 Found 193 hits with Last Name = 'fato' and Initial = 'r'
Found 193 hits with Last Name = 'fato' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50278990

(CHEMBL4164524)Show InChI InChI=1S/C18H21Cl2N3O2/c19-16-11-15(12-17(20)23-16)22-18(24)25-10-6-2-5-9-21-13-14-7-3-1-4-8-14/h1,3-4,7-8,11-12,21H,2,5-6,9-10,13H2,(H,22,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50278986

(CHEMBL4161860)Show InChI InChI=1S/C19H23Cl2N3O/c20-17-12-16(13-18(21)24-17)19(25)23-11-7-2-1-6-10-22-14-15-8-4-3-5-9-15/h3-5,8-9,12-13,22H,1-2,6-7,10-11,14H2,(H,23,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50278968
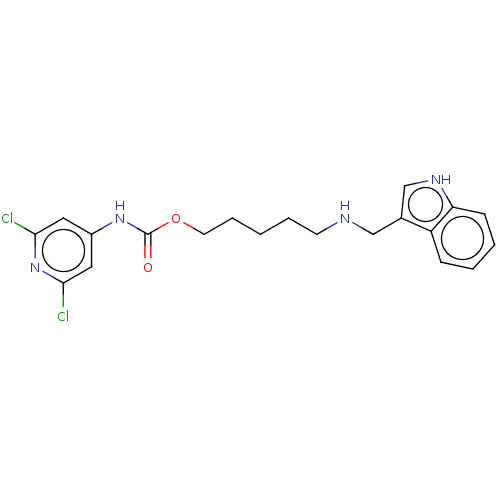
(CHEMBL4164186)Show SMILES Clc1cc(NC(=O)OCCCCCNCc2c[nH]c3ccccc23)cc(Cl)n1 Show InChI InChI=1S/C20H22Cl2N4O2/c21-18-10-15(11-19(22)26-18)25-20(27)28-9-5-1-4-8-23-12-14-13-24-17-7-3-2-6-16(14)17/h2-3,6-7,10-11,13,23-24H,1,4-5,8-9,12H2,(H,25,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50278987
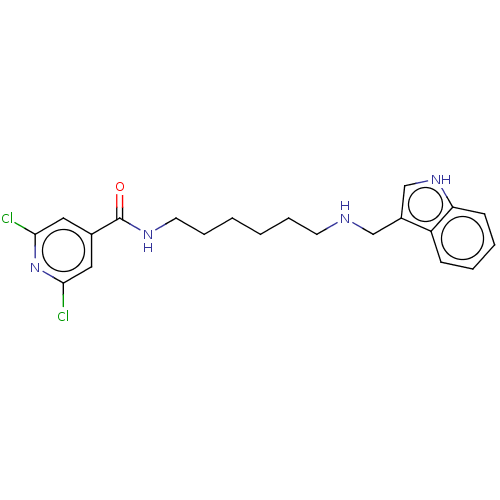
(CHEMBL4165299)Show SMILES Clc1cc(cc(Cl)n1)C(=O)NCCCCCCNCc1c[nH]c2ccccc12 Show InChI InChI=1S/C21H24Cl2N4O/c22-19-11-15(12-20(23)27-19)21(28)25-10-6-2-1-5-9-24-13-16-14-26-18-8-4-3-7-17(16)18/h3-4,7-8,11-12,14,24,26H,1-2,5-6,9-10,13H2,(H,25,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50278969

(CHEMBL4160790)Show InChI InChI=1S/C19H23Cl2N3O2/c20-17-12-16(13-18(21)24-17)23-19(25)26-11-7-2-1-6-10-22-14-15-8-4-3-5-9-15/h3-5,8-9,12-13,22H,1-2,6-7,10-11,14H2,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50278984

