 Found 485 hits with Last Name = 'dvorak' and Initial = 'l'
Found 485 hits with Last Name = 'dvorak' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346209

(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371662
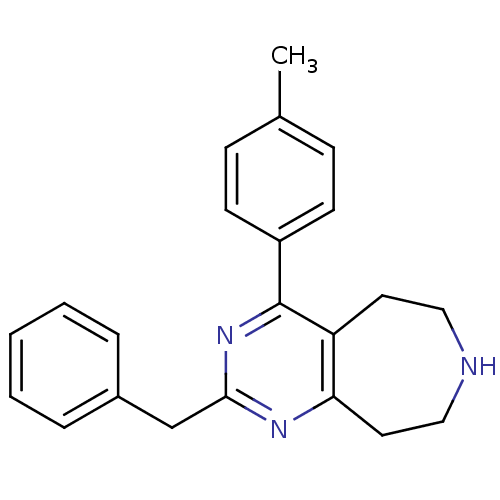
(CHEMBL269974)Show InChI InChI=1S/C22H23N3/c1-16-7-9-18(10-8-16)22-19-11-13-23-14-12-20(19)24-21(25-22)15-17-5-3-2-4-6-17/h2-10,23H,11-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371305
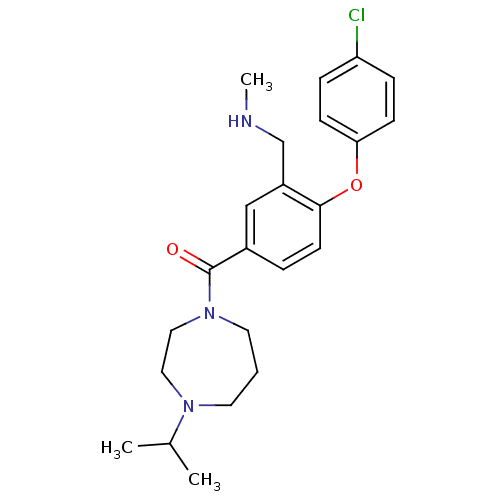
(CHEMBL272077)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-11-4-12-27(14-13-26)23(28)18-5-10-22(19(15-18)16-25-3)29-21-8-6-20(24)7-9-21/h5-10,15,17,25H,4,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200636
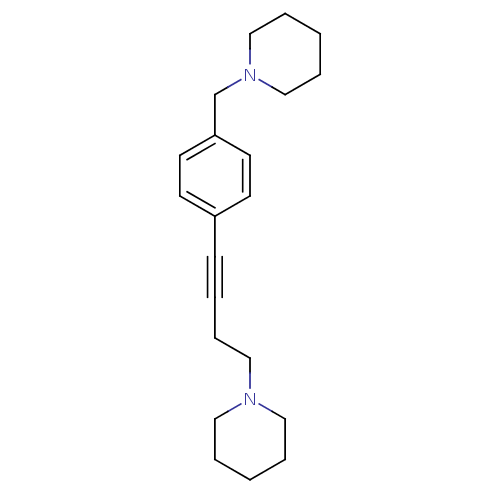
(1-(4-(4-(piperidin-1-ylmethyl)phenyl)but-3-ynyl)pi...)Show InChI InChI=1S/C21H30N2/c1-4-14-22(15-5-1)16-8-3-9-20-10-12-21(13-11-20)19-23-17-6-2-7-18-23/h10-13H,1-2,4-8,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414797
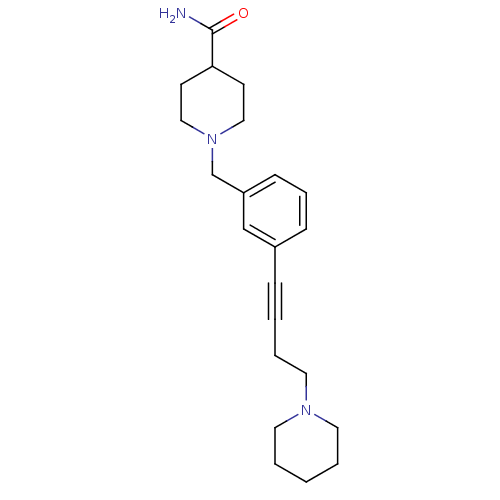
(CHEMBL582977)Show InChI InChI=1S/C22H31N3O/c23-22(26)21-10-15-25(16-11-21)18-20-9-6-8-19(17-20)7-2-5-14-24-12-3-1-4-13-24/h6,8-9,17,21H,1,3-5,10-16,18H2,(H2,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371682
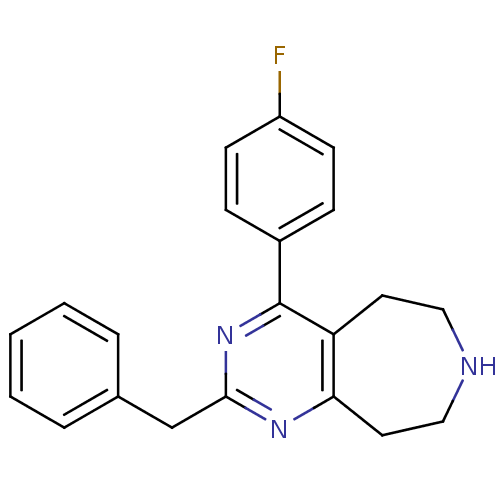
(CHEMBL270188)Show InChI InChI=1S/C21H20FN3/c22-17-8-6-16(7-9-17)21-18-10-12-23-13-11-19(18)24-20(25-21)14-15-4-2-1-3-5-15/h1-9,23H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414804
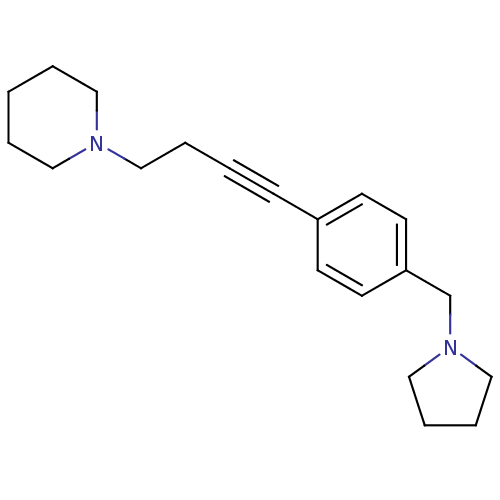
(CHEMBL574712)Show InChI InChI=1S/C20H28N2/c1-3-13-21(14-4-1)15-5-2-8-19-9-11-20(12-10-19)18-22-16-6-7-17-22/h9-12H,1,3-7,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414811
