 Found 183 hits with Last Name = 'earl' and Initial = 'ra'
Found 183 hits with Last Name = 'earl' and Initial = 'ra' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50041291
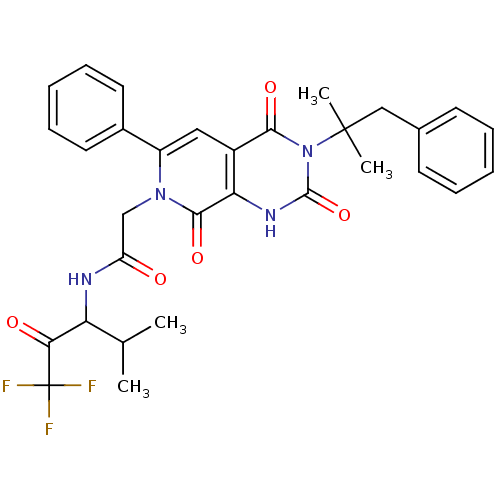
(2-[(R)-3-(1,1-Dimethyl-2-phenyl-ethyl)-2,4,8-triox...)Show SMILES CC(C)C(NC(=O)Cn1c(cc2c([nH]c(=O)n(c2=O)C(C)(C)Cc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C31H31F3N4O5/c1-18(2)24(26(40)31(32,33)34)35-23(39)17-37-22(20-13-9-6-10-14-20)15-21-25(28(37)42)36-29(43)38(27(21)41)30(3,4)16-19-11-7-5-8-12-19/h5-15,18,24H,16-17H2,1-4H3,(H,35,39)(H,36,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037348
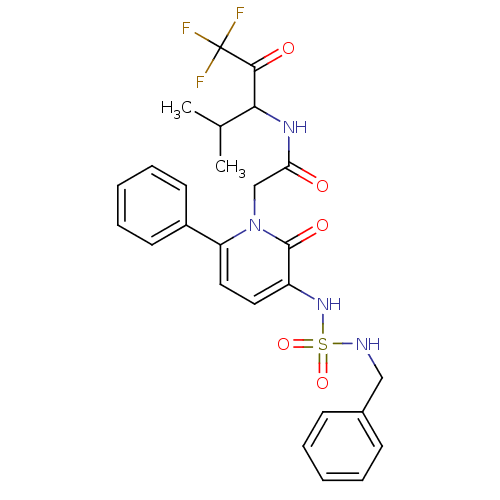
(1N-(3,3,3-trifluoro-1-isopropyl-2-oxopropyl)-2-(3-...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NS(=O)(=O)NCc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H27F3N4O5S/c1-17(2)23(24(35)26(27,28)29)31-22(34)16-33-21(19-11-7-4-8-12-19)14-13-20(25(33)36)32-39(37,38)30-15-18-9-5-3-6-10-18/h3-14,17,23,30,32H,15-16H2,1-2H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036127
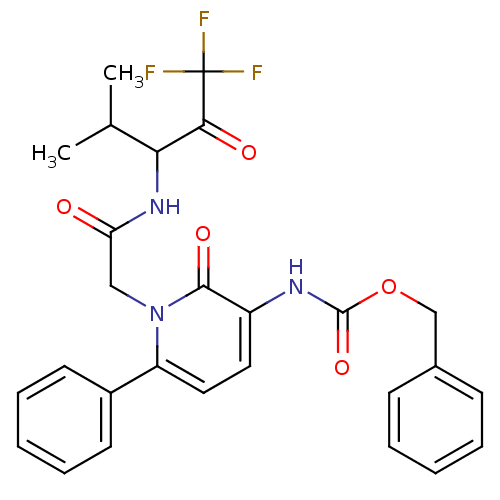
(CHEMBL11391 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro-...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NC(=O)OCc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C27H26F3N3O5/c1-17(2)23(24(35)27(28,29)30)32-22(34)15-33-21(19-11-7-4-8-12-19)14-13-20(25(33)36)31-26(37)38-16-18-9-5-3-6-10-18/h3-14,17,23H,15-16H2,1-2H3,(H,31,37)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037341
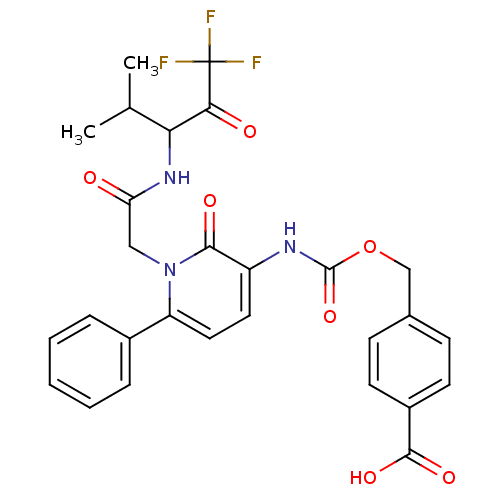
(4-{2-Oxo-6-phenyl-1-[(3,3,3-trifluoro-1-isopropyl-...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NC(=O)OCc2ccc(cc2)C(O)=O)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C28H26F3N3O7/c1-16(2)23(24(36)28(29,30)31)33-22(35)14-34-21(18-6-4-3-5-7-18)13-12-20(25(34)37)32-27(40)41-15-17-8-10-19(11-9-17)26(38)39/h3-13,16,23H,14-15H2,1-2H3,(H,32,40)(H,33,35)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036112