(CHEMBL4168710)Show InChI InChI=1S/C19H23Cl2N3O3/c1-26-16-8-4-3-7-14(16)13-22-9-5-2-6-10-27-19(25)23-15-11-17(20)24-18(21)12-15/h3-4,7-8,11-12,22H,2,5-6,9-10,13H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50278970
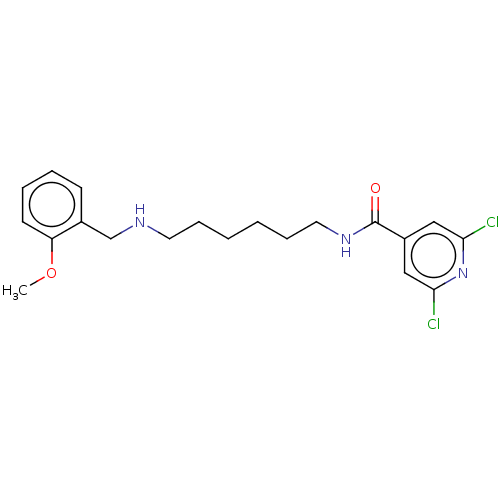
(CHEMBL4175914)Show InChI InChI=1S/C20H25Cl2N3O2/c1-27-17-9-5-4-8-15(17)14-23-10-6-2-3-7-11-24-20(26)16-12-18(21)25-19(22)13-16/h4-5,8-9,12-13,23H,2-3,6-7,10-11,14H2,1H3,(H,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50278983

(CHEMBL4172510)Show InChI InChI=1S/C18H22BrN3O2/c19-16-14-21-11-9-17(16)22-18(23)24-12-6-2-5-10-20-13-15-7-3-1-4-8-15/h1,3-4,7-9,11,14,20H,2,5-6,10,12-13H2,(H,21,22,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50278487