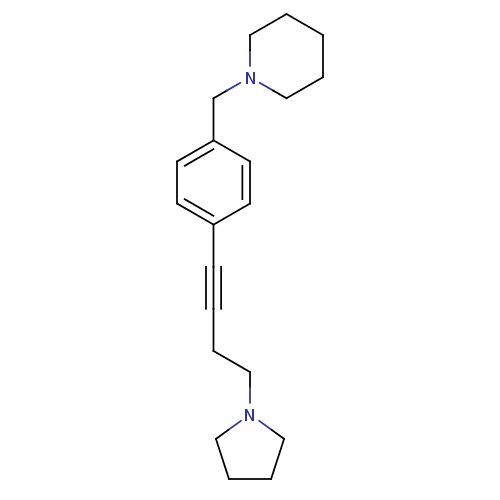
(CHEMBL574721)Show InChI InChI=1S/C20H28N2/c1-3-16-22(17-4-1)18-20-11-9-19(10-12-20)8-2-5-13-21-14-6-7-15-21/h9-12H,1,3-7,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371669
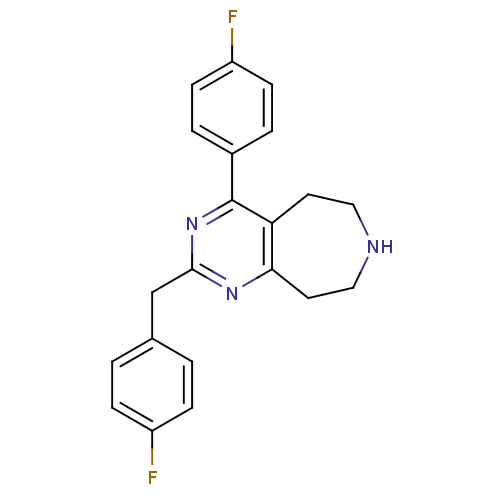
(CHEMBL402164)Show InChI InChI=1S/C21H19F2N3/c22-16-5-1-14(2-6-16)13-20-25-19-10-12-24-11-9-18(19)21(26-20)15-3-7-17(23)8-4-15/h1-8,24H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346205
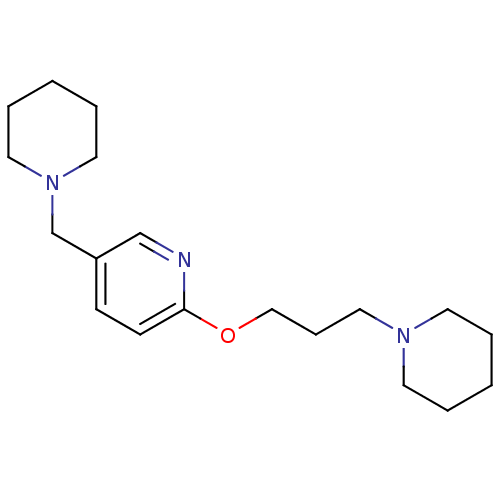
(5-Piperidin-1-ylmethyl-2-(3-piperidin-1-yl-propoxy...)Show InChI InChI=1S/C19H31N3O/c1-3-10-21(11-4-1)14-7-15-23-19-9-8-18(16-20-19)17-22-12-5-2-6-13-22/h8-9,16H,1-7,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217589
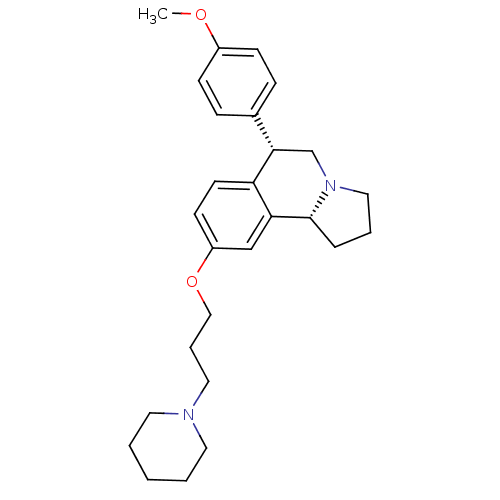
((6S,10bR)-6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)[C@@H]1CN2CCC[C@@H]2c2cc(OCCCN3CCCCC3)ccc12 Show InChI InChI=1S/C27H36N2O2/c1-30-22-10-8-21(9-11-22)26-20-29-17-5-7-27(29)25-19-23(12-13-24(25)26)31-18-6-16-28-14-3-2-4-15-28/h8-13,19,26-27H,2-7,14-18,20H2,1H3/t26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371294
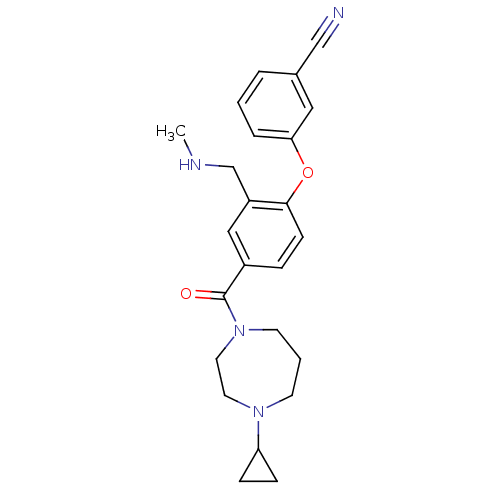
(CHEMBL257208)Show SMILES CNCc1cc(ccc1Oc1cccc(c1)C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-19(6-9-23(20)30-22-5-2-4-18(14-22)16-25)24(29)28-11-3-10-27(12-13-28)21-7-8-21/h2,4-6,9,14-15,21,26H,3,7-8,10-13,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414798
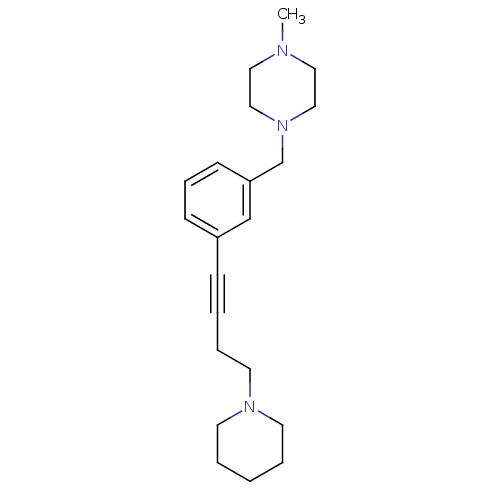
(CHEMBL575172)Show InChI InChI=1S/C21H31N3/c1-22-14-16-24(17-15-22)19-21-10-7-9-20(18-21)8-3-6-13-23-11-4-2-5-12-23/h7,9-10,18H,2,4-6,11-17,19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414800