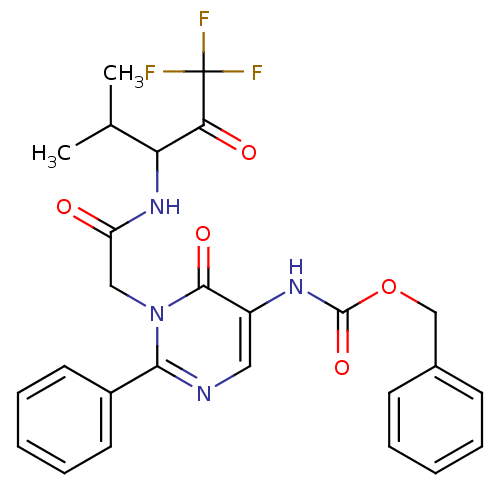
(CHEMBL11403 | {6-Oxo-2-phenyl-1-[(3,3,3-trifluoro-...)Show SMILES CC(C)C(NC(=O)Cn1c(ncc(NC(=O)OCc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H25F3N4O5/c1-16(2)21(22(35)26(27,28)29)32-20(34)14-33-23(18-11-7-4-8-12-18)30-13-19(24(33)36)31-25(37)38-15-17-9-5-3-6-10-17/h3-13,16,21H,14-15H2,1-2H3,(H,31,37)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037375
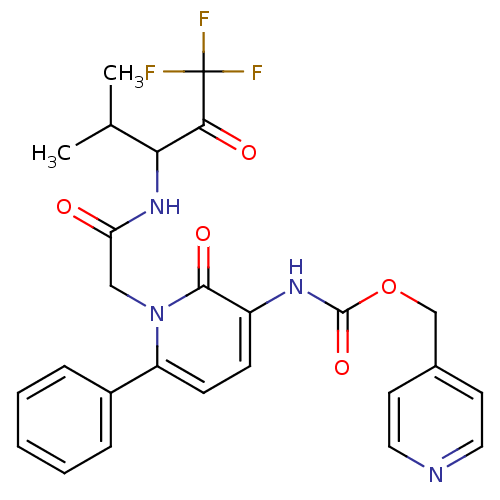
(CHEMBL275043 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NC(=O)OCc2ccncc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H25F3N4O5/c1-16(2)22(23(35)26(27,28)29)32-21(34)14-33-20(18-6-4-3-5-7-18)9-8-19(24(33)36)31-25(37)38-15-17-10-12-30-13-11-17/h3-13,16,22H,14-15H2,1-2H3,(H,31,37)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036103

(CHEMBL11098 | {2-(4-Fluoro-phenyl)-6-oxo-1-[(3,3,3...)Show SMILES CC(C)C(NC(=O)Cn1c(ncc(NC(=O)OCc2ccccc2)c1=O)-c1ccc(F)cc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H24F4N4O5/c1-15(2)21(22(36)26(28,29)30)33-20(35)13-34-23(17-8-10-18(27)11-9-17)31-12-19(24(34)37)32-25(38)39-14-16-6-4-3-5-7-16/h3-12,15,21H,13-14H2,1-2H3,(H,32,38)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036078
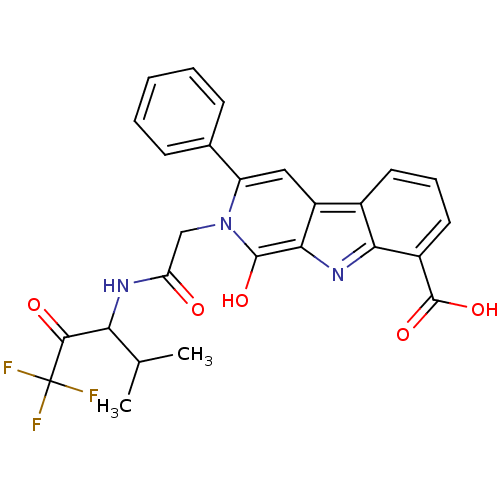
(1-Oxo-3-phenyl-2-[(3,3,3-trifluoro-1-isopropyl-2-o...)Show SMILES CC(C)C(NC(=O)Cn1c(O)c2nc3c(cccc3c2cc1-c1ccccc1)C(O)=O)C(=O)C(F)(F)F Show InChI InChI=1S/C26H22F3N3O5/c1-13(2)20(23(34)26(27,28)29)30-19(33)12-32-18(14-7-4-3-5-8-14)11-17-15-9-6-10-16(25(36)37)21(15)31-22(17)24(32)35/h3-11,13,20,35H,12H2,1-2H3,(H,30,33)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037342
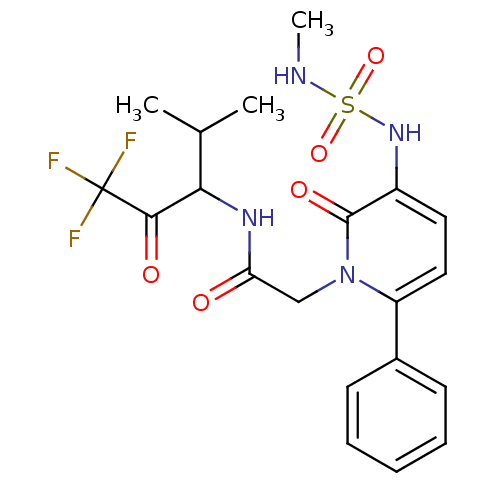
(1N-(3,3,3-trifluoro-1-isopropyl-2-oxopropyl)-2-(3-...)Show SMILES CNS(=O)(=O)Nc1ccc(-c2ccccc2)n(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)c1=O Show InChI InChI=1S/C20H23F3N4O5S/c1-12(2)17(18(29)20(21,22)23)25-16(28)11-27-15(13-7-5-4-6-8-13)10-9-14(19(27)30)26-33(31,32)24-3/h4-10,12,17,24,26H,11H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036122
