 Found 77 hits with Last Name = 'fanjul' and Initial = 'an'
Found 77 hits with Last Name = 'fanjul' and Initial = 'an' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29864
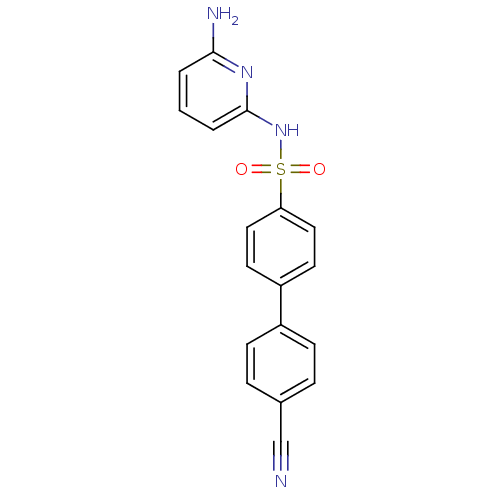
(N-(Pyridin-2-yl) arylsulfonamide, 26)Show SMILES Nc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C18H14N4O2S/c19-12-13-4-6-14(7-5-13)15-8-10-16(11-9-15)25(23,24)22-18-3-1-2-17(20)21-18/h1-11H,(H3,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29863

(N-(Pyridin-2-yl) arylsulfonamide, 25)Show SMILES CC(C)c1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C21H19N3O2S/c1-15(2)20-4-3-5-21(23-20)24-27(25,26)19-12-10-18(11-13-19)17-8-6-16(14-22)7-9-17/h3-13,15H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29862
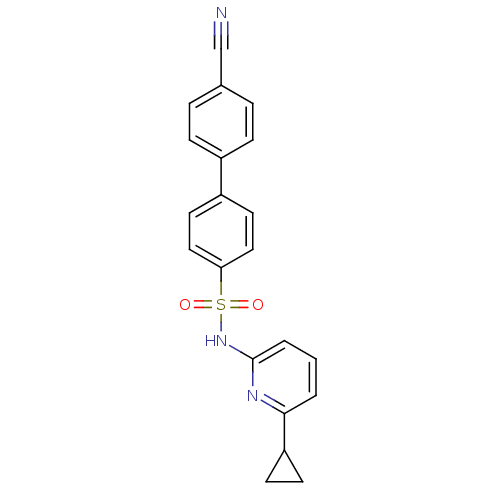
(N-(Pyridin-2-yl) arylsulfonamide, 24)Show SMILES O=S(=O)(Nc1cccc(n1)C1CC1)c1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C21H17N3O2S/c22-14-15-4-6-16(7-5-15)17-10-12-19(13-11-17)27(25,26)24-21-3-1-2-20(23-21)18-8-9-18/h1-7,10-13,18H,8-9H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29860
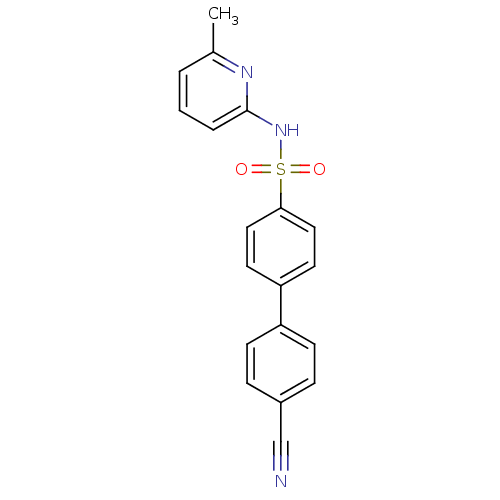
(N-(Pyridin-2-yl) arylsulfonamide, 22)Show SMILES Cc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C19H15N3O2S/c1-14-3-2-4-19(21-14)22-25(23,24)18-11-9-17(10-12-18)16-7-5-15(13-20)6-8-16/h2-12H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29866