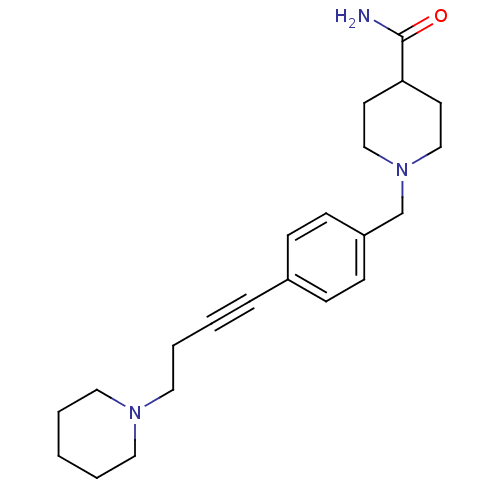
(CHEMBL583182)Show InChI InChI=1S/C22H31N3O/c23-22(26)21-11-16-25(17-12-21)18-20-9-7-19(8-10-20)6-2-5-15-24-13-3-1-4-14-24/h7-10,21H,1,3-5,11-18H2,(H2,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414792
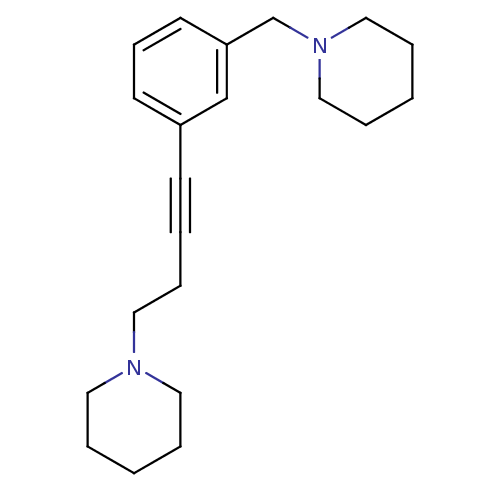
(CHEMBL573817)Show InChI InChI=1S/C21H30N2/c1-4-13-22(14-5-1)15-8-3-10-20-11-9-12-21(18-20)19-23-16-6-2-7-17-23/h9,11-12,18H,1-2,4-8,13-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371681
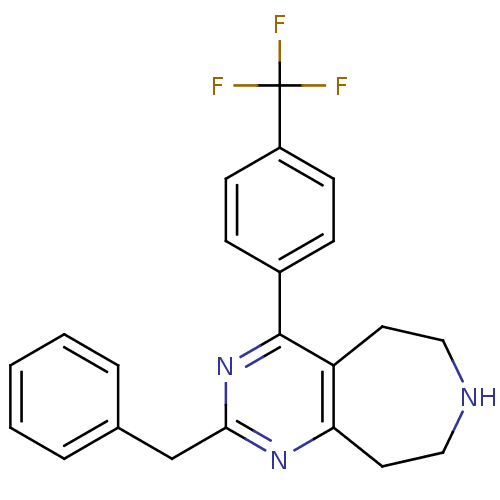
(CHEMBL271418)Show SMILES FC(F)(F)c1ccc(cc1)-c1nc(Cc2ccccc2)nc2CCNCCc12 Show InChI InChI=1S/C22H20F3N3/c23-22(24,25)17-8-6-16(7-9-17)21-18-10-12-26-13-11-19(18)27-20(28-21)14-15-4-2-1-3-5-15/h1-9,26H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346208
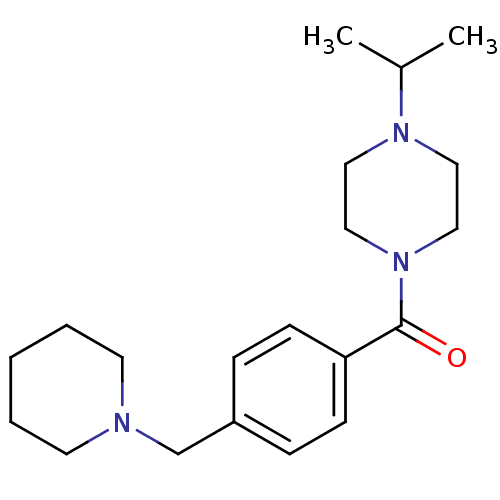
((1-isopropylpiperidin-4-yl)(4-(piperidin-1-ylmethy...)Show InChI InChI=1S/C20H31N3O/c1-17(2)22-12-14-23(15-13-22)20(24)19-8-6-18(7-9-19)16-21-10-4-3-5-11-21/h6-9,17H,3-5,10-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371290
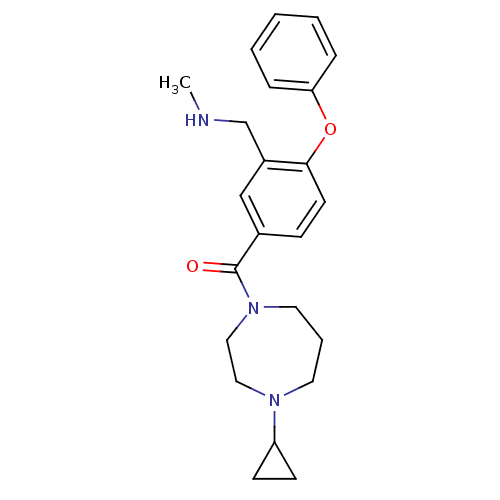
(CHEMBL401683)Show SMILES CNCc1cc(ccc1Oc1ccccc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H29N3O2/c1-24-17-19-16-18(8-11-22(19)28-21-6-3-2-4-7-21)23(27)26-13-5-12-25(14-15-26)20-9-10-20/h2-4,6-8,11,16,20,24H,5,9-10,12-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371289
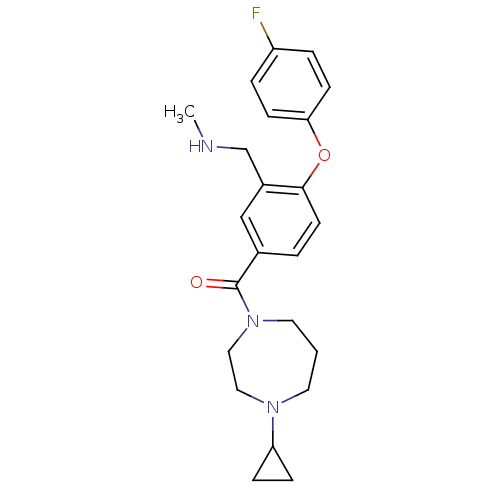
(CHEMBL258349)Show SMILES CNCc1cc(ccc1Oc1ccc(F)cc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-15-17(3-10-22(18)29-21-8-4-19(24)5-9-21)23(28)27-12-2-11-26(13-14-27)20-6-7-20/h3-5,8-10,15,20,25H,2,6-7,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217576