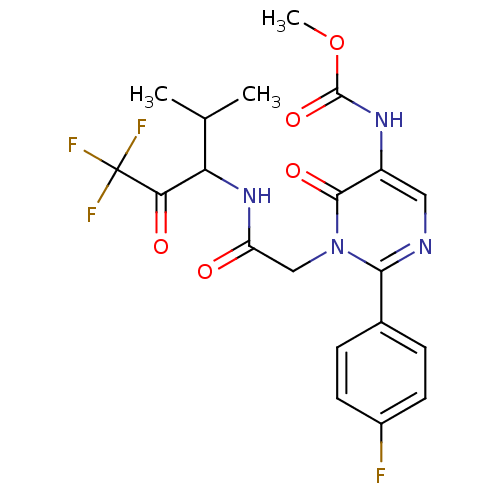
(CHEMBL11336 | {2-(4-Fluoro-phenyl)-6-oxo-1-[(3,3,3...)Show SMILES COC(=O)Nc1cnc(-c2ccc(F)cc2)n(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)c1=O Show InChI InChI=1S/C20H20F4N4O5/c1-10(2)15(16(30)20(22,23)24)27-14(29)9-28-17(11-4-6-12(21)7-5-11)25-8-13(18(28)31)26-19(32)33-3/h4-8,10,15H,9H2,1-3H3,(H,26,32)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50036127

(CHEMBL11391 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro-...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NC(=O)OCc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C27H26F3N3O5/c1-17(2)23(24(35)27(28,29)30)32-22(34)15-33-21(19-11-7-4-8-12-19)14-13-20(25(33)36)31-26(37)38-16-18-9-5-3-6-10-18/h3-14,17,23H,15-16H2,1-2H3,(H,31,37)(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
The compound was evaluated for the binding affinity against bovine pancreatic chymotrypsinogen |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036093
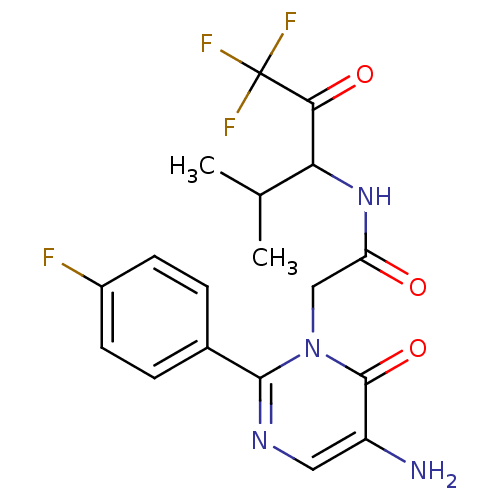
(2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...)Show SMILES CC(C)C(NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1)C(=O)C(F)(F)F Show InChI InChI=1S/C18H18F4N4O3/c1-9(2)14(15(28)18(20,21)22)25-13(27)8-26-16(24-7-12(23)17(26)29)10-3-5-11(19)6-4-10/h3-7,9,14H,8,23H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50453643
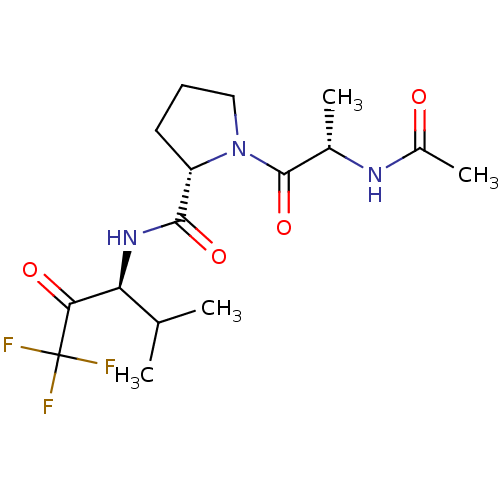
(CHEMBL2371666)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(C)=O)C(=O)C(F)(F)F Show InChI InChI=1S/C16H24F3N3O4/c1-8(2)12(13(24)16(17,18)19)21-14(25)11-6-5-7-22(11)15(26)9(3)20-10(4)23/h8-9,11-12H,5-7H2,1-4H3,(H,20,23)(H,21,25)/t9-,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037115
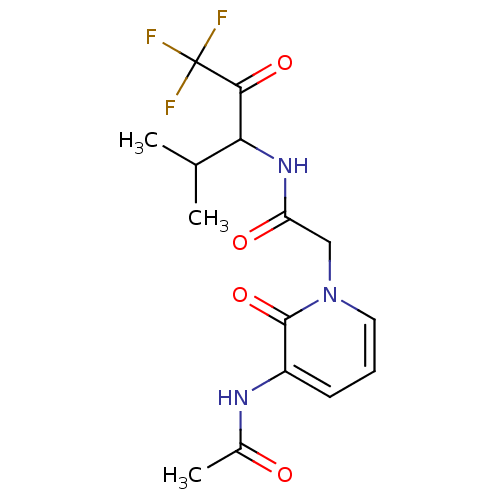