(N-(Pyridin-2-yl) arylsulfonamide, 28)Show SMILES CCNc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C20H18N4O2S/c1-2-22-19-4-3-5-20(23-19)24-27(25,26)18-12-10-17(11-13-18)16-8-6-15(14-21)7-9-16/h3-13H,2H2,1H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29858
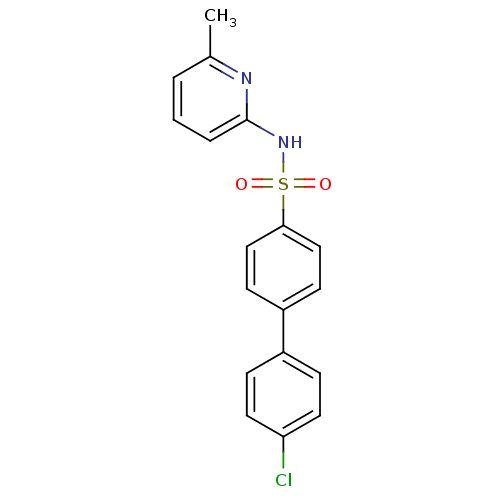
(N-(Pyridin-2-yl) arylsulfonamide, 20)Show SMILES Cc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(Cl)cc2)n1 Show InChI InChI=1S/C18H15ClN2O2S/c1-13-3-2-4-18(20-13)21-24(22,23)17-11-7-15(8-12-17)14-5-9-16(19)10-6-14/h2-12H,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29861
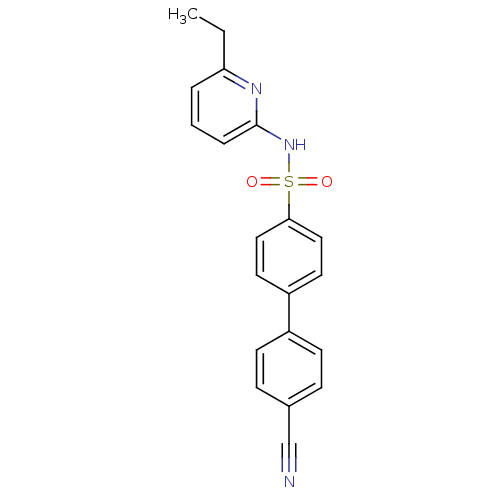
(N-(Pyridin-2-yl) arylsulfonamide, 23)Show SMILES CCc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C20H17N3O2S/c1-2-18-4-3-5-20(22-18)23-26(24,25)19-12-10-17(11-13-19)16-8-6-15(14-21)7-9-16/h3-13H,2H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | -46.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29865
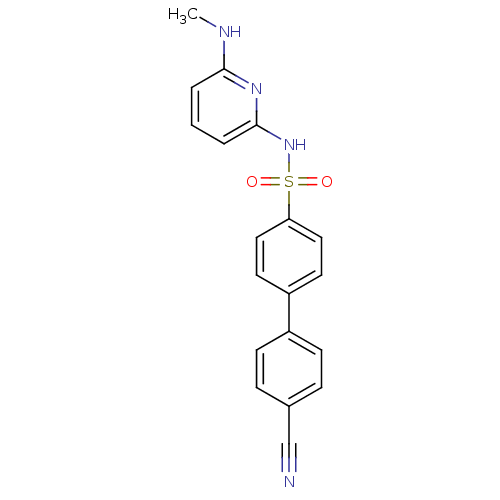
(N-(Pyridin-2-yl) arylsulfonamide, 27)Show SMILES CNc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C19H16N4O2S/c1-21-18-3-2-4-19(22-18)23-26(24,25)17-11-9-16(10-12-17)15-7-5-14(13-20)6-8-15/h2-12H,1H3,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | -45.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29857
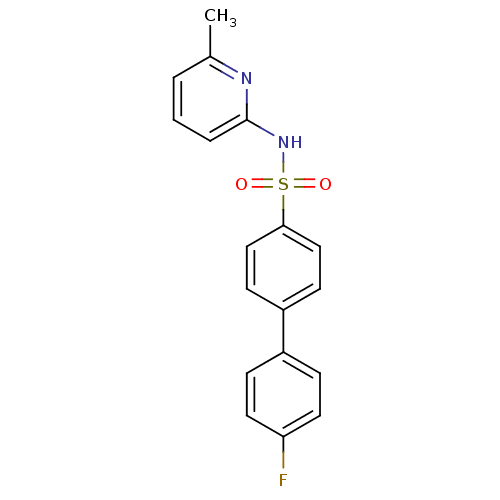
(N-(Pyridin-2-yl) arylsulfonamide, 19)Show SMILES Cc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(F)cc2)n1 Show InChI InChI=1S/C18H15FN2O2S/c1-13-3-2-4-18(20-13)21-24(22,23)17-11-7-15(8-12-17)14-5-9-16(19)10-6-14/h2-12H,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29856
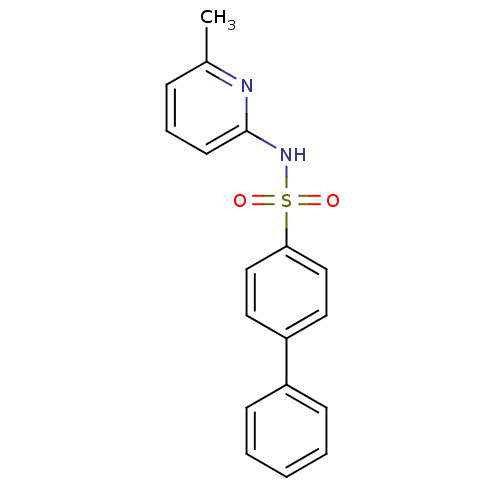
(N-(Pyridin-2-yl) arylsulfonamide, 18)Show InChI InChI=1S/C18H16N2O2S/c1-14-6-5-9-18(19-14)20-23(21,22)17-12-10-16(11-13-17)15-7-3-2-4-8-15/h2-13H,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | -41.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29859
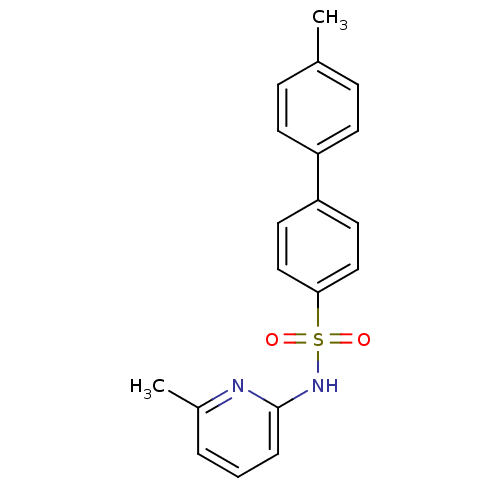
(N-(Pyridin-2-yl) arylsulfonamide, 21)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Nc1cccc(C)n1 Show InChI InChI=1S/C19H18N2O2S/c1-14-6-8-16(9-7-14)17-10-12-18(13-11-17)24(22,23)21-19-5-3-4-15(2)20-19/h3-13H,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 62 | -40.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29854
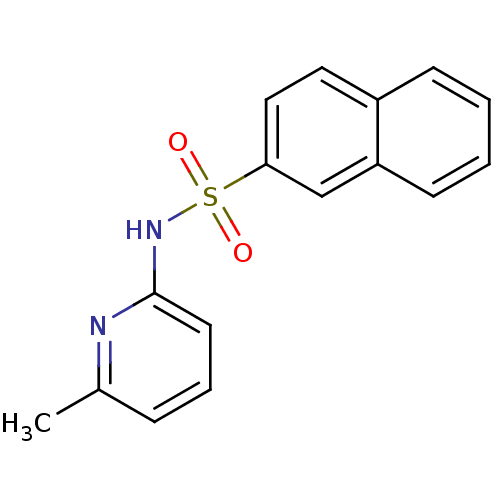
(N-(Pyridin-2-yl) arylsulfonamide, 16)Show InChI InChI=1S/C16H14N2O2S/c1-12-5-4-8-16(17-12)18-21(19,20)15-10-9-13-6-2-3-7-14(13)11-15/h2-11H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 84 | -40.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29850
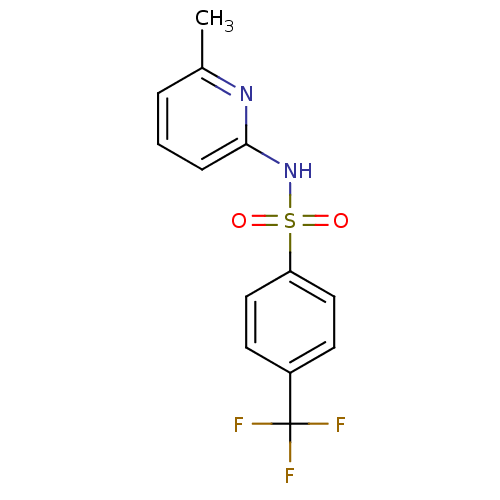
(N-(Pyridin-2-yl) arylsulfonamide, 12)Show InChI InChI=1S/C13H11F3N2O2S/c1-9-3-2-4-12(17-9)18-21(19,20)11-7-5-10(6-8-11)13(14,15)16/h2-8H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 108 | -39.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29849
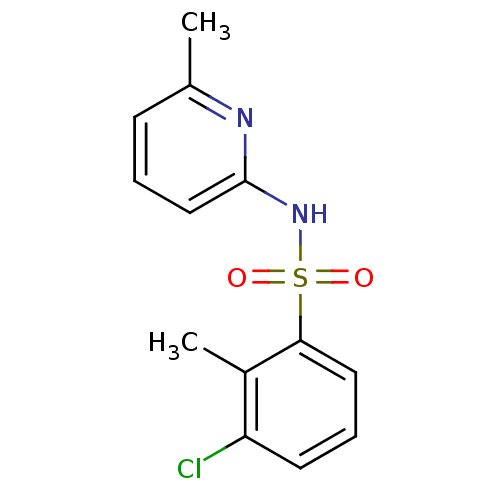
(N-(Pyridin-2-yl) arylsulfonamide, 11)Show InChI InChI=1S/C13H13ClN2O2S/c1-9-5-3-8-13(15-9)16-19(17,18)12-7-4-6-11(14)10(12)2/h3-8H,1-2H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 169 | -38.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29843
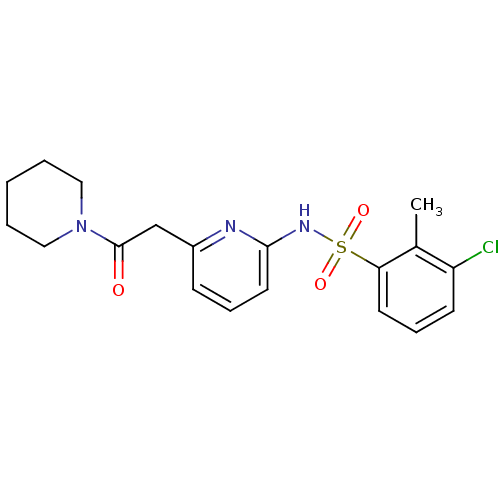
(N-(Pyridin-2-yl) arylsulfonamide, 3)Show SMILES Cc1c(Cl)cccc1S(=O)(=O)Nc1cccc(CC(=O)N2CCCCC2)n1 Show InChI InChI=1S/C19H22ClN3O3S/c1-14-16(20)8-6-9-17(14)27(25,26)22-18-10-5-7-15(21-18)13-19(24)23-11-3-2-4-12-23/h5-10H,2-4,11-13H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 169 | -38.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29841
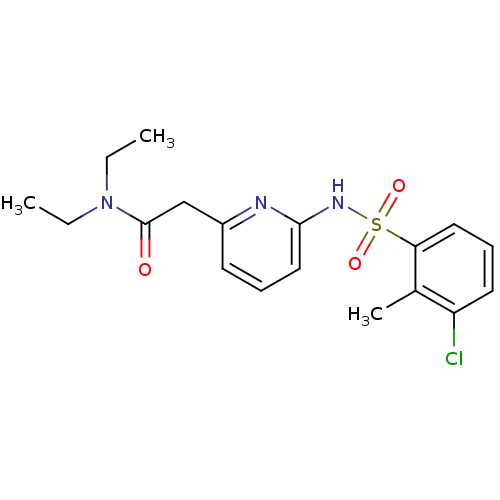
(N-(Pyridin-2-yl) arylsulfonamide, 1)Show SMILES CCN(CC)C(=O)Cc1cccc(NS(=O)(=O)c2cccc(Cl)c2C)n1 Show InChI InChI=1S/C18H22ClN3O3S/c1-4-22(5-2)18(23)12-14-8-6-11-17(20-14)21-26(24,25)16-10-7-9-15(19)13(16)3/h6-11H,4-5,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 287 | -37.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356991