(CHEMBL236046 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2O2S/c1-24-18-20-17-19(5-3-4-12-25-13-15-26-16-14-25)6-11-23(20)27-21-7-9-22(28-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human SERT |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414813

(CHEMBL573328)Show InChI InChI=1S/C23H35N3/c1-21(2)26-18-16-25(17-19-26)20-23-11-9-22(10-12-23)8-4-7-15-24-13-5-3-6-14-24/h9-12,21H,3,5-7,13-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414799

(CHEMBL573815)Show InChI InChI=1S/C21H30N2O/c24-21-11-16-23(17-12-21)18-20-9-7-19(8-10-20)6-2-5-15-22-13-3-1-4-14-22/h7-10,21,24H,1,3-5,11-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371287
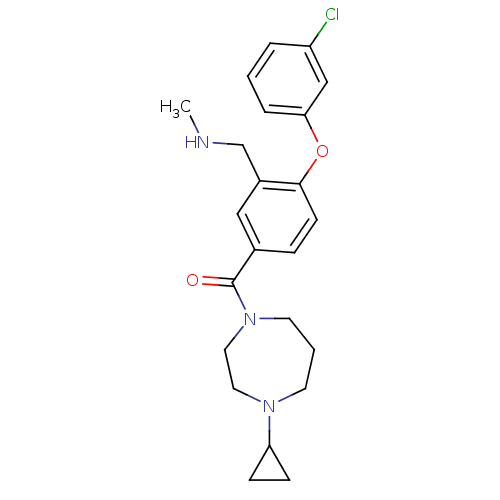
(CHEMBL272699)Show SMILES CNCc1cc(ccc1Oc1cccc(Cl)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28ClN3O2/c1-25-16-18-14-17(6-9-22(18)29-21-5-2-4-19(24)15-21)23(28)27-11-3-10-26(12-13-27)20-7-8-20/h2,4-6,9,14-15,20,25H,3,7-8,10-13,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346207
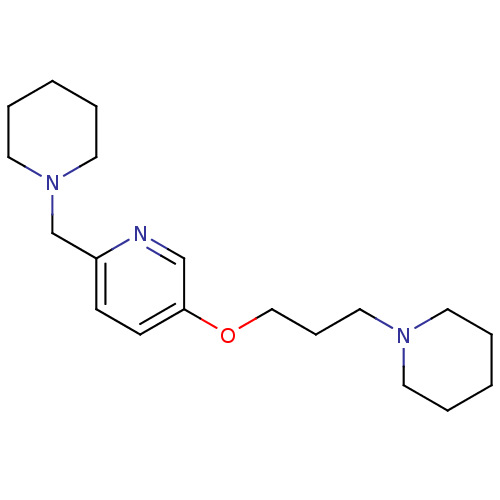
(2-Piperdin-1-ylmethyl-5-(3-piperdin-1-yl-propoxy)-...)Show InChI InChI=1S/C19H31N3O/c1-3-10-21(11-4-1)14-7-15-23-19-9-8-18(20-16-19)17-22-12-5-2-6-13-22/h8-9,16H,1-7,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414806
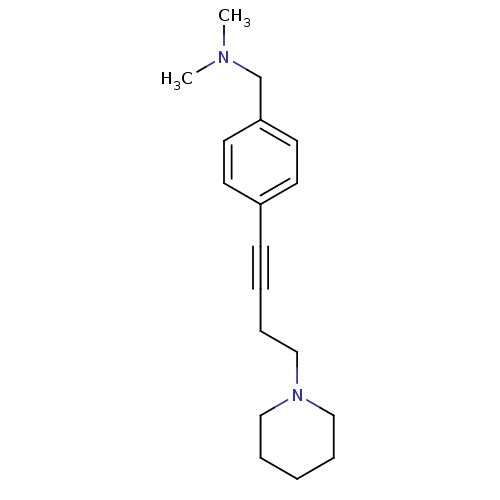
(CHEMBL572845)Show InChI InChI=1S/C18H26N2/c1-19(2)16-18-11-9-17(10-12-18)8-4-7-15-20-13-5-3-6-14-20/h9-12H,3,5-7,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414803
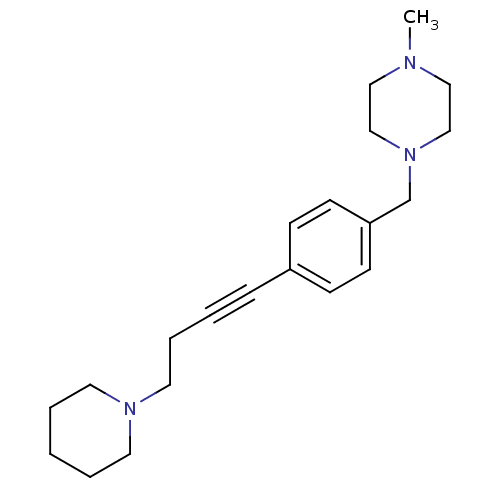