(2-(3-Acetylamino-2-oxo-2H-pyridin-1-yl)-N-(3,3,3-t...)Show SMILES CC(C)C(NC(=O)Cn1cccc(NC(C)=O)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C15H18F3N3O4/c1-8(2)12(13(24)15(16,17)18)20-11(23)7-21-6-4-5-10(14(21)25)19-9(3)22/h4-6,8,12H,7H2,1-3H3,(H,19,22)(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50001786
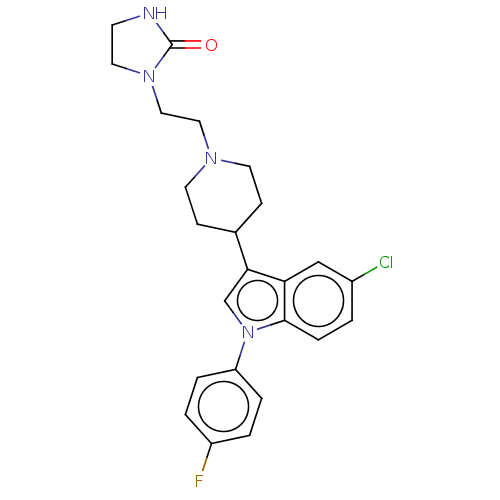
(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50127999
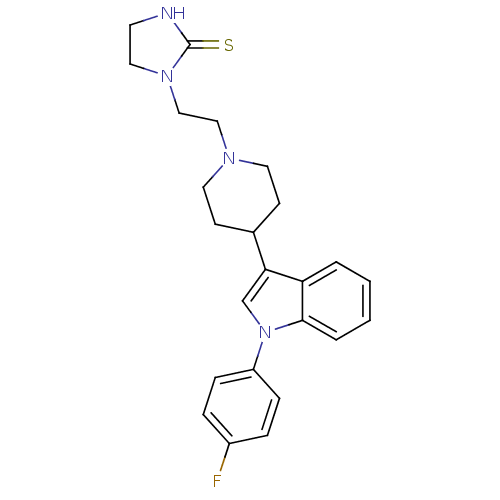
(1-(2-{4-[1-(4-Fluoro-phenyl)-1H-indol-3-yl]-piperi...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=S)CC2)c2ccccc12 Show InChI InChI=1S/C24H27FN4S/c25-19-5-7-20(8-6-19)29-17-22(21-3-1-2-4-23(21)29)18-9-12-27(13-10-18)15-16-28-14-11-26-24(28)30/h1-8,17-18H,9-16H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50001778
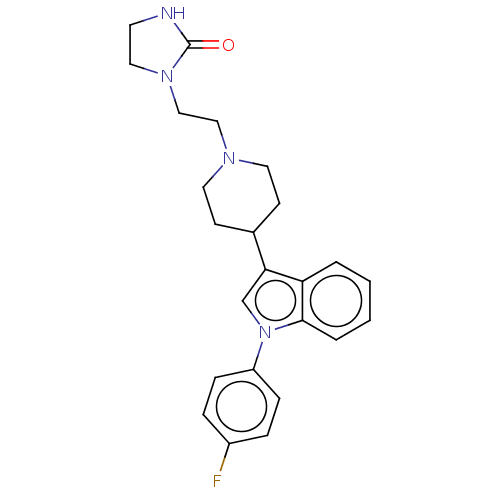
((Lu 23-086)1-(2-{4-[1-(4-Fluoro-phenyl)-1H-indol-3...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2ccccc12 Show InChI InChI=1S/C24H27FN4O/c25-19-5-7-20(8-6-19)29-17-22(21-3-1-2-4-23(21)29)18-9-12-27(13-10-18)15-16-28-14-11-26-24(28)30/h1-8,17-18H,9-16H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50002002

(1-(2-{4-[5-Chloro-1-(4-fluoro-phenyl)-1H-indol-3-y...)Show SMILES Fc1ccc(cc1)-n1cc(C2=CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 |t:11| Show InChI InChI=1S/C24H24ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-7,15-16H,8-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031720
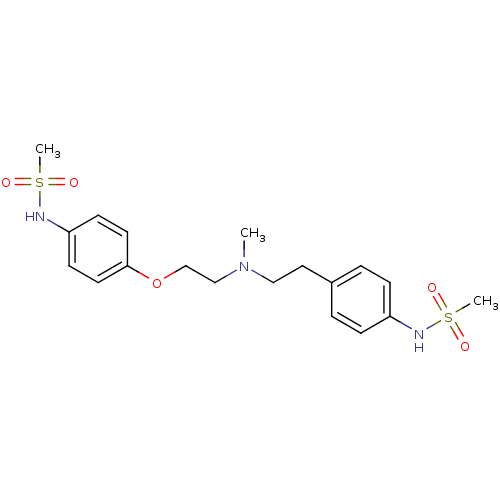
((Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-p...)Show SMILES CN(CCOc1ccc(NS(C)(=O)=O)cc1)CCc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50053194
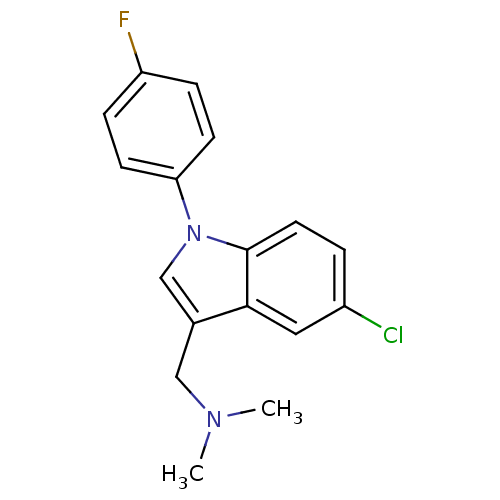
(CHEMBL301956 | [5-Chloro-1-(4-fluoro-phenyl)-1H-in...)Show InChI InChI=1S/C17H16ClFN2/c1-20(2)10-12-11-21(15-6-4-14(19)5-7-15)17-8-3-13(18)9-16(12)17/h3-9,11H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50334150
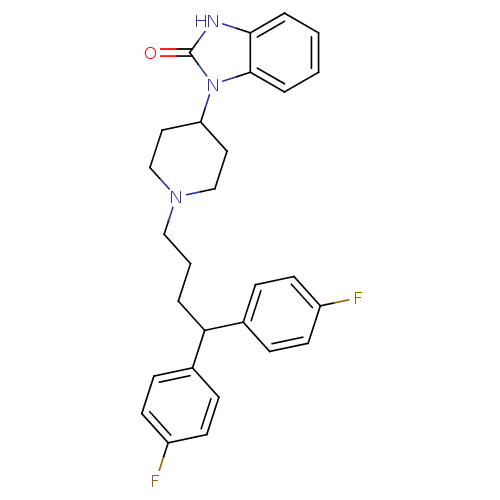