(CHEMBL1916247)Show SMILES CC1(C)[C@H](NC(=O)c2cnc3ncccn23)C(C)(C)[C@H]1Oc1ccc(C#N)c(Cl)c1 |r,wU:3.3,wD:19.22,(4.89,-35.35,;5.3,-33.87,;3.8,-34.26,;6.84,-33.87,;8.19,-34.61,;8.22,-36.15,;6.91,-36.95,;9.57,-36.9,;9.76,-38.42,;11.28,-38.71,;12.02,-37.36,;13.51,-37.02,;13.97,-35.55,;12.92,-34.42,;11.41,-34.77,;10.97,-36.24,;6.84,-32.33,;7.23,-30.83,;8.32,-32.72,;5.31,-32.33,;3.98,-31.55,;3.98,-30.01,;2.65,-29.25,;2.65,-27.71,;3.99,-26.94,;4,-25.4,;4,-23.87,;5.32,-27.71,;6.66,-26.95,;5.32,-29.25,)| Show InChI InChI=1S/C22H22ClN5O2/c1-21(2)18(27-17(29)16-12-26-20-25-8-5-9-28(16)20)22(3,4)19(21)30-14-7-6-13(11-24)15(23)10-14/h5-10,12,18-19H,1-4H3,(H,27,29)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356988

(CHEMBL1916245)Show SMILES Cn1cc(cn1)C(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(Cl)c2)C1(C)C |r,wU:9.9,wD:13.14,(37.34,-24.69,;35.8,-24.71,;34.88,-23.47,;33.42,-23.97,;33.44,-25.5,;34.91,-25.96,;32.07,-23.22,;30.75,-24.02,;32.04,-21.68,;30.69,-20.94,;29.15,-20.94,;28.74,-22.42,;27.65,-21.33,;29.16,-19.4,;27.82,-18.62,;27.83,-17.08,;26.5,-16.32,;26.5,-14.78,;27.84,-14.01,;27.85,-12.47,;27.85,-10.94,;29.17,-14.79,;30.51,-14.02,;29.16,-16.32,;30.69,-19.4,;31.08,-17.9,;32.17,-19.79,)| Show InChI InChI=1S/C20H23ClN4O2/c1-19(2)17(24-16(26)13-10-23-25(5)11-13)20(3,4)18(19)27-14-7-6-12(9-22)15(21)8-14/h6-8,10-11,17-18H,1-5H3,(H,24,26)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356990
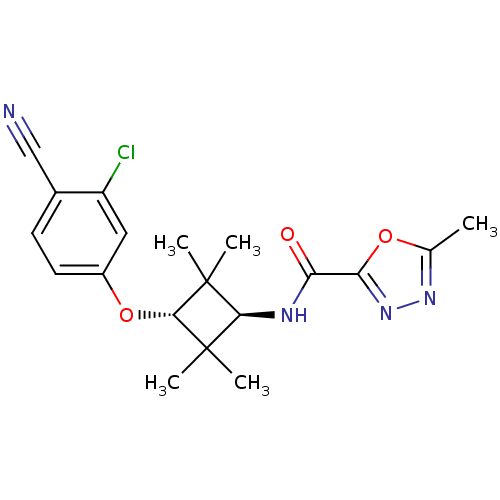
(CHEMBL1916246)Show SMILES Cc1nnc(o1)C(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(Cl)c2)C1(C)C |r,wU:9.9,wD:13.14,(2.34,-36.66,;.8,-36.67,;-.09,-37.93,;-1.56,-37.47,;-1.58,-35.94,;-.12,-35.44,;-2.93,-35.19,;-4.25,-35.99,;-2.96,-33.65,;-4.31,-32.91,;-5.85,-32.91,;-6.26,-34.39,;-7.35,-33.3,;-5.85,-31.37,;-7.18,-30.59,;-7.17,-29.05,;-8.5,-28.29,;-8.51,-26.75,;-7.17,-25.98,;-7.16,-24.44,;-7.15,-22.91,;-5.83,-26.75,;-4.5,-25.99,;-5.84,-28.29,;-4.31,-31.37,;-3.92,-29.87,;-2.83,-31.76,)| Show InChI InChI=1S/C19H21ClN4O3/c1-10-23-24-15(26-10)14(25)22-16-18(2,3)17(19(16,4)5)27-12-7-6-11(9-21)13(20)8-12/h6-8,16-17H,1-5H3,(H,22,25)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356980
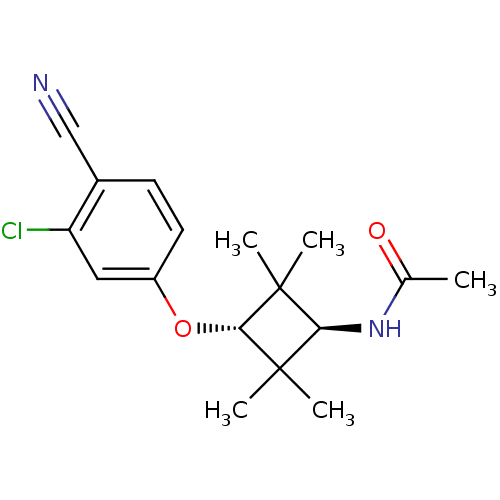
(CHEMBL1916237)Show SMILES CC(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(Cl)c2)C1(C)C |r,wU:4.3,wD:8.8,(9.36,-7.82,;8.01,-7.08,;6.7,-7.87,;7.98,-5.54,;6.63,-4.79,;5.09,-4.79,;4.68,-6.27,;3.59,-5.18,;5.1,-3.25,;3.77,-2.48,;3.77,-.94,;2.44,-.17,;2.44,1.36,;3.78,2.14,;3.79,3.68,;3.79,5.21,;5.11,1.36,;6.45,2.13,;5.11,-.18,;6.63,-3.25,;7.02,-1.75,;8.11,-3.64,)| Show InChI InChI=1S/C17H21ClN2O2/c1-10(21)20-14-16(2,3)15(17(14,4)5)22-12-7-6-11(9-19)13(18)8-12/h6-8,14-15H,1-5H3,(H,20,21)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356987