(CHEMBL3585376)Show SMILES CN(C)CCOc1cc2ccc(OC3=CC(=O)c4cc5ccccc5cc4C3=O)cc2oc1=O |t:13| Show InChI InChI=1S/C27H21NO6/c1-28(2)9-10-32-25-13-18-7-8-19(14-23(18)34-27(25)31)33-24-15-22(29)20-11-16-5-3-4-6-17(16)12-21(20)26(24)30/h3-8,11-15H,9-10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of 125-I echistatin from Vitronectin receptor (alpha v beta3) |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50278488
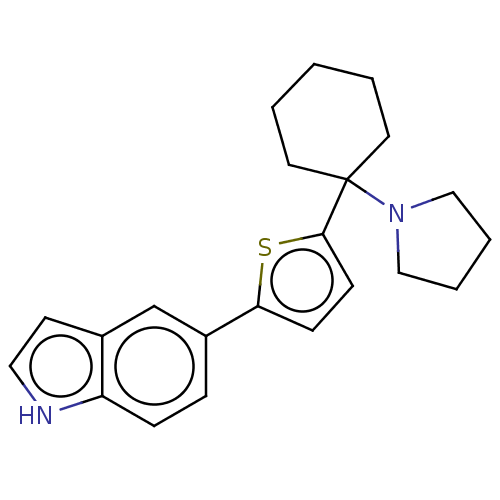
(CHEMBL4159241)Show SMILES C1CCN(C1)C1(CCCCC1)c1ccc(s1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C22H26N2S/c1-2-11-22(12-3-1,24-14-4-5-15-24)21-9-8-20(25-21)18-6-7-19-17(16-18)10-13-23-19/h6-10,13,16,23H,1-5,11-12,14-15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as... |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50278486
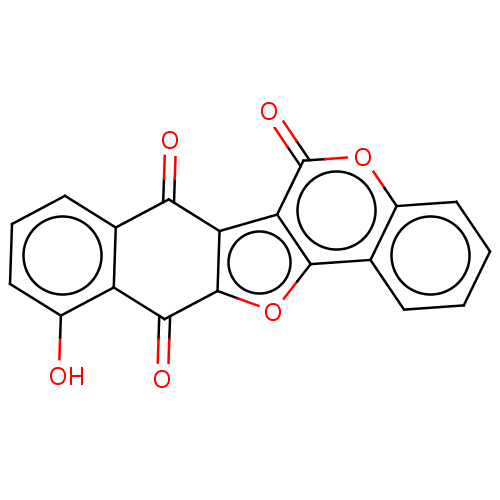
(CHEMBL4160100)Show SMILES Oc1cccc2C(=O)c3c(oc4c3c(=O)oc3ccccc43)C(=O)c12 Show InChI InChI=1S/C19H8O6/c20-10-6-3-5-9-12(10)16(22)18-13(15(9)21)14-17(25-18)8-4-1-2-7-11(8)24-19(14)23/h1-7,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione d... |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM77970
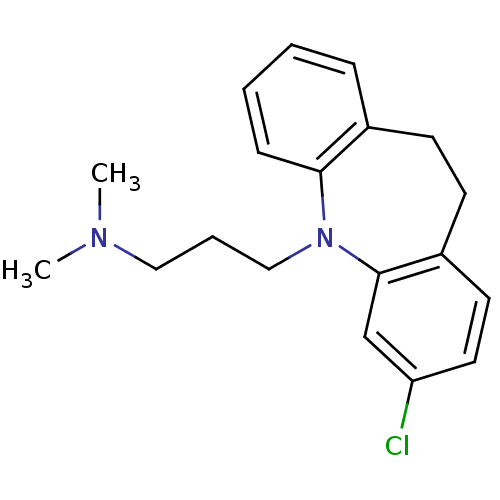
(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as... |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50278489
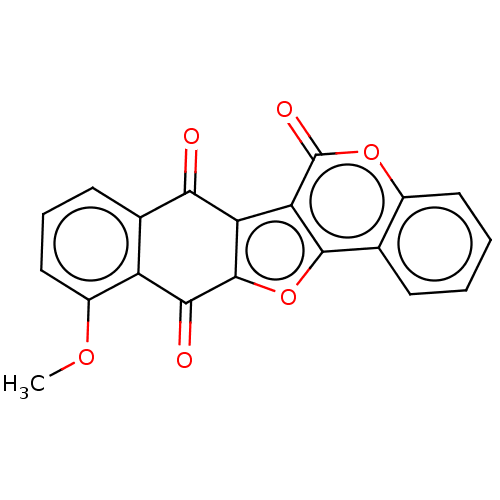
(CHEMBL4170316)Show SMILES COc1cccc2C(=O)c3c(oc4c3c(=O)oc3ccccc43)C(=O)c12 Show InChI InChI=1S/C20H10O6/c1-24-12-8-4-6-10-13(12)17(22)19-14(16(10)21)15-18(26-19)9-5-2-3-7-11(9)25-20(15)23/h2-8H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothi... |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10512
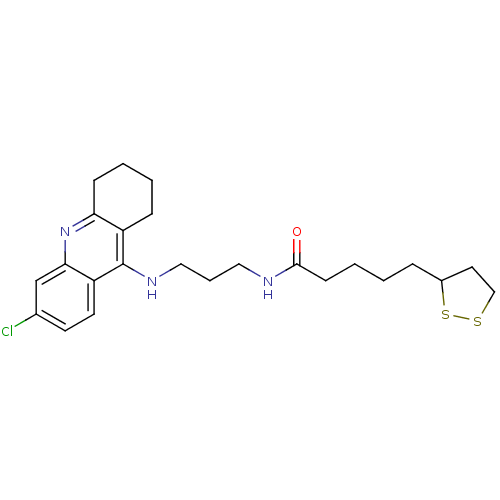
(CHEMBL194823 | N-{3-[(6-chloro-1,2,3,4-tetrahydroa...)Show SMILES Clc1ccc2c(NCCCNC(=O)CCCCC3CCSS3)c3CCCCc3nc2c1 Show InChI InChI=1S/C24H32ClN3OS2/c25-17-10-11-20-22(16-17)28-21-8-3-2-7-19(21)24(20)27-14-5-13-26-23(29)9-4-1-6-18-12-15-30-31-18/h10-11,16,18H,1-9,12-15H2,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.253 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's method |
J Med Chem 52: 7883-6 (2009)
Article DOI: 10.1021/jm901123n
BindingDB Entry DOI: 10.7270/Q2571C2D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028685