(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50127982
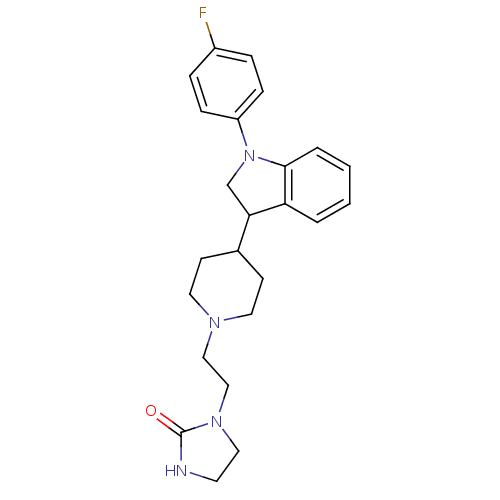
(1-(2-{4-[1-(4-Fluoro-phenyl)-2,3-dihydro-1H-indol-...)Show SMILES Fc1ccc(cc1)N1CC(C2CCN(CCN3CCNC3=O)CC2)c2ccccc12 Show InChI InChI=1S/C24H29FN4O/c25-19-5-7-20(8-6-19)29-17-22(21-3-1-2-4-23(21)29)18-9-12-27(13-10-18)15-16-28-14-11-26-24(28)30/h1-8,18,22H,9-17H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128003
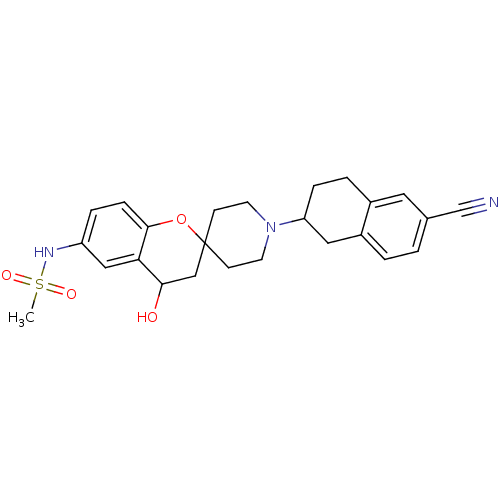
(CHEMBL52627 | MK-499 | N-[1'-(6-cyano-1,2,3,4-tetr...)Show SMILES CS(=O)(=O)Nc1ccc2OC3(CCN(CC3)C3CCc4cc(ccc4C3)C#N)CC(O)c2c1 Show InChI InChI=1S/C25H29N3O4S/c1-33(30,31)27-20-5-7-24-22(14-20)23(29)15-25(32-24)8-10-28(11-9-25)21-6-4-18-12-17(16-26)2-3-19(18)13-21/h2-3,5,7,12,14,21,23,27,29H,4,6,8-11,13,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50127983
(4-(5-Chloro-3-{1-[2-(2-oxo-imidazolidin-1-yl)-ethy...)Show SMILES COC(=O)c1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C26H29ClN4O3/c1-34-25(32)19-2-5-21(6-3-19)31-17-23(22-16-20(27)4-7-24(22)31)18-8-11-29(12-9-18)14-15-30-13-10-28-26(30)33/h2-7,16-18H,8-15H2,1H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50136106

(4-(6-Benzyl-benzo[1,3]dioxol-5-yl)-benzenesulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc2OCOc2cc1Cc1ccccc1 Show InChI InChI=1S/C20H17NO4S/c21-26(22,23)17-8-6-15(7-9-17)18-12-20-19(24-13-25-20)11-16(18)10-14-4-2-1-3-5-14/h1-9,11-12H,10,13H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 2
(Homo sapiens (Human)) | BDBM50072147
((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of the dual specificity kinase MEK-2 |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50136109
(5-Cyclohexylidenemethyl-6-(4-methanesulfonyl-pheny...)Show SMILES [#6]S(=O)(=O)c1ccc(cc1)-c1cc2-[#8]-[#6]-[#8]-c2cc1\[#6]=[#6]-1\[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C21H22O4S/c1-26(22,23)18-9-7-16(8-10-18)19-13-21-20(24-14-25-21)12-17(19)11-15-5-3-2-4-6-15/h7-13H,2-6,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 2 (COX-2) by enzyme Assay |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50072147

