

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255582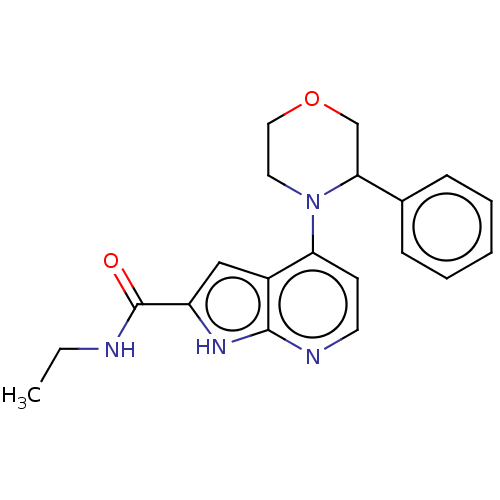 (CHEMBL4078783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255603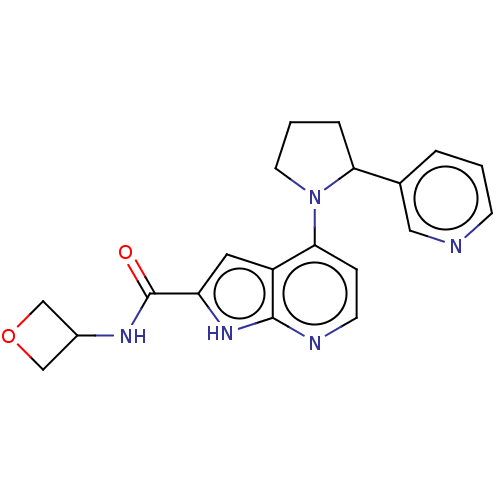 (CHEMBL4083992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255566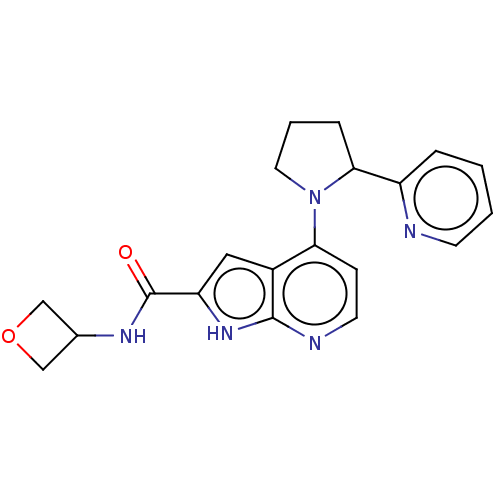 (CHEMBL4094252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255598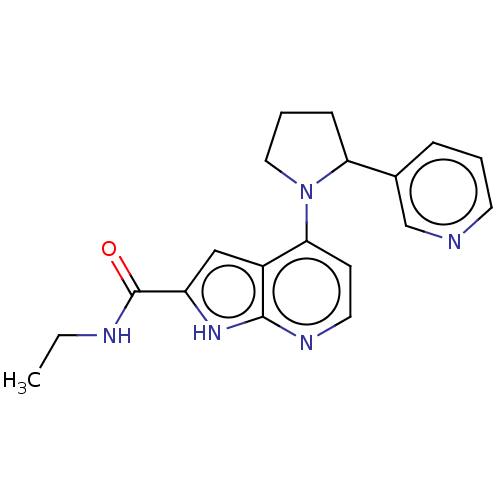 (CHEMBL4064004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255568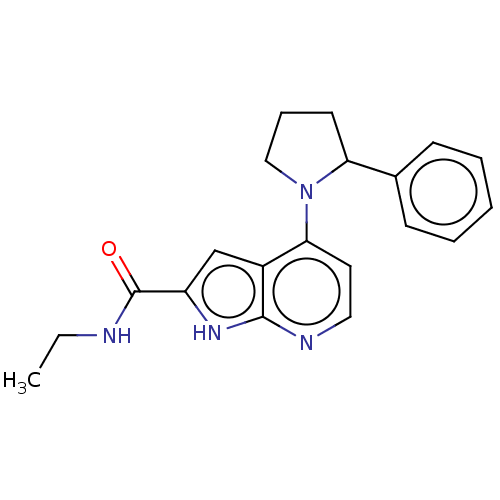 (CHEMBL4091768) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255621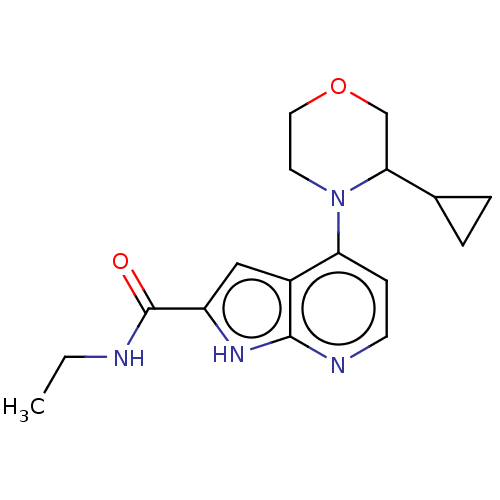 (CHEMBL4070624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255622 (CHEMBL4067896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255624 (CHEMBL4101983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255581 (CHEMBL4073623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255583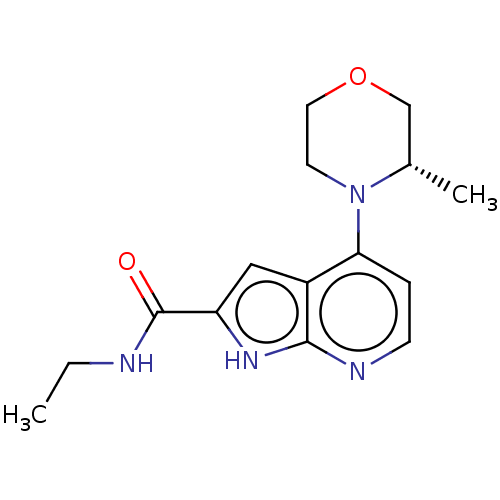 (CHEMBL4096813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255623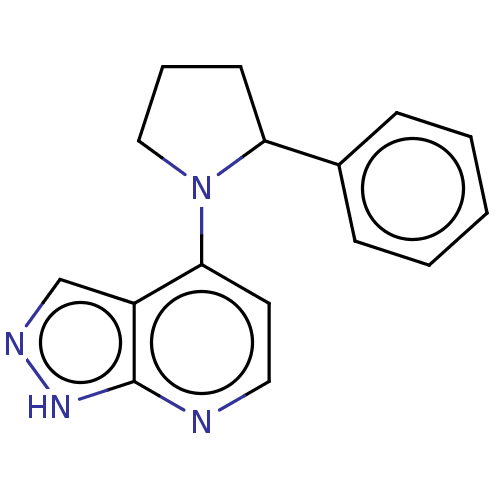 (CHEMBL4072758) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255585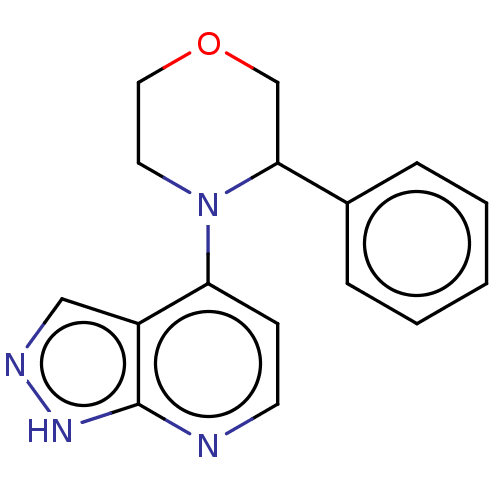 (CHEMBL4094381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM568667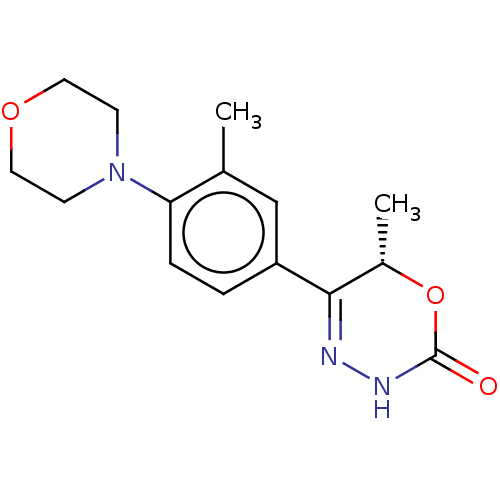 ((6S)-6-Methyl-5-[4-(morpholin-4-yl)-3-(trifluorome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255615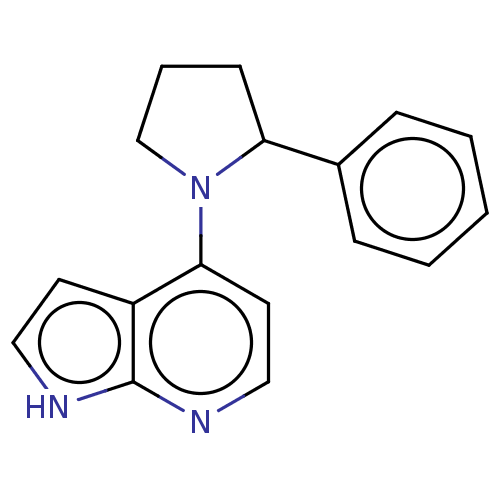 (CHEMBL4061204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255584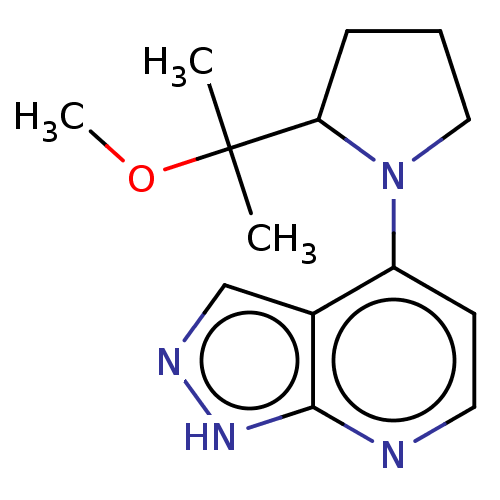 (CHEMBL4089106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM568667 ((6S)-6-Methyl-5-[4-(morpholin-4-yl)-3-(trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298092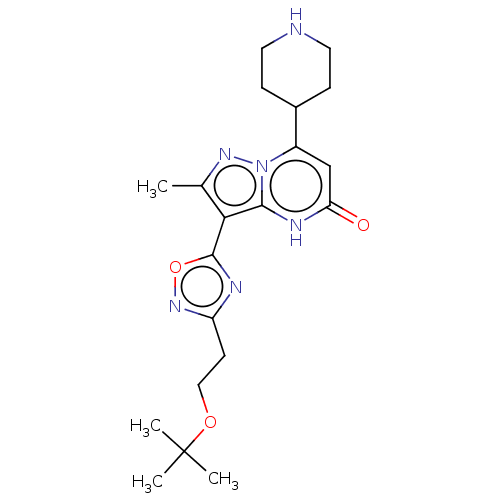 (3-[3-(2-tert-butoxyethyl)-1,2,4-oxadiazol-5-yl]-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM568827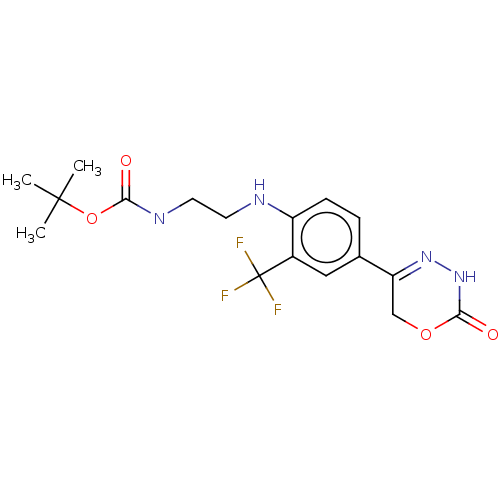 (US11427553, Example Intermediate 67) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM568827 (US11427553, Example Intermediate 67) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM568669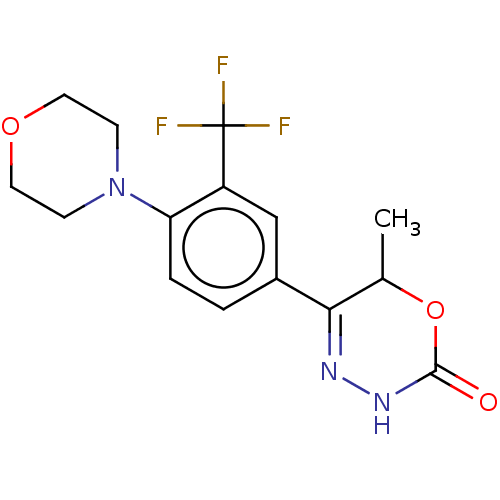 ((rac)-6-Methyl-5-(4-morpholino-3-(trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50615689 (CHEMBL5283715) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50615690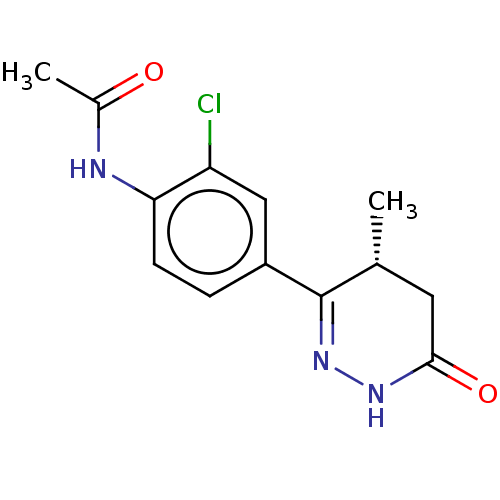 (CHEMBL5286823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298254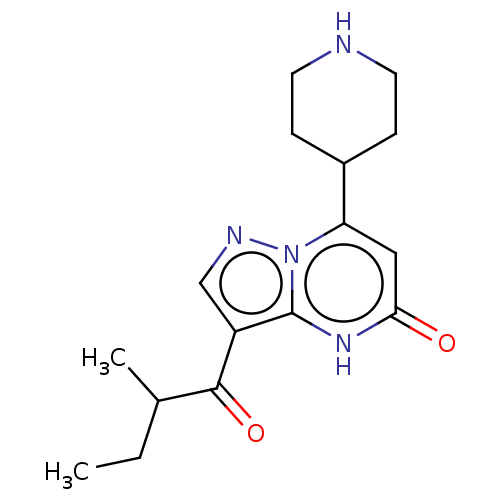 (3-(2-methylbutanoyl)-7-(piperidin-4-yl)pyrazolo[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298255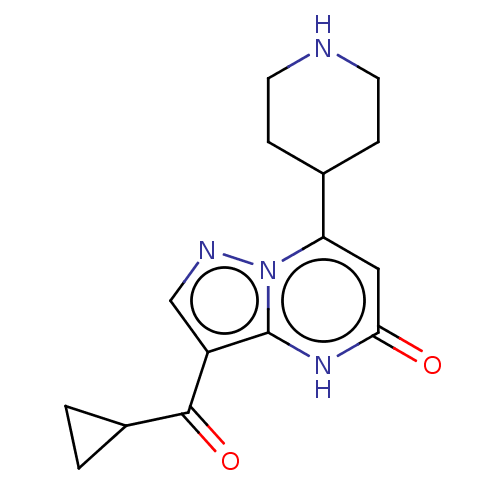 (3-(cyclopropylcarbonyl)-7-(piperidin-4-yl)pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298041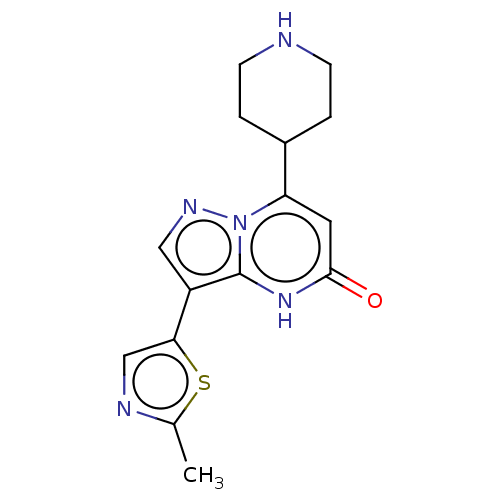 (3-(2-methyl-1,3-thiazol-5-yl)-7-(piperidin-4-yl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297950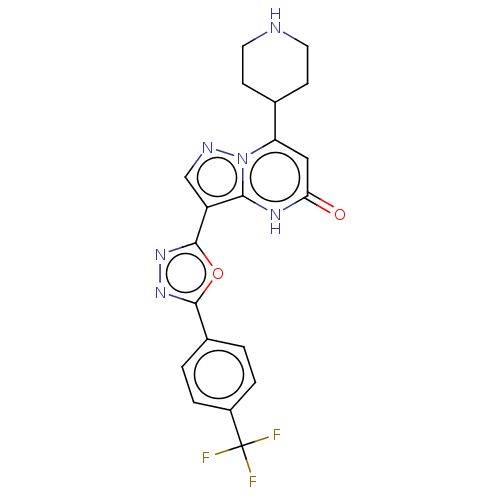 (7-(piperidin-4-yl)-3-{5-[4-(trifluoromethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM568817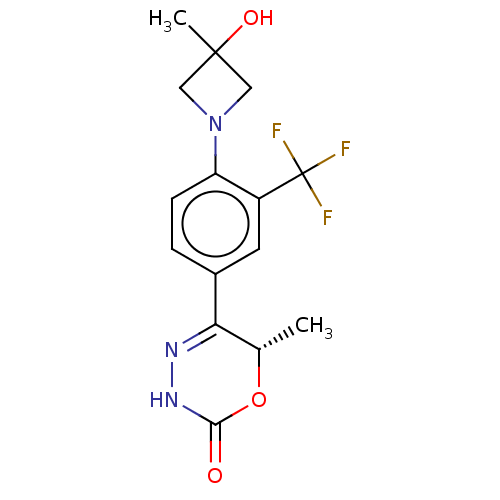 ((6S)-5-[4-(3-Hydroxy-3-methylazetidin-1-yl)-3-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM568764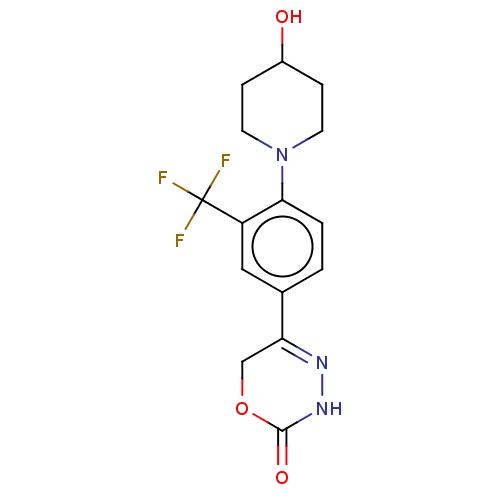 (5-[4-(4-Hydroxypiperidin-1-yl)-3-(trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298115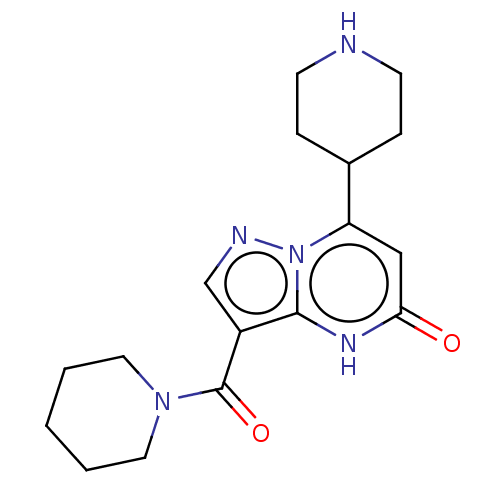 (7-(piperidin-4-yl)-3-(piperidin-1-ylcarbonyl)pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298123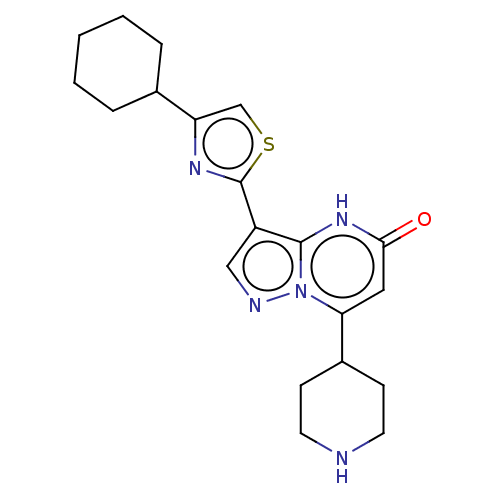 (3-(4-cyclohexyl-1,3-thiazol-2-yl)-7-(piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298009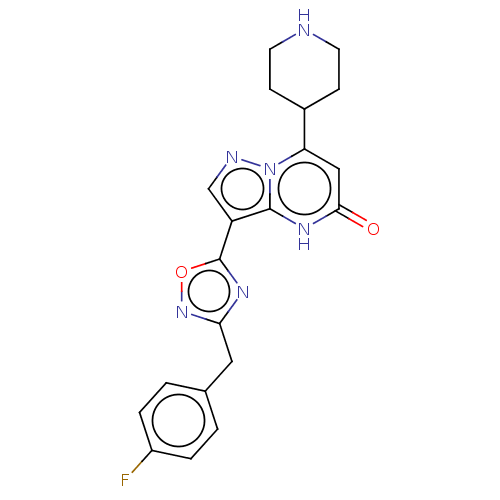 (3-[3-(4-fluorobenzyl)-1,2,4-oxadiazol-5-yl]-7-(pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM50615690 (CHEMBL5286823) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50615688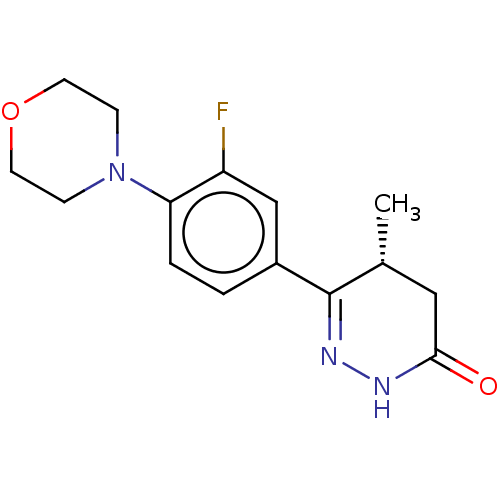 (CHEMBL5280568) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM568809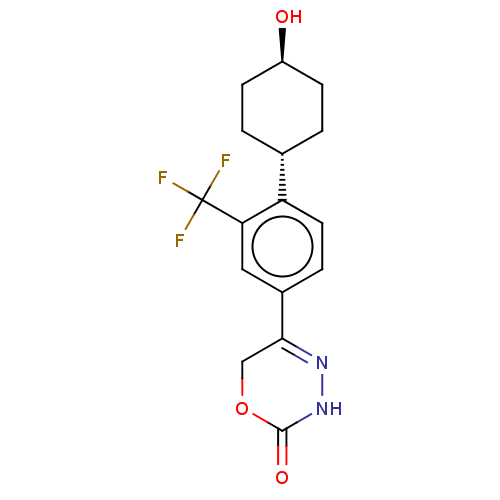 ((trans)-5-{4-[4-hydroxycyclohexyl]-3-(trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290292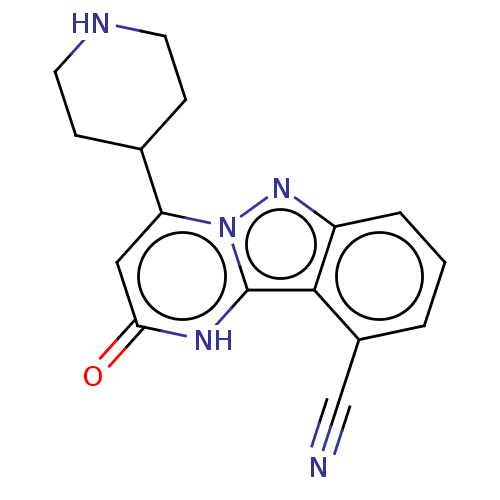 (2-Oxo-4-(piperidin-4-yl)-1,2-dihydropyrimido[1,2-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290292 (2-Oxo-4-(piperidin-4-yl)-1,2-dihydropyrimido[1,2-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255617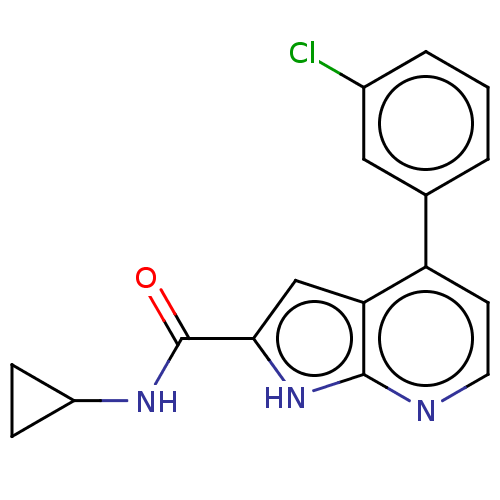 (CHEMBL4095468) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298184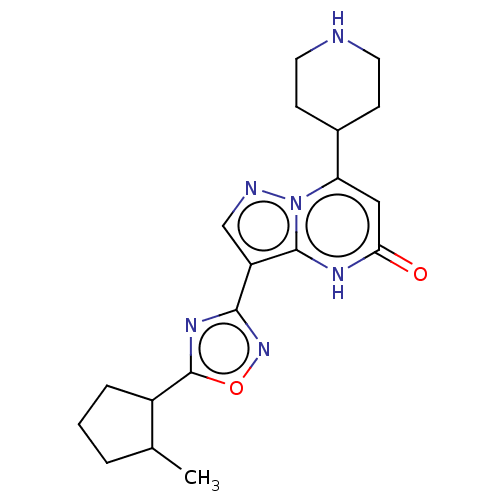 (Rel-3-{5-[(1R,2S)-2-methylcyclopentyl]-1,2,4-oxadi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298237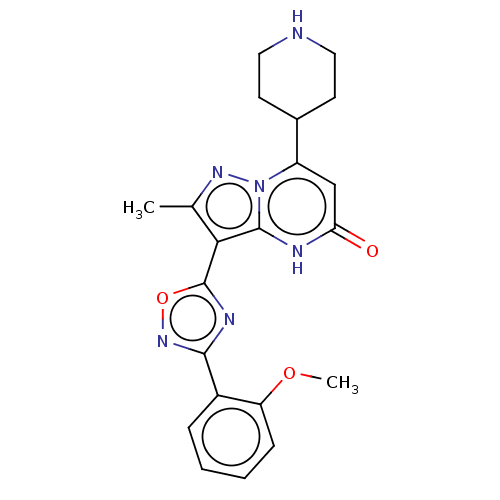 (3-[3-(2-methoxyphenyl)-1,2,4-oxadiazol-5-yl]-2-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298073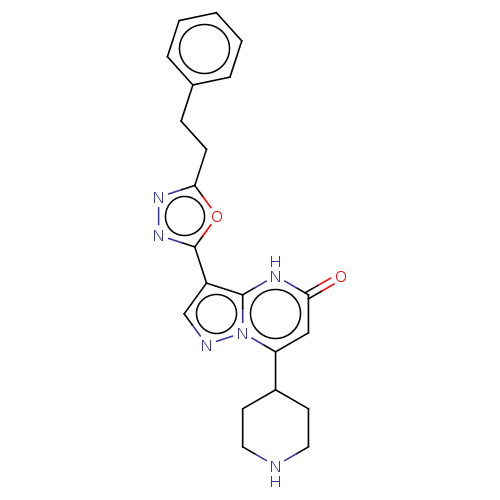 (3-[5-(2-phenylethyl)-1,3,4-oxadiazol-2-yl]-7-(pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298246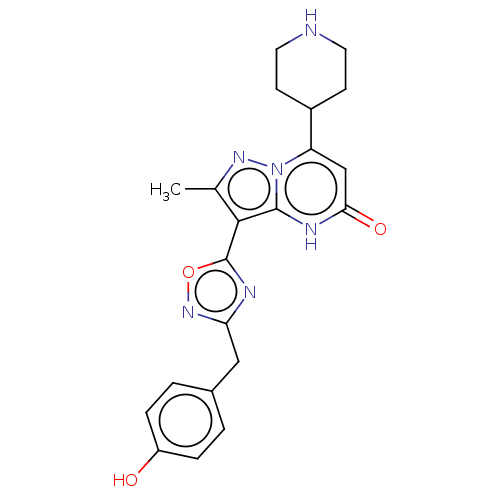 (3-[3-(4-hydroxybenzyl)-1,2,4-oxadiazol-5-yl]-2-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM568817 ((6S)-5-[4-(3-Hydroxy-3-methylazetidin-1-yl)-3-(tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290336 (10-Chloro-8-methyl-4-(piperidin-4-yl)pyrimido[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297791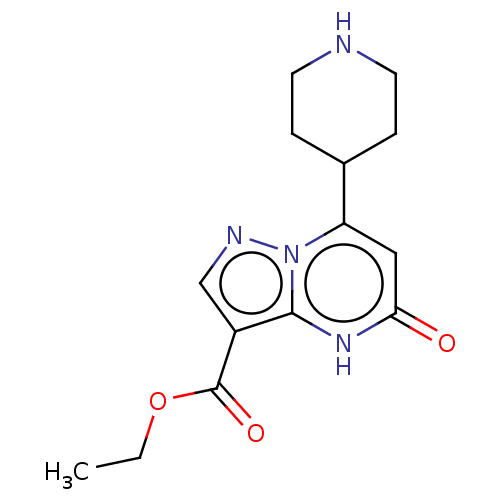 (Ethyl 5-oxo-7-(piperidin-4-yl)-4,5-dihydropyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290334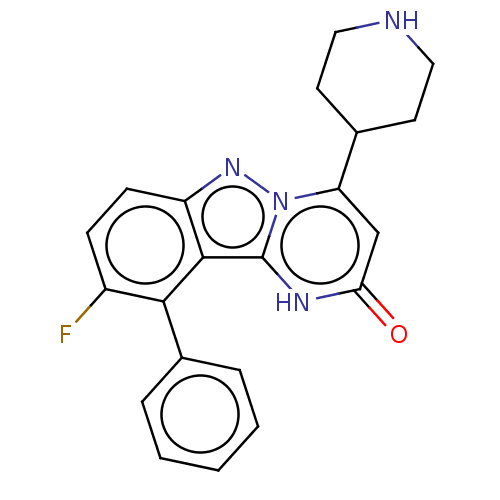 (9-Fluoro-10-phenyl-4-(piperidin-4-yl)pyrimido[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290336 (10-Chloro-8-methyl-4-(piperidin-4-yl)pyrimido[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298253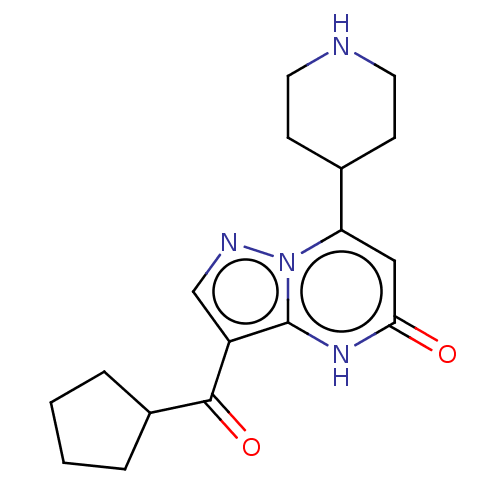 (3-(cyclopentylcarbonyl)-7-(piperidin-4-yl)pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298170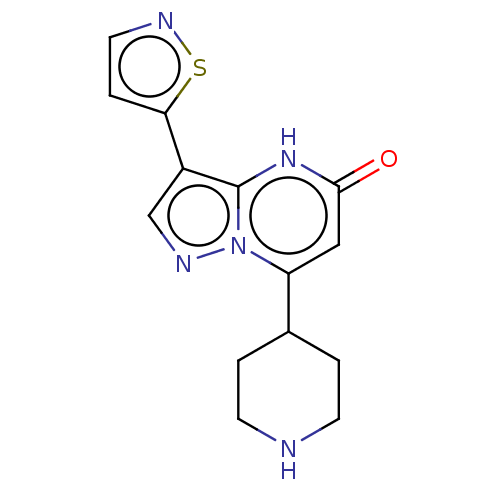 (7-(piperidin-4-yl)-3-(1,2-thiazol-5-yl)pyrazolo[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298096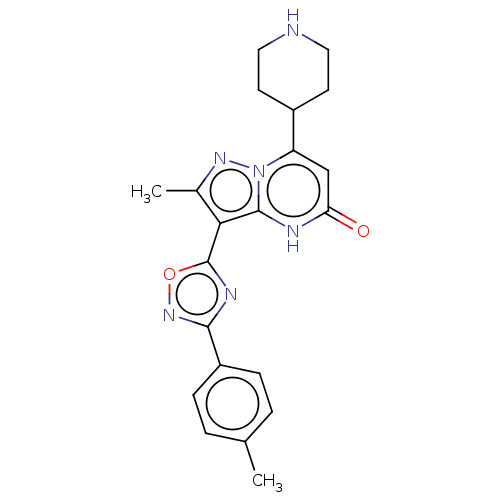 (2-methyl-3-[3-(4-methylphenyl)-1,2,4-oxadiazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298098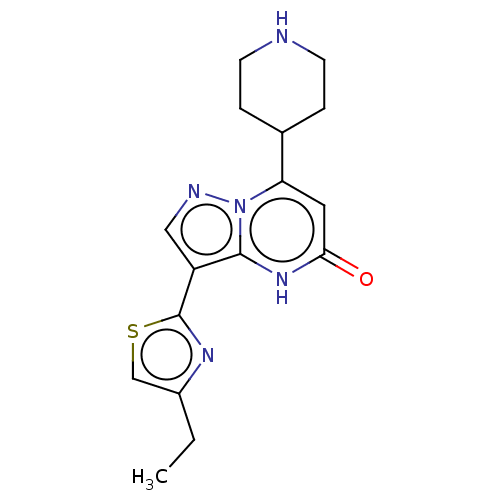 (3-(4-ethyl-1,3-thiazol-2-yl)-7-(piperidin-4-yl)pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1574 total ) | Next | Last >> |