(CHEMBL573321)Show InChI InChI=1S/C21H31N3/c1-22-15-17-24(18-16-22)19-21-10-8-20(9-11-21)7-3-6-14-23-12-4-2-5-13-23/h8-11H,2,4-6,12-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371314

(CHEMBL402949)Show SMILES CNCc1cc(ccc1Oc1ccccc1F)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-15-17(7-10-21(18)29-22-6-3-2-5-20(22)24)23(28)27-12-4-11-26(13-14-27)19-8-9-19/h2-3,5-7,10,15,19,25H,4,8-9,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50548822
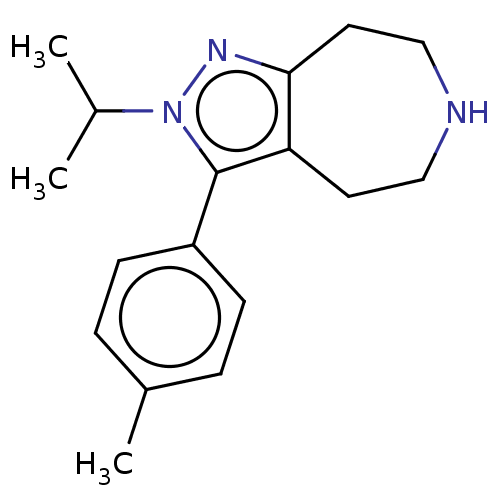
(CHEMBL4746737) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-ketanserin from human 5-HT2A receptor by competitive radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127669
BindingDB Entry DOI: 10.7270/Q2VX0M4X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371315

(CHEMBL273136)Show SMILES CNCc1cc(ccc1Oc1cccc(OC)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H31N3O3/c1-25-17-19-15-18(7-10-23(19)30-22-6-3-5-21(16-22)29-2)24(28)27-12-4-11-26(13-14-27)20-8-9-20/h3,5-7,10,15-16,20,25H,4,8-9,11-14,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50371670
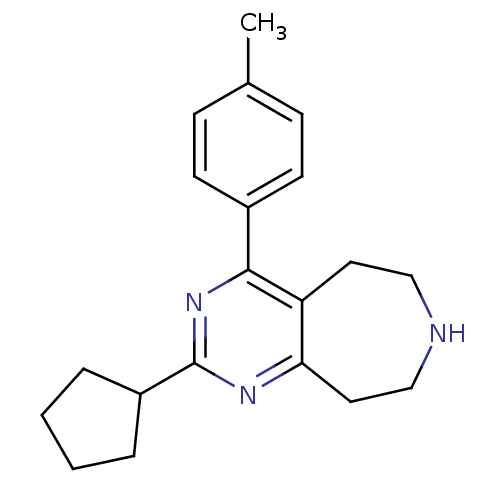
(CHEMBL272082)Show InChI InChI=1S/C20H25N3/c1-14-6-8-15(9-7-14)19-17-10-12-21-13-11-18(17)22-20(23-19)16-4-2-3-5-16/h6-9,16,21H,2-5,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5HT2B receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50371281
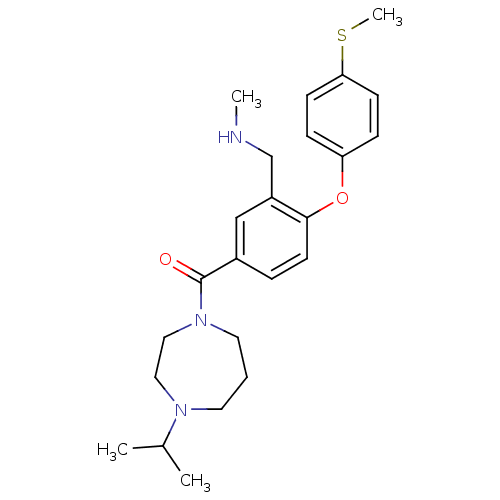
(CHEMBL267267)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C24H33N3O2S/c1-18(2)26-12-5-13-27(15-14-26)24(28)19-6-11-23(20(16-19)17-25-3)29-21-7-9-22(30-4)10-8-21/h6-11,16,18,25H,5,12-15,17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of rat SERT |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371280