((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of the dual specificity kinase MEK-1 |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50127989
(1-{2-[4-(5-Chloro-1-phenyl-1H-indol-3-yl)-piperidi...)Show SMILES Clc1ccc2n(cc(C3CCN(CCN4CCNC4=O)CC3)c2c1)-c1ccccc1 Show InChI InChI=1S/C24H27ClN4O/c25-19-6-7-23-21(16-19)22(17-29(23)20-4-2-1-3-5-20)18-8-11-27(12-9-18)14-15-28-13-10-26-24(28)30/h1-7,16-18H,8-15H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128000
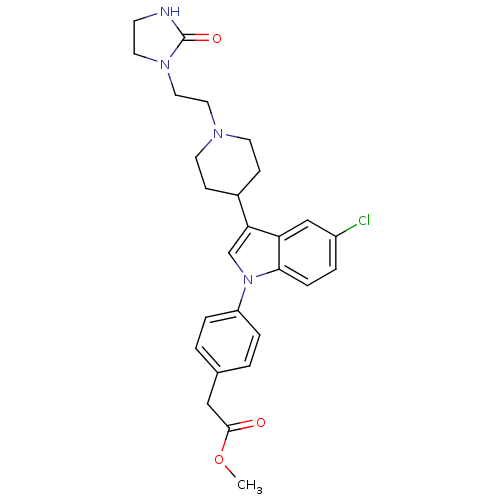
(CHEMBL415944 | [4-(5-Chloro-3-{1-[2-(2-oxo-imidazo...)Show SMILES COC(=O)Cc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C27H31ClN4O3/c1-35-26(33)16-19-2-5-22(6-3-19)32-18-24(23-17-21(28)4-7-25(23)32)20-8-11-30(12-9-20)14-15-31-13-10-29-27(31)34/h2-7,17-18,20H,8-16H2,1H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128001
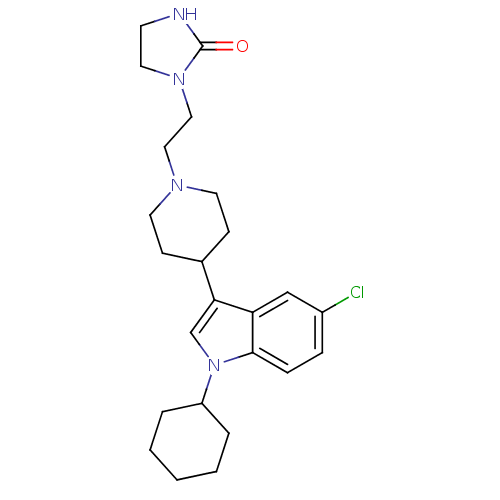
(1-{2-[4-(5-Chloro-1-cyclohexyl-1H-indol-3-yl)-pipe...)Show SMILES Clc1ccc2n(cc(C3CCN(CCN4CCNC4=O)CC3)c2c1)C1CCCCC1 Show InChI InChI=1S/C24H33ClN4O/c25-19-6-7-23-21(16-19)22(17-29(23)20-4-2-1-3-5-20)18-8-11-27(12-9-18)14-15-28-13-10-26-24(28)30/h6-7,16-18,20H,1-5,8-15H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM81939
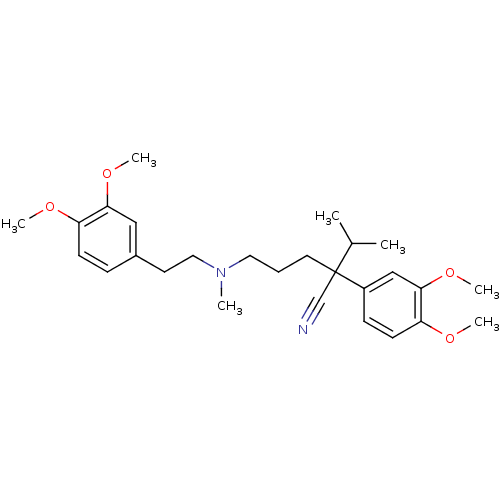
(CAS_52-53-9 | NSC_62969 | VERAPAMIL)Show SMILES COc1ccc(CCN(C)CCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC Show InChI InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50136108
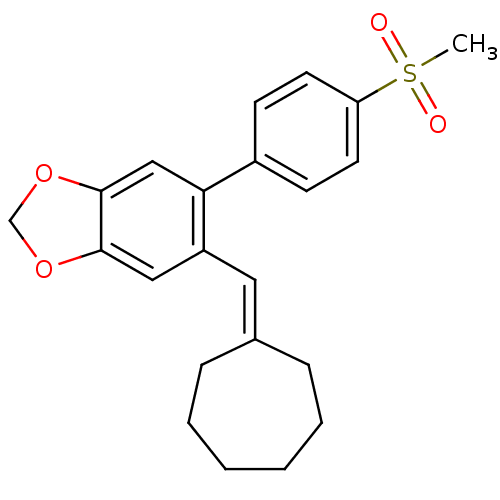
(5-Cycloheptylidenemethyl-6-(4-methanesulfonyl-phen...)Show SMILES [#6]S(=O)(=O)c1ccc(cc1)-c1cc2-[#8]-[#6]-[#8]-c2cc1\[#6]=[#6]-1/[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C22H24O4S/c1-27(23,24)19-10-8-17(9-11-19)20-14-22-21(25-15-26-22)13-18(20)12-16-6-4-2-3-5-7-16/h8-14H,2-7,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50072147