(CHEMBL1916244)Show SMILES CC1(C)[C@H](NC(=O)c2cn[nH]c2)C(C)(C)[C@H]1Oc1ccc(C#N)c(Cl)c1 |r,wU:3.3,wD:15.17,(20.6,-21.31,;21.02,-19.83,;19.51,-20.22,;22.56,-19.83,;23.9,-20.58,;23.93,-22.12,;22.62,-22.91,;25.28,-22.86,;25.3,-24.4,;26.77,-24.86,;27.66,-23.6,;26.74,-22.36,;22.56,-18.29,;22.94,-16.79,;24.03,-18.68,;21.02,-18.29,;19.69,-17.52,;19.69,-15.98,;18.36,-15.22,;18.36,-13.68,;19.7,-12.91,;19.71,-11.37,;19.71,-9.84,;21.03,-13.68,;22.37,-12.91,;21.03,-15.22,)| Show InChI InChI=1S/C19H21ClN4O2/c1-18(2)16(24-15(25)12-9-22-23-10-12)19(3,4)17(18)26-13-6-5-11(8-21)14(20)7-13/h5-7,9-10,16-17H,1-4H3,(H,22,23)(H,24,25)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356989

(CHEMBL1914477)Show SMILES Cc1cc(nn1C)C(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(Cl)c2)C1(C)C |r,wU:10.10,wD:14.15,(44.07,-26.91,;43.58,-25.45,;42.11,-24.99,;42.09,-23.45,;43.55,-22.96,;44.47,-24.19,;46.01,-24.17,;40.74,-22.71,;39.42,-23.5,;40.71,-21.17,;39.36,-20.42,;37.82,-20.42,;37.41,-21.9,;36.32,-20.81,;37.83,-18.88,;36.49,-18.11,;36.5,-16.57,;35.17,-15.81,;35.17,-14.27,;36.51,-13.5,;36.52,-11.96,;36.52,-10.43,;37.84,-14.27,;39.18,-13.51,;37.83,-15.81,;39.36,-18.88,;39.75,-17.38,;40.84,-19.27,)| Show InChI InChI=1S/C21H25ClN4O2/c1-12-9-16(25-26(12)6)17(27)24-18-20(2,3)19(21(18,4)5)28-14-8-7-13(11-23)15(22)10-14/h7-10,18-19H,1-6H3,(H,24,27)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356995
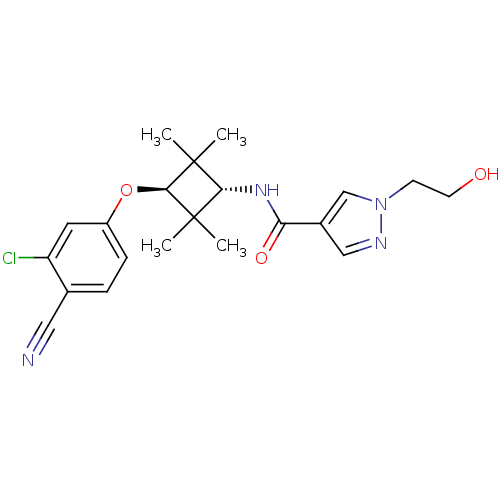
(CHEMBL1916251)Show SMILES CC1(C)[C@H](NC(=O)c2cnn(CCO)c2)C(C)(C)[C@H]1Oc1ccc(C#N)c(Cl)c1 |r,wU:3.3,wD:18.20,(6.41,-47.96,;6.82,-46.48,;5.32,-46.87,;8.36,-46.48,;9.71,-47.22,;9.74,-48.76,;8.42,-49.56,;11.09,-49.51,;11.11,-51.04,;12.58,-51.5,;13.47,-50.25,;15.01,-50.23,;15.79,-51.56,;17.33,-51.54,;12.55,-49.01,;8.36,-44.94,;8.75,-43.44,;9.84,-45.33,;6.82,-44.94,;5.49,-44.16,;5.5,-42.62,;4.17,-41.86,;4.16,-40.33,;5.5,-39.55,;5.51,-38.01,;5.52,-36.48,;6.84,-40.33,;8.17,-39.56,;6.83,-41.87,)| Show InChI InChI=1S/C21H25ClN4O3/c1-20(2)18(25-17(28)14-11-24-26(12-14)7-8-27)21(3,4)19(20)29-15-6-5-13(10-23)16(22)9-15/h5-6,9,11-12,18-19,27H,7-8H2,1-4H3,(H,25,28)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50379780
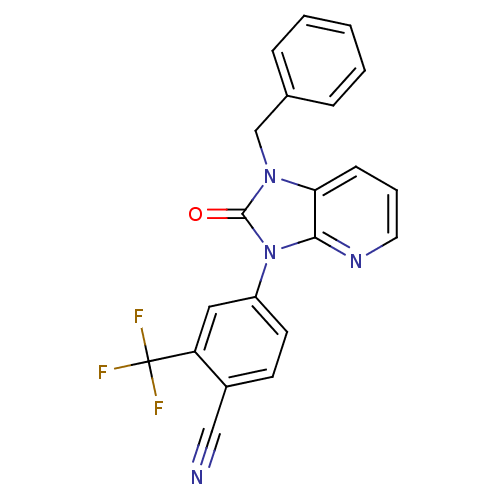
(CHEMBL2011371)Show SMILES FC(F)(F)c1cc(ccc1C#N)-n1c2ncccc2n(Cc2ccccc2)c1=O Show InChI InChI=1S/C21H13F3N4O/c22-21(23,24)17-11-16(9-8-15(17)12-25)28-19-18(7-4-10-26-19)27(20(28)29)13-14-5-2-1-3-6-14/h1-11H,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human LNAR cells expressing ARE-PSA-LUC by luminescence analysis in presence of R1881 |
Bioorg Med Chem Lett 22: 2572-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.116
BindingDB Entry DOI: 10.7270/Q2XP75Z5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50363360
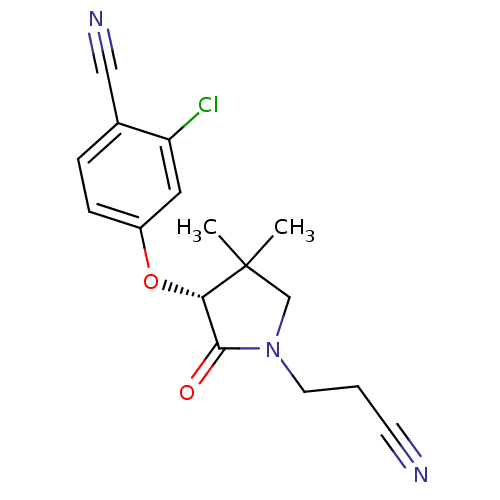
(CHEMBL1947185)Show SMILES CC1(C)CN(CCC#N)C(=O)[C@@H]1Oc1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C16H16ClN3O2/c1-16(2)10-20(7-3-6-18)15(21)14(16)22-12-5-4-11(9-19)13(17)8-12/h4-5,8,14H,3,7,10H2,1-2H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in human LNAR cells assessed as inhibition of R1881-induced luciferase activity by spectrophotomet... |
Bioorg Med Chem Lett 22: 1230-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.068
BindingDB Entry DOI: 10.7270/Q29S1RG3 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356992
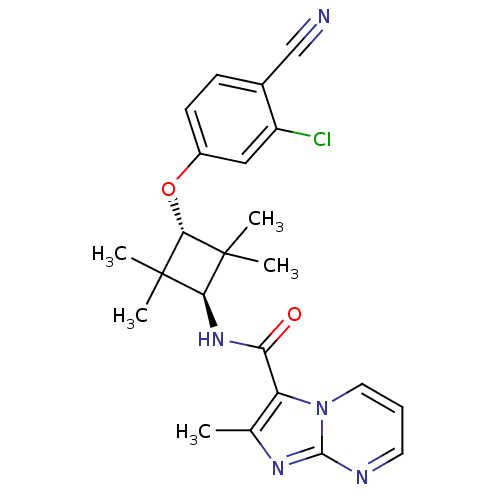
(CHEMBL1916248)Show SMILES Cc1nc2ncccn2c1C(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(Cl)c2)C1(C)C |r,wU:13.14,wD:17.19,(22.86,-40.64,;23.98,-39.59,;25.49,-39.88,;26.24,-38.53,;27.73,-38.19,;28.18,-36.72,;27.13,-35.59,;25.63,-35.94,;25.19,-37.41,;23.79,-38.07,;22.44,-37.32,;21.12,-38.12,;22.41,-35.78,;21.06,-35.04,;19.52,-35.04,;19.11,-36.52,;18.02,-35.43,;19.52,-33.5,;18.19,-32.72,;18.2,-31.18,;16.87,-30.42,;16.87,-28.88,;18.21,-28.11,;18.21,-26.57,;18.22,-25.04,;19.54,-28.89,;20.87,-28.12,;19.53,-30.42,;21.06,-33.5,;21.45,-32,;22.54,-33.89,)| Show InChI InChI=1S/C23H24ClN5O2/c1-13-17(29-10-6-9-26-21(29)27-13)18(30)28-19-22(2,3)20(23(19,4)5)31-15-8-7-14(12-25)16(24)11-15/h6-11,19-20H,1-5H3,(H,28,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356981