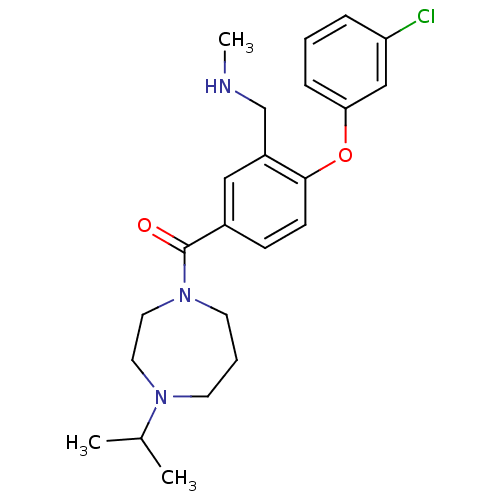
(CHEMBL272289)Show SMILES CNCc1cc(ccc1Oc1cccc(Cl)c1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-10-5-11-27(13-12-26)23(28)18-8-9-22(19(14-18)16-25-3)29-21-7-4-6-20(24)15-21/h4,6-9,14-15,17,25H,5,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371286
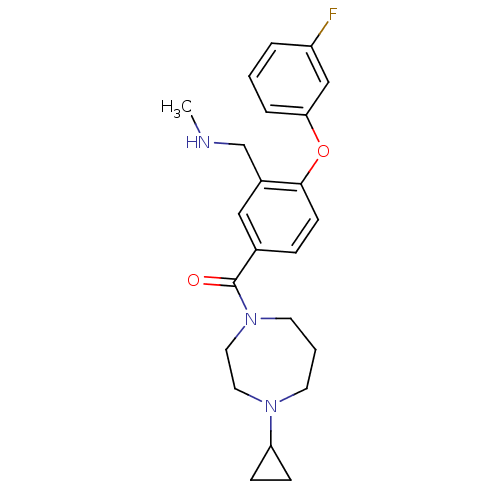
(CHEMBL401867)Show SMILES CNCc1cc(ccc1Oc1cccc(F)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-14-17(6-9-22(18)29-21-5-2-4-19(24)15-21)23(28)27-11-3-10-26(12-13-27)20-7-8-20/h2,4-6,9,14-15,20,25H,3,7-8,10-13,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50255823

((2-((1-isopropylpiperidin-4-yl)methoxy)-1-methyl-1...)Show InChI InChI=1S/C20H27N3O2/c1-15(2)23-11-9-16(10-12-23)14-25-20-21-13-18(22(20)3)19(24)17-7-5-4-6-8-17/h4-8,13,15-16H,9-12,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 19: 903-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.114
BindingDB Entry DOI: 10.7270/Q2X63MTD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371291

(CHEMBL257009)Show SMILES CNCc1cc(ccc1Oc1ccccc1C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-18(7-10-23(20)30-22-6-3-2-5-19(22)16-25)24(29)28-12-4-11-27(13-14-28)21-8-9-21/h2-3,5-7,10,15,21,26H,4,8-9,11-14,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50548821
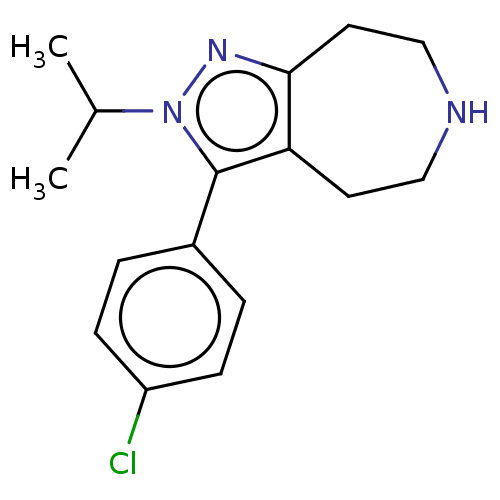
(CHEMBL4784845) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-ketanserin from human 5-HT2A receptor by competitive radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127669