((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
AP-1 suppression activity using freshly prepared DMSO stock |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50002009
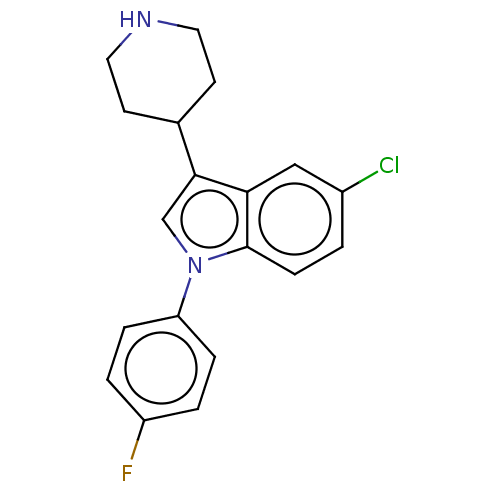
(5-Chloro-1-(4-fluoro-phenyl)-3-piperidin-4-yl-1H-i...)Show InChI InChI=1S/C19H18ClFN2/c20-14-1-6-19-17(11-14)18(13-7-9-22-10-8-13)12-23(19)16-4-2-15(21)3-5-16/h1-6,11-13,22H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1 |
Bioorg Med Chem Lett 13: 1829-35 (2003)
BindingDB Entry DOI: 10.7270/Q2TX3FX0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50168167
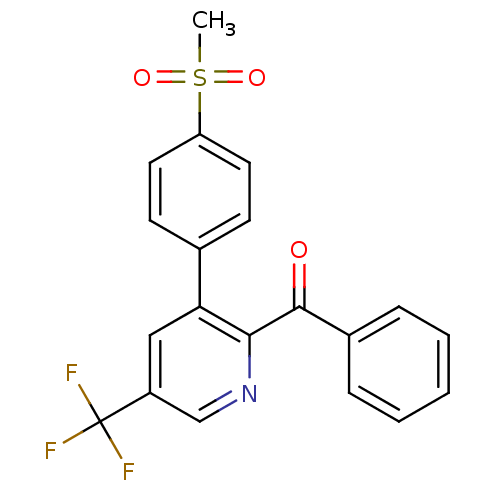
(CHEMBL364966 | [3-(4-Methanesulfonyl-phenyl)-5-tri...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(cnc1C(=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C20H14F3NO3S/c1-28(26,27)16-9-7-13(8-10-16)17-11-15(20(21,22)23)12-24-18(17)19(25)14-5-3-2-4-6-14/h2-12H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 48: 3930-4 (2005)
Article DOI: 10.1021/jm0582064
BindingDB Entry DOI: 10.7270/Q28C9VSZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human blood |
J Med Chem 47: 2180-93 (2004)
Article DOI: 10.1021/jm030276s
BindingDB Entry DOI: 10.7270/Q2P55MZF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 14: 6049-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.073
BindingDB Entry DOI: 10.7270/Q2Q81CJ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50072163
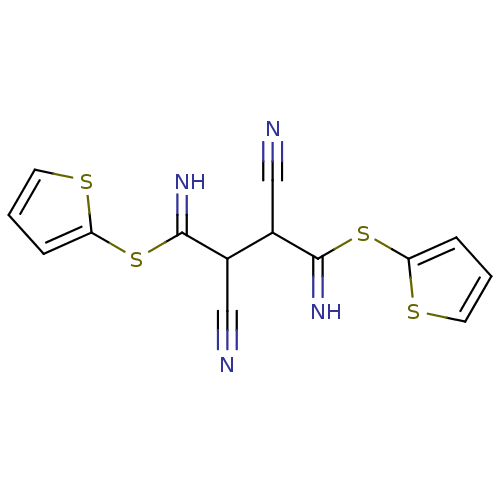
(2,3-Bis-[1-amino-1-(thiophen-2-ylsulfanyl)-meth-(Z...)Show InChI InChI=1S/C14H10N4S4/c15-7-9(13(17)21-11-3-1-5-19-11)10(8-16)14(18)22-12-4-2-6-20-12/h1-6,9-10,17-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of the dual specificity kinase MEK |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50072154
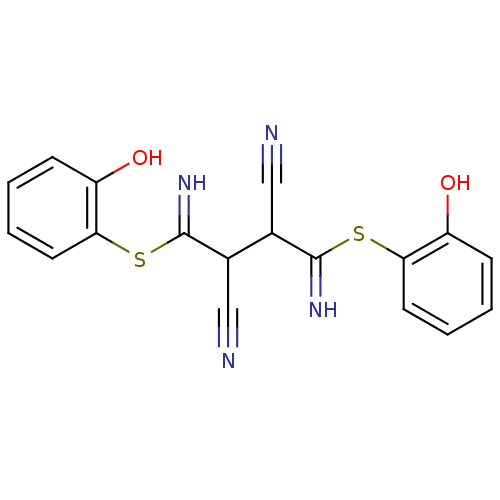