(CHEMBL1916238)Show SMILES CC(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(C)c2)C1(C)C |r,wU:4.3,wD:8.8,(19.82,-7.76,;18.47,-7.01,;17.15,-7.81,;18.44,-5.47,;17.09,-4.73,;15.55,-4.73,;15.14,-6.21,;14.05,-5.12,;15.55,-3.19,;14.22,-2.41,;14.22,-.87,;12.89,-.11,;12.89,1.43,;14.23,2.2,;14.24,3.74,;14.24,5.27,;15.56,1.42,;16.9,2.19,;15.56,-.11,;17.09,-3.19,;17.48,-1.69,;18.57,-3.58,)| Show InChI InChI=1S/C18H24N2O2/c1-11-9-14(8-7-13(11)10-19)22-16-17(3,4)15(18(16,5)6)20-12(2)21/h7-9,15-16H,1-6H3,(H,20,21)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50363350

(CHEMBL1946809)Show SMILES CC1(C)CN(Cc2nnc(o2)-c2ccccc2)C(=O)[C@@H]1Oc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C23H19F3N4O3/c1-22(2)13-30(12-18-28-29-20(33-18)14-6-4-3-5-7-14)21(31)19(22)32-16-9-8-15(11-27)17(10-16)23(24,25)26/h3-10,19H,12-13H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in human LNAR cells assessed as inhibition of R1881-induced luciferase activity by spectrophotomet... |
Bioorg Med Chem Lett 22: 1230-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.068
BindingDB Entry DOI: 10.7270/Q29S1RG3 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356986

(CHEMBL1916243)Show SMILES CC1(C)[C@H](NC(=O)c2ccn[nH]2)C(C)(C)[C@H]1Oc1ccc(C#N)c(Cl)c1 |r,wU:3.3,wD:15.17,(11.11,-20.91,;11.53,-19.43,;10.03,-19.82,;13.07,-19.43,;14.42,-20.17,;14.45,-21.71,;13.13,-22.51,;15.79,-22.46,;15.81,-23.99,;17.28,-24.45,;18.17,-23.2,;17.26,-21.96,;13.07,-17.89,;13.46,-16.39,;14.54,-18.28,;11.53,-17.89,;10.2,-17.11,;10.2,-15.57,;8.87,-14.81,;8.87,-13.27,;10.21,-12.5,;10.22,-10.96,;10.22,-9.43,;11.54,-13.28,;12.88,-12.51,;11.54,-14.81,)| Show InChI InChI=1S/C19H21ClN4O2/c1-18(2)16(23-15(25)14-7-8-22-24-14)19(3,4)17(18)26-12-6-5-11(10-21)13(20)9-12/h5-9,16-17H,1-4H3,(H,22,24)(H,23,25)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50363349

(CHEMBL1946807)Show SMILES CC(N1CC(C)(C)C(Oc2ccc(C#N)c(c2)C(F)(F)F)C1=O)c1nnc(o1)-c1cccc(F)c1 Show InChI InChI=1S/C24H20F4N4O3/c1-13(20-30-31-21(35-20)14-5-4-6-16(25)9-14)32-12-23(2,3)19(22(32)33)34-17-8-7-15(11-29)18(10-17)24(26,27)28/h4-10,13,19H,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in human LNAR cells assessed as inhibition of R1881-induced luciferase activity by spectrophotomet... |
Bioorg Med Chem Lett 22: 1230-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.068
BindingDB Entry DOI: 10.7270/Q29S1RG3 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50379772

(CHEMBL2011381)Show SMILES COc1cc(ccc1C#N)N1C(=O)C(C)(C)c2cc(ccc12)-c1cccnc1OC Show InChI InChI=1S/C24H21N3O3/c1-24(2)19-12-15(18-6-5-11-26-22(18)30-4)8-10-20(19)27(23(24)28)17-9-7-16(14-25)21(13-17)29-3/h5-13H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human LNAR cells expressing ARE-PSA-LUC by luminescence analysis in presence of R1881 |
Bioorg Med Chem Lett 22: 2572-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.116
BindingDB Entry DOI: 10.7270/Q2XP75Z5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356985