BindingDB Entry DOI: 10.7270/Q2VX0M4X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217569
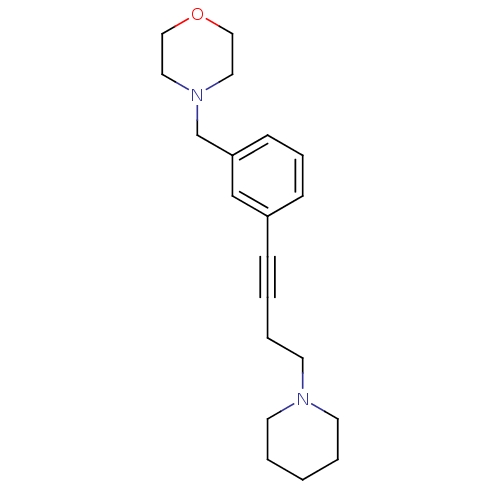
(4-(3-(4-(piperidin-1-yl)but-1-ynyl)benzyl)morpholi...)Show InChI InChI=1S/C20H28N2O/c1-3-10-21(11-4-1)12-5-2-7-19-8-6-9-20(17-19)18-22-13-15-23-16-14-22/h6,8-9,17H,1,3-5,10-16,18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50548821

(CHEMBL4784845) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-ketanserin from human 5-HT2B receptor by competitive radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127669
BindingDB Entry DOI: 10.7270/Q2VX0M4X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50548831
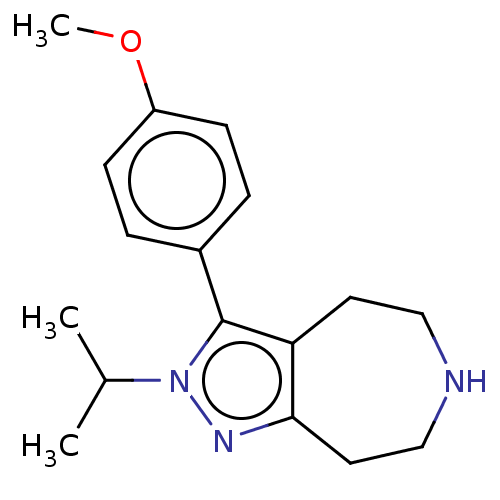
(CHEMBL4752099) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-ketanserin from human 5-HT2B receptor by competitive radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127669
BindingDB Entry DOI: 10.7270/Q2VX0M4X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT7 receptor |
Bioorg Med Chem Lett 21: 42-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.078
BindingDB Entry DOI: 10.7270/Q2M908Z5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50548818

(CHEMBL4782005) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-ketanserin from human 5-HT2A receptor by competitive radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127669
BindingDB Entry DOI: 10.7270/Q2VX0M4X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50255824
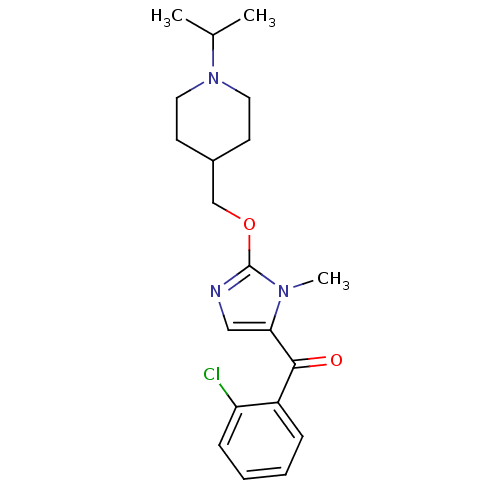
((2-chlorophenyl)(2-((1-isopropylpiperidin-4-yl)met...)Show InChI InChI=1S/C20H26ClN3O2/c1-14(2)24-10-8-15(9-11-24)13-26-20-22-12-18(23(20)3)19(25)16-6-4-5-7-17(16)21/h4-7,12,14-15H,8-11,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 19: 903-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.114