(2,3-Bis-[1-amino-1-(2-hydroxy-phenylsulfanyl)-meth...)Show InChI InChI=1S/C18H14N4O2S2/c19-9-11(17(21)25-15-7-3-1-5-13(15)23)12(10-20)18(22)26-16-8-4-2-6-14(16)24/h1-8,11-12,21-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of the dual specificity kinase MEK |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50072147

((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
AP-1 suppression activity using one week old DMSO stock |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50072147

((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
AP-1 suppression activity using one month old DMSO stock |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50072166
(2,3-Bis-[1-amino-1-(4-hydroxy-phenylsulfanyl)-meth...)Show SMILES Oc1ccc(SC(=N)C(C#N)C(C#N)C(=N)Sc2ccc(O)cc2)cc1 Show InChI InChI=1S/C18H14N4O2S2/c19-9-15(17(21)25-13-5-1-11(23)2-6-13)16(10-20)18(22)26-14-7-3-12(24)4-8-14/h1-8,15-16,21-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of the dual specificity kinase MEK |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50144866
(1-Cyclohexyl-5-(4-methanesulfonyl-phenyl)-3-nitroo...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(CO[N+]([O-])=O)nn1C1CCCCC1 Show InChI InChI=1S/C17H21N3O5S/c1-26(23,24)16-9-7-13(8-10-16)17-11-14(12-25-20(21)22)18-19(17)15-5-3-2-4-6-15/h7-11,15H,2-6,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human blood |
J Med Chem 47: 2180-93 (2004)
Article DOI: 10.1021/jm030276s
BindingDB Entry DOI: 10.7270/Q2P55MZF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50072154

(2,3-Bis-[1-amino-1-(2-hydroxy-phenylsulfanyl)-meth...)Show InChI InChI=1S/C18H14N4O2S2/c19-9-11(17(21)25-15-7-3-1-5-13(15)23)12(10-20)18(22)26-16-8-4-2-6-14(16)24/h1-8,11-12,21-24H | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Ability to antagonise AP-1 transcriptional activity |
Bioorg Med Chem Lett 8: 2839-44 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2HNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data