(CHEMBL1916242)Show SMILES CC1(C)[C@H](NC(=O)c2cnccn2)C(C)(C)[C@H]1Oc1ccc(C#N)c(Cl)c1 |r,wU:3.3,wD:16.18,(2.03,-20.07,;2.44,-18.6,;.94,-18.99,;3.98,-18.6,;5.33,-19.34,;5.36,-20.88,;4.04,-21.68,;6.71,-21.62,;6.73,-23.16,;8.08,-23.91,;9.4,-23.11,;9.36,-21.56,;8.01,-20.83,;3.98,-17.06,;4.37,-15.56,;5.46,-17.45,;2.44,-17.05,;1.11,-16.28,;1.12,-14.74,;-.21,-13.98,;-.21,-12.44,;1.13,-11.67,;1.13,-10.13,;1.14,-8.6,;2.46,-12.44,;3.79,-11.68,;2.45,-13.98,)| Show InChI InChI=1S/C20H21ClN4O2/c1-19(2)17(25-16(26)15-11-23-7-8-24-15)20(3,4)18(19)27-13-6-5-12(10-22)14(21)9-13/h5-9,11,17-18H,1-4H3,(H,25,26)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356996

(CHEMBL1916252)Show SMILES CC1(C)[C@H](NC(=O)c2ccn(CCC#N)n2)C(C)(C)[C@H]1Oc1ccc(C#N)c(Cl)c1 |r,wU:3.3,wD:19.21,(16.79,-47.6,;17.21,-46.12,;15.7,-46.51,;18.75,-46.12,;20.09,-46.86,;20.12,-48.4,;18.81,-49.2,;21.47,-49.15,;21.49,-50.68,;22.96,-51.14,;23.85,-49.88,;25.39,-49.87,;26.18,-51.19,;27.72,-51.18,;29.25,-51.16,;22.93,-48.65,;18.75,-44.58,;19.13,-43.08,;20.22,-44.97,;17.21,-44.58,;15.88,-43.8,;15.88,-42.26,;14.55,-41.5,;14.55,-39.96,;15.89,-39.19,;15.9,-37.65,;15.9,-36.12,;17.22,-39.96,;18.56,-39.2,;17.22,-41.5,)| Show InChI InChI=1S/C22H24ClN5O2/c1-21(2)19(26-18(29)17-8-11-28(27-17)10-5-9-24)22(3,4)20(21)30-15-7-6-14(13-25)16(23)12-15/h6-8,11-12,19-20H,5,10H2,1-4H3,(H,26,29)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50363352

(CHEMBL1946622)Show SMILES CC1(C)CNC(=O)[C@@H]1Oc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C14H13F3N2O2/c1-13(2)7-19-12(20)11(13)21-9-4-3-8(6-18)10(5-9)14(15,16)17/h3-5,11H,7H2,1-2H3,(H,19,20)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in human LNAR cells assessed as inhibition of R1881-induced luciferase activity by spectrophotomet... |
Bioorg Med Chem Lett 22: 1230-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.068
BindingDB Entry DOI: 10.7270/Q29S1RG3 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356982

(CHEMBL1916239)Show SMILES CC(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(c2)C(F)(F)F)C1(C)C |r,wU:4.3,wD:8.8,(30.13,-7.85,;28.78,-7.11,;27.46,-7.9,;28.75,-5.57,;27.4,-4.82,;25.86,-4.82,;25.45,-6.3,;24.36,-5.21,;25.86,-3.28,;24.53,-2.51,;24.54,-.97,;23.21,-.2,;23.2,1.33,;24.54,2.11,;24.55,3.65,;24.56,5.17,;25.88,1.33,;25.87,-.21,;27.21,2.1,;28.54,1.32,;27.22,3.64,;28.54,2.88,;27.4,-3.28,;27.79,-1.78,;28.88,-3.67,)| Show InChI InChI=1S/C18H21F3N2O2/c1-10(24)23-14-16(2,3)15(17(14,4)5)25-12-7-6-11(9-22)13(8-12)18(19,20)21/h6-8,14-15H,1-5H3,(H,23,24)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50379771

(CHEMBL2011377)Show SMILES Clc1cc(ccc1C#N)-n1c2ccccc2n(Cc2nc(no2)-c2ccccn2)c1=O Show InChI InChI=1S/C22H13ClN6O2/c23-16-11-15(9-8-14(16)12-24)29-19-7-2-1-6-18(19)28(22(29)30)13-20-26-21(27-31-20)17-5-3-4-10-25-17/h1-11H,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human LNAR cells expressing ARE-PSA-LUC by luminescence analysis in presence of R1881 |
Bioorg Med Chem Lett 22: 2572-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.116
BindingDB Entry DOI: 10.7270/Q2XP75Z5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50363354

(CHEMBL1946811)Show SMILES CC1(C)CN(CCC#N)C(=O)[C@@H]1Oc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C17H16F3N3O2/c1-16(2)10-23(7-3-6-21)15(24)14(16)25-12-5-4-11(9-22)13(8-12)17(18,19)20/h4-5,8,14H,3,7,10H2,1-2H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in human LNAR cells assessed as inhibition of R1881-induced luciferase activity by spectrophotomet... |
Bioorg Med Chem Lett 22: 1230-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.068
BindingDB Entry DOI: 10.7270/Q29S1RG3 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356994
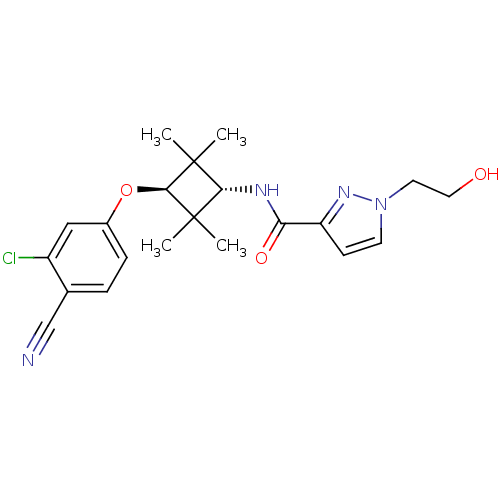
(CHEMBL1916250)Show SMILES CC1(C)[C@H](NC(=O)c2ccn(CCO)n2)C(C)(C)[C@H]1Oc1ccc(C#N)c(Cl)c1 |r,wU:3.3,wD:18.20,(-6.58,-47.2,;-6.17,-45.72,;-7.67,-46.11,;-4.63,-45.72,;-3.28,-46.46,;-3.25,-48,;-4.57,-48.8,;-1.9,-48.75,;-1.88,-50.28,;-.41,-50.74,;.48,-49.48,;2.02,-49.47,;2.8,-50.79,;4.34,-50.78,;-.44,-48.25,;-4.63,-44.18,;-4.24,-42.68,;-3.15,-44.57,;-6.17,-44.18,;-7.5,-43.4,;-7.49,-41.86,;-8.82,-41.1,;-8.82,-39.56,;-7.48,-38.79,;-7.48,-37.25,;-7.47,-35.72,;-6.15,-39.56,;-4.82,-38.8,;-6.16,-41.1,)| Show InChI InChI=1S/C21H25ClN4O3/c1-20(2)18(24-17(28)16-7-8-26(25-16)9-10-27)21(3,4)19(20)29-14-6-5-13(12-23)15(22)11-14/h5-8,11,18-19,27H,9-10H2,1-4H3,(H,24,28)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356993

