11H2,1-4H3/t15-,16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329080

(CHEMBL1270378 | N2-(2,6-dimorpholinopyrimidin-4-yl...)Show SMILES CN(c1ccnc(Nc2cc(nc(n2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C24H28N10O2/c1-32(19-4-2-3-18-17(19)16-26-31-18)21-5-6-25-23(29-21)27-20-15-22(33-7-11-35-12-8-33)30-24(28-20)34-9-13-36-14-10-34/h2-6,15-16H,7-14H2,1H3,(H,26,31)(H,25,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084974

(CHEMBL3427402)Show SMILES Cc1cc(OC(F)(F)F)ccc1-c1c(Oc2ccc(\C=C\C(O)=O)cc2)c2ccc(O)cc2oc1=O |(5.33,.26,;5.33,-.77,;6.66,-1.54,;6.66,-3.08,;8,-3.85,;9.33,-3.08,;9.33,-2.06,;10.4,-2.47,;10.22,-3.6,;5.33,-3.85,;3.99,-3.08,;4,-1.54,;2.66,-.77,;1.33,-1.54,;1.33,-3.08,;0,-3.85,;0,-5.39,;-1.33,-6.16,;-2.66,-5.39,;-4,-6.16,;-5.33,-5.39,;-6.67,-6.16,;-6.67,-7.39,;-7.74,-5.54,;-2.67,-3.85,;-1.33,-3.08,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-3.75,1.39,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.73,1.38,)| Show InChI InChI=1S/C26H17F3O7/c1-14-12-18(36-26(27,28)29)8-10-19(14)23-24(20-9-5-16(30)13-21(20)35-25(23)33)34-17-6-2-15(3-7-17)4-11-22(31)32/h2-13,30H,1H3,(H,31,32)/b11-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Suppression of ER alpha in human MCF7 cells after 24 hrs by immunostaining analysis |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084966
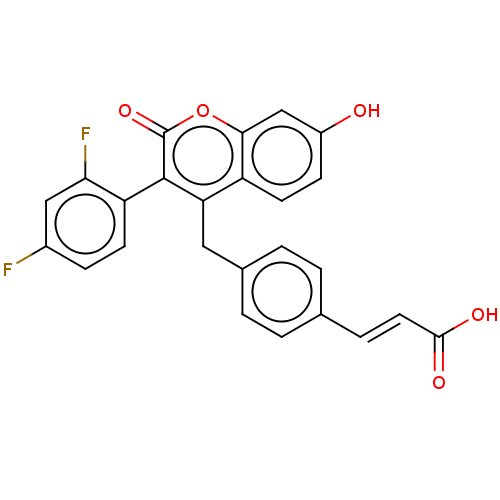
(CHEMBL3427394)Show SMILES OC(=O)\C=C\c1ccc(Cc2c(-c3ccc(F)cc3F)c(=O)oc3cc(O)ccc23)cc1 |(-6.67,7.39,;-6.67,6.16,;-7.74,5.54,;-5.33,5.39,;-4,6.16,;-2.66,5.39,;-1.33,6.16,;0,5.39,;0,3.85,;1.33,3.08,;1.33,1.54,;2.66,.77,;4,1.54,;3.99,3.08,;5.33,3.85,;6.66,3.08,;7.73,3.7,;6.66,1.54,;5.33,.77,;5.33,-.46,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-3.75,-1.39,;-2.68,.77,;-1.33,1.54,;,.77,;-1.33,3.08,;-2.67,3.85,)| Show InChI InChI=1S/C25H16F2O5/c26-16-6-8-19(21(27)12-16)24-20(18-9-7-17(28)13-22(18)32-25(24)31)11-15-3-1-14(2-4-15)5-10-23(29)30/h1-10,12-13,28H,11H2,(H,29,30)/b10-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay |
J Med Chem 58: 3522-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00066
BindingDB Entry DOI: 10.7270/Q2M32XGF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373864

(CHEMBL402339)Show SMILES COc1cc(Nc2ncnc3n[nH]c(OCCN4CCC(O)CC4)c23)ccc1OCc1cccc(F)c1 Show InChI InChI=1S/C26H29FN6O4/c1-35-22-14-19(5-6-21(22)37-15-17-3-2-4-18(27)13-17)30-24-23-25(29-16-28-24)31-32-26(23)36-12-11-33-9-7-20(34)8-10-33/h2-6,13-14,16,20,34H,7-12,15H2,1H3,(H2,28,29,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data