

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50225280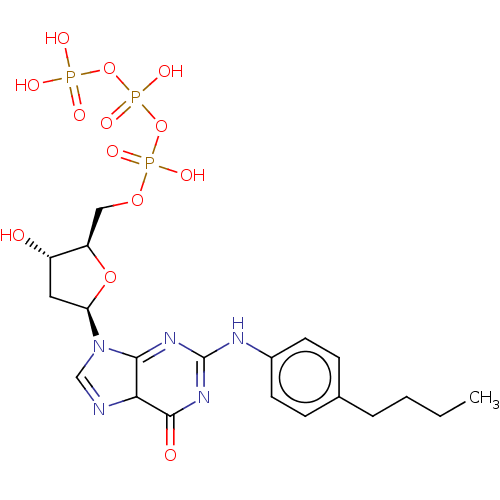 (CHEMBL3144215) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025592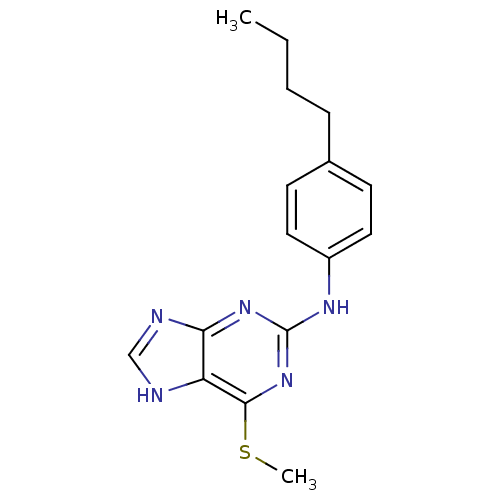 ((4-Butyl-phenyl)-(6-methylsulfanyl-9H-purin-2-yl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025600 ((4-Butyl-phenyl)-(6-methoxy-9H-purin-2-yl)-amine |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025598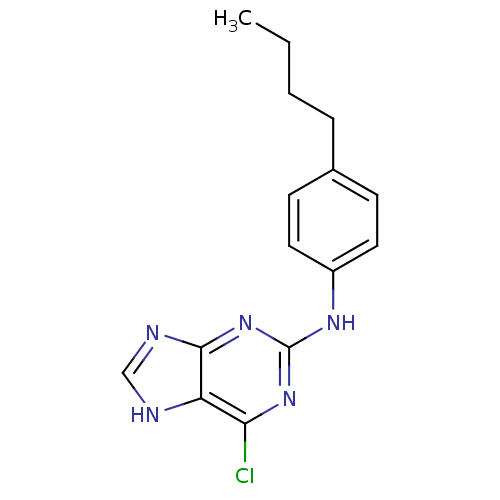 ((4-Butyl-phenyl)-(6-chloro-9H-purin-2-yl)-amine | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025597 ((4-Butyl-phenyl)-(9H-purin-2-yl)-amine | CHEMBL278...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50013074 (2-(4-Butyl-phenylamino)-1,9-dihydro-purin-6-one | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50013074 (2-(4-Butyl-phenylamino)-1,9-dihydro-purin-6-one | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025593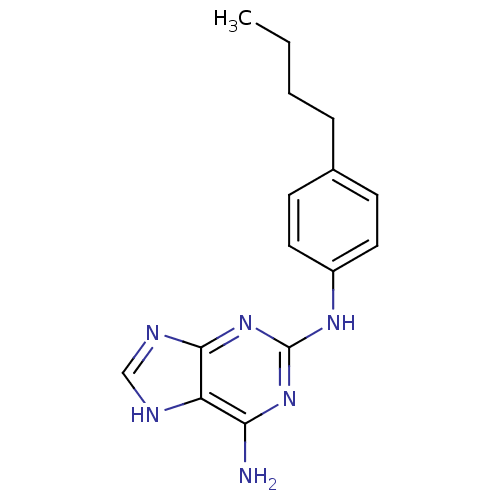 (CHEMBL16122 | N*2*-(4-Butyl-phenyl)-9H-purine-2,6-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50405103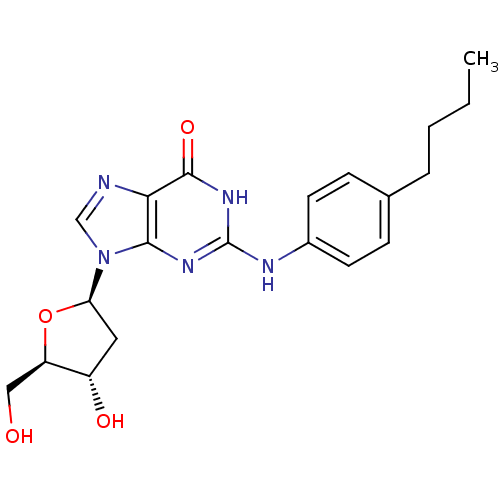 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025595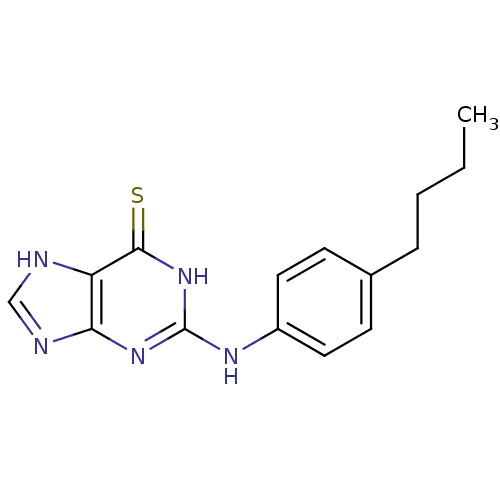 (2-(4-Butyl-phenylamino)-1,9-dihydro-purine-6-thion...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50405103 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50405103 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50013075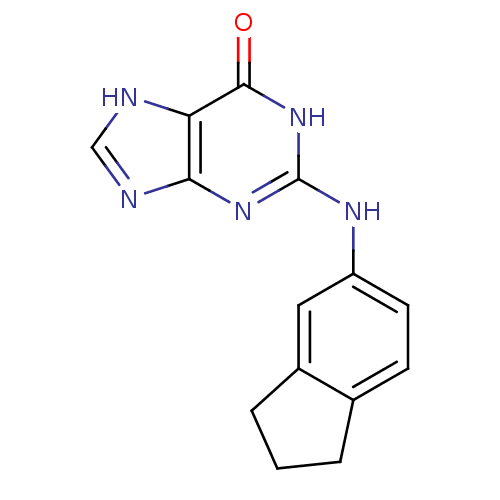 (2-(Indan-5-ylamino)-1,9-dihydro-purin-6-one | CHEM...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50013093 (2-(3-Ethyl-4-methyl-phenylamino)-1,9-dihydro-purin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.76E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||