(CHEMBL1916249)Show SMILES COCCn1ccc(n1)C(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(Cl)c2)C1(C)C |r,wU:12.12,wD:16.17,(43.88,-41.99,;43.1,-40.67,;41.56,-40.68,;40.78,-39.36,;39.24,-39.37,;38.34,-40.63,;36.87,-40.17,;36.86,-38.63,;38.32,-38.14,;35.51,-37.89,;34.19,-38.69,;35.48,-36.35,;34.13,-35.61,;32.59,-35.61,;32.18,-37.08,;31.09,-36,;32.59,-34.06,;31.26,-33.29,;31.26,-31.75,;29.93,-30.99,;29.93,-29.45,;31.27,-28.68,;31.28,-27.14,;31.28,-25.61,;32.6,-29.45,;33.94,-28.69,;32.6,-30.99,;34.13,-34.07,;34.52,-32.57,;35.61,-34.46,)| Show InChI InChI=1S/C22H27ClN4O3/c1-21(2)19(25-18(28)17-8-9-27(26-17)10-11-29-5)22(3,4)20(21)30-15-7-6-14(13-24)16(23)12-15/h6-9,12,19-20H,10-11H2,1-5H3,(H,25,28)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356999

(CHEMBL1916412)Show SMILES Cc1nc(O[C@H]2C(C)(C)[C@H](NC(=O)c3cnc4ncccn34)C2(C)C)ccc1C#N |r,wU:9.9,wD:5.4,(28.73,2.19,;27.4,1.42,;27.39,-.11,;26.05,-.87,;26.05,-2.41,;27.38,-3.19,;27.38,-4.73,;26.97,-6.21,;25.88,-5.12,;28.92,-4.73,;30.27,-5.47,;30.3,-7.01,;28.98,-7.81,;31.65,-7.76,;31.84,-9.28,;33.35,-9.57,;34.09,-8.22,;35.59,-7.88,;36.04,-6.41,;34.99,-5.28,;33.49,-5.63,;33.04,-7.1,;28.92,-3.19,;29.31,-1.69,;30.4,-3.58,;24.72,-.11,;24.72,1.43,;26.06,2.2,;26.07,3.74,;26.08,5.27,)| Show InChI InChI=1S/C22H24N6O2/c1-13-14(11-23)7-8-16(26-13)30-19-21(2,3)18(22(19,4)5)27-17(29)15-12-25-20-24-9-6-10-28(15)20/h6-10,12,18-19H,1-5H3,(H,27,29)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356983

(CHEMBL1916240)Show SMILES CC(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(cc2Cl)C#N)C1(C)C |r,wU:4.3,wD:8.8,(43.08,-6.9,;41.73,-6.16,;40.41,-6.95,;41.7,-4.62,;40.35,-3.87,;38.81,-3.87,;38.4,-5.35,;37.31,-4.26,;38.82,-2.33,;37.48,-1.56,;37.49,-.02,;38.82,.74,;38.83,2.28,;37.5,3.06,;36.16,2.28,;36.16,.74,;34.83,-.03,;37.51,4.59,;37.51,6.12,;40.35,-2.33,;40.74,-.83,;41.83,-2.72,)| Show InChI InChI=1S/C17H21ClN2O2/c1-10(21)20-14-16(2,3)15(17(14,4)5)22-13-7-6-11(9-19)8-12(13)18/h6-8,14-15H,1-5H3,(H,20,21)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356997
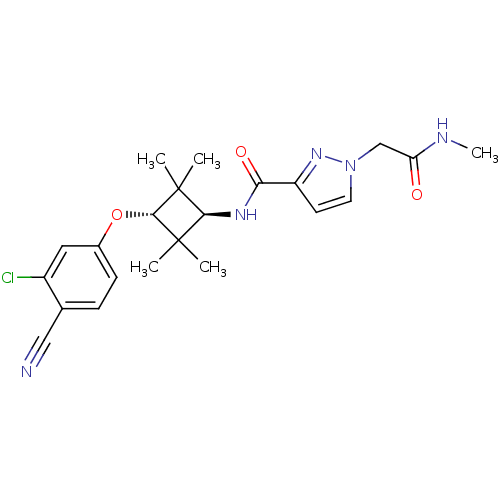
(CHEMBL1916253)Show SMILES CNC(=O)Cn1ccc(n1)C(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(Cl)c2)C1(C)C |r,wU:13.13,wD:17.18,(4.4,-9.25,;3.62,-7.92,;2.08,-7.94,;1.32,-9.28,;1.3,-6.61,;-.24,-6.63,;-1.14,-7.88,;-2.61,-7.42,;-2.62,-5.89,;-1.16,-5.39,;-3.97,-5.15,;-5.29,-5.94,;-4,-3.61,;-5.35,-2.86,;-6.89,-2.86,;-7.3,-4.34,;-8.39,-3.25,;-6.89,-1.32,;-8.22,-.55,;-8.22,.99,;-9.55,1.76,;-9.55,3.29,;-8.21,4.07,;-8.2,5.61,;-8.2,7.13,;-6.88,3.29,;-5.54,4.06,;-6.88,1.75,;-5.35,-1.32,;-4.96,.18,;-3.87,-1.71,)| Show InChI InChI=1S/C22H26ClN5O3/c1-21(2)19(26-18(30)16-8-9-28(27-16)12-17(29)25-5)22(3,4)20(21)31-14-7-6-13(11-24)15(23)10-14/h6-10,19-20H,12H2,1-5H3,(H,25,29)(H,26,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50379773

(CHEMBL2011380)Show SMILES CNC(=O)c1ccc(cc1F)-c1ccc2N(C(=O)C(C)(C)c2c1)c1ccc(C#N)c(OC)c1 Show InChI InChI=1S/C26H22FN3O3/c1-26(2)20-11-15(16-6-9-19(21(27)12-16)24(31)29-3)7-10-22(20)30(25(26)32)18-8-5-17(14-28)23(13-18)33-4/h5-13H,1-4H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human LNAR cells expressing ARE-PSA-LUC by luminescence analysis in presence of R1881 |
Bioorg Med Chem Lett 22: 2572-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.116
BindingDB Entry DOI: 10.7270/Q2XP75Z5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50379783
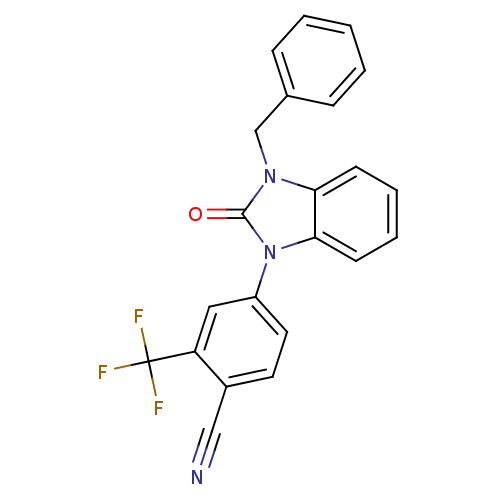
(CHEMBL2011362)Show SMILES FC(F)(F)c1cc(ccc1C#N)-n1c2ccccc2n(Cc2ccccc2)c1=O Show InChI InChI=1S/C22H14F3N3O/c23-22(24,25)18-12-17(11-10-16(18)13-26)28-20-9-5-4-8-19(20)27(21(28)29)14-15-6-2-1-3-7-15/h1-12H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human LNAR cells expressing ARE-PSA-LUC by luminescence analysis in presence of R1881 |
Bioorg Med Chem Lett 22: 2572-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.116
BindingDB Entry DOI: 10.7270/Q2XP75Z5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356998