BindingDB Entry DOI: 10.7270/Q2X63MTD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371297
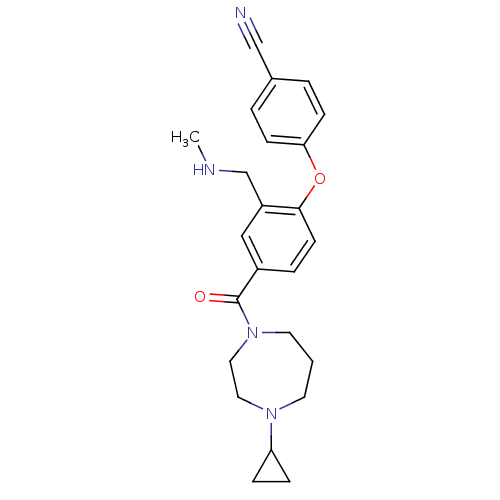
(CHEMBL404572)Show SMILES CNCc1cc(ccc1Oc1ccc(cc1)C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-19(5-10-23(20)30-22-8-3-18(16-25)4-9-22)24(29)28-12-2-11-27(13-14-28)21-6-7-21/h3-5,8-10,15,21,26H,2,6-7,11-14,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371281

(CHEMBL267267)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C24H33N3O2S/c1-18(2)26-12-5-13-27(15-14-26)24(28)19-6-11-23(20(16-19)17-25-3)29-21-7-9-22(30-4)10-8-21/h6-11,16,18,25H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371311
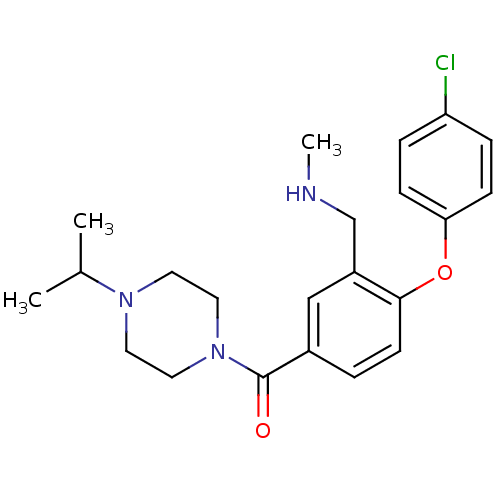
(CHEMBL256044)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCN(CC1)C(C)C Show InChI InChI=1S/C22H28ClN3O2/c1-16(2)25-10-12-26(13-11-25)22(27)17-4-9-21(18(14-17)15-24-3)28-20-7-5-19(23)6-8-20/h4-9,14,16,24H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371284
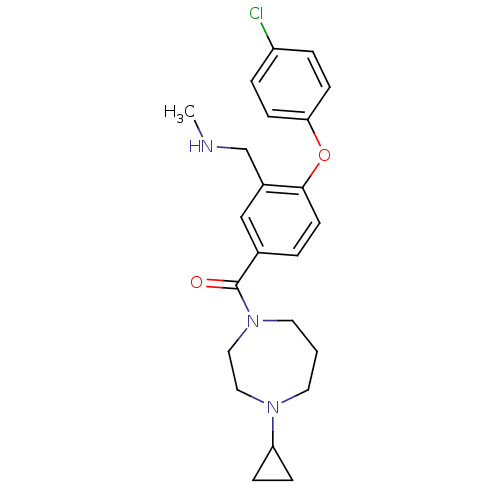
(CHEMBL257909)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28ClN3O2/c1-25-16-18-15-17(3-10-22(18)29-21-8-4-19(24)5-9-21)23(28)27-12-2-11-26(13-14-27)20-6-7-20/h3-5,8-10,15,20,25H,2,6-7,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346199

((4-Isopropyl-piperazin-1-yl)-(5-piperidin-1-ylmeth...)Show InChI InChI=1S/C18H29N5O/c1-15(2)22-8-10-23(11-9-22)18(24)17-13-19-16(12-20-17)14-21-6-4-3-5-7-21/h12-13,15H,3-11,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data