(CHEMBL1916254)Show SMILES CN(C)C(=O)Cn1ccc(n1)C(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(Cl)c2)C1(C)C |r,wU:14.14,wD:18.19,(20.82,-9.72,;20.04,-8.4,;20.79,-7.05,;18.5,-8.41,;17.74,-9.75,;17.71,-7.09,;16.17,-7.1,;15.28,-8.36,;13.81,-7.9,;13.79,-6.36,;15.26,-5.87,;12.45,-5.62,;11.13,-6.42,;12.42,-4.08,;11.07,-3.34,;9.53,-3.34,;9.12,-4.81,;8.03,-3.73,;9.53,-1.79,;8.2,-1.02,;8.2,.52,;6.87,1.28,;6.87,2.82,;8.21,3.59,;8.22,5.13,;8.22,6.66,;9.54,2.82,;10.88,3.58,;9.54,1.28,;11.07,-1.8,;11.46,-.3,;12.55,-2.19,)| Show InChI InChI=1S/C23H28ClN5O3/c1-22(2)20(26-19(31)17-9-10-29(27-17)13-18(30)28(5)6)23(3,4)21(22)32-15-8-7-14(12-25)16(24)11-15/h7-11,20-21H,13H2,1-6H3,(H,26,31)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50356979
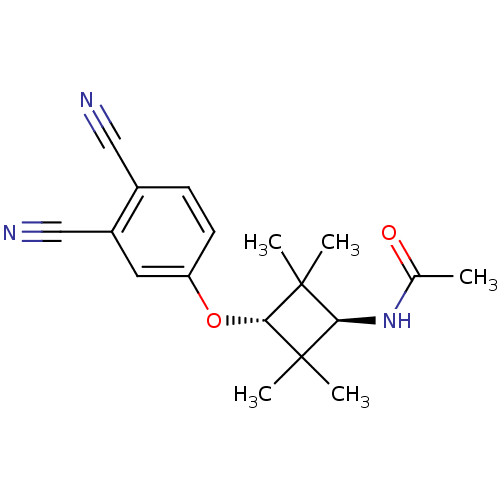
(CHEMBL1916236)Show SMILES CC(=O)N[C@H]1C(C)(C)[C@H](Oc2ccc(C#N)c(c2)C#N)C1(C)C |r,wU:4.3,wD:8.8,(-1.54,-7.24,;-2.89,-6.5,;-4.21,-7.3,;-2.92,-4.96,;-4.27,-4.22,;-5.81,-4.22,;-6.22,-5.69,;-7.31,-4.6,;-5.81,-2.67,;-7.14,-1.9,;-7.14,-.36,;-8.47,.4,;-8.47,1.94,;-7.13,2.71,;-7.12,4.26,;-7.11,5.79,;-5.79,1.94,;-5.8,.4,;-4.45,2.7,;-3.12,3.47,;-4.27,-2.68,;-3.88,-1.17,;-2.79,-3.06,)| Show InChI InChI=1S/C18H21N3O2/c1-11(22)21-15-17(2,3)16(18(15,4)5)23-14-7-6-12(9-19)13(8-14)10-20/h6-8,15-16H,1-5H3,(H,21,22)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay |
J Med Chem 54: 7693-704 (2011)
Article DOI: 10.1021/jm201059s
BindingDB Entry DOI: 10.7270/Q2348KS9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50379775
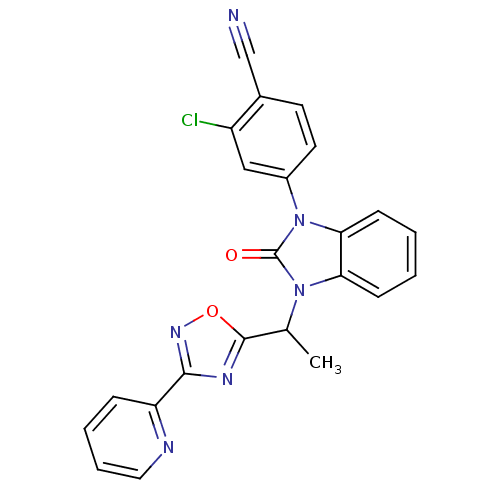
(CHEMBL2011378)Show SMILES CC(c1nc(no1)-c1ccccn1)n1c2ccccc2n(-c2ccc(C#N)c(Cl)c2)c1=O Show InChI InChI=1S/C23H15ClN6O2/c1-14(22-27-21(28-32-22)18-6-4-5-11-26-18)29-19-7-2-3-8-20(19)30(23(29)31)16-10-9-15(13-25)17(24)12-16/h2-12,14H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human LNAR cells expressing ARE-PSA-LUC by luminescence analysis in presence of R1881 |
Bioorg Med Chem Lett 22: 2572-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.116
BindingDB Entry DOI: 10.7270/Q2XP75Z5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50379775

(CHEMBL2011378)Show SMILES CC(c1nc(no1)-c1ccccn1)n1c2ccccc2n(-c2ccc(C#N)c(Cl)c2)c1=O Show InChI InChI=1S/C23H15ClN6O2/c1-14(22-27-21(28-32-22)18-6-4-5-11-26-18)29-19-7-2-3-8-20(19)30(23(29)31)16-10-9-15(13-25)17(24)12-16/h2-12,14H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human LNAR cells expressing ARE-PSA-LUC by luminescence analysis in presence of R1881 |
Bioorg Med Chem Lett 22: 2572-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.116
BindingDB Entry DOI: 10.7270/Q2XP75Z5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50379774
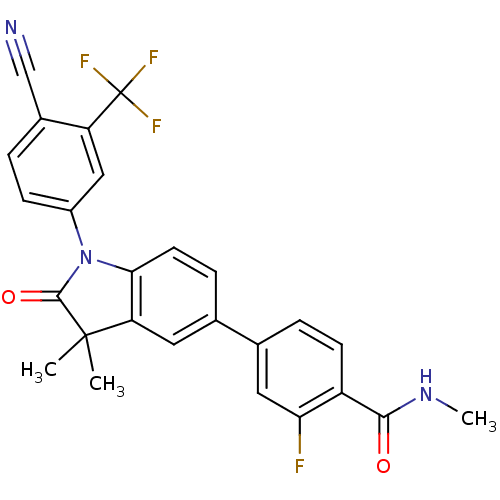
(CHEMBL2011379)Show SMILES CNC(=O)c1ccc(cc1F)-c1ccc2N(C(=O)C(C)(C)c2c1)c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C26H19F4N3O2/c1-25(2)20-10-14(15-5-8-18(21(27)11-15)23(34)32-3)6-9-22(20)33(24(25)35)17-7-4-16(13-31)19(12-17)26(28,29)30/h4-12H,1-3H3,(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human LNAR cells expressing ARE-PSA-LUC by luminescence analysis in presence of R1881 |
Bioorg Med Chem Lett 22: 2572-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.116
BindingDB Entry DOI: 10.7270/Q2XP75Z5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50363355

(CHEMBL1946812)Show SMILES CC1(C)CN(CCO)C(=O)[C@@H]1Oc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C16H17F3N2O3/c1-15(2)9-21(5-6-22)14(23)13(15)24-11-4-3-10(8-20)12(7-11)16(17,18)19/h3-4,7,13,22H,5-6,9H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in human LNAR cells assessed as inhibition of R1881-induced luciferase activity by spectrophotomet... |
Bioorg Med Chem Lett 22: 1230-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.068
BindingDB Entry DOI: 10.7270/Q29S1RG3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data