(CHEMBL3356536)Show SMILES Oc1cccc2C(=O)C(NCCNc3c4CCCCc4nc4cc(Cl)ccc34)=CC(=O)c12 |c:30| Show InChI InChI=1S/C25H22ClN3O3/c26-14-8-9-16-19(12-14)29-18-6-2-1-4-15(18)24(16)28-11-10-27-20-13-22(31)23-17(25(20)32)5-3-7-21(23)30/h3,5,7-9,12-13,27,30H,1-2,4,6,10-11H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50231951
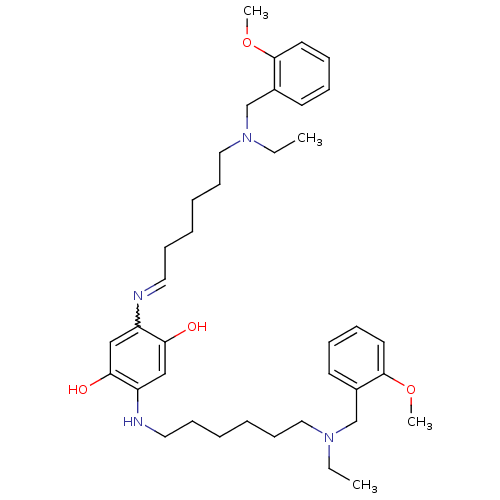
(2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-19-11-13-21-37(31)45-3)25-17-9-7-15-23-39-33-27-36(44)34(28-35(33)43)40-24-16-8-10-18-26-42(6-2)30-32-20-12-14-22-38(32)46-4/h11-14,19-23,27-28,40,43-44H,5-10,15-18,24-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's method |
J Med Chem 52: 7883-6 (2009)
Article DOI: 10.1021/jm901123n
BindingDB Entry DOI: 10.7270/Q2571C2D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50231951

(2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-19-11-13-21-37(31)45-3)25-17-9-7-15-23-39-33-27-36(44)34(28-35(33)43)40-24-16-8-10-18-26-42(6-2)30-32-20-12-14-22-38(32)46-4/h11-14,19-23,27-28,40,43-44H,5-10,15-18,24-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028681
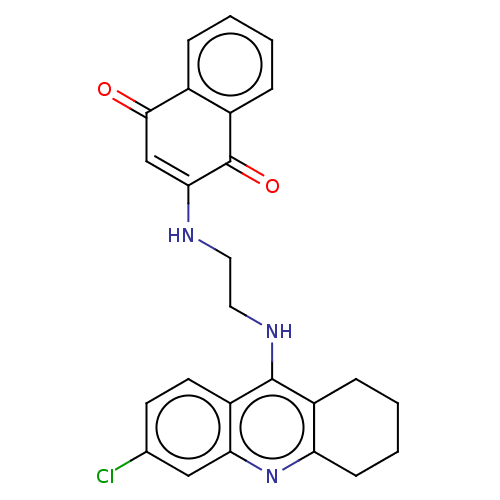
(CHEMBL3356532)Show SMILES Clc1ccc2c(NCCNC3=CC(=O)c4ccccc4C3=O)c3CCCCc3nc2c1 |t:10| Show InChI InChI=1S/C25H22ClN3O2/c26-15-9-10-19-21(13-15)29-20-8-4-3-7-18(20)24(19)28-12-11-27-22-14-23(30)16-5-1-2-6-17(16)25(22)31/h1-2,5-6,9-10,13-14,27H,3-4,7-8,11-12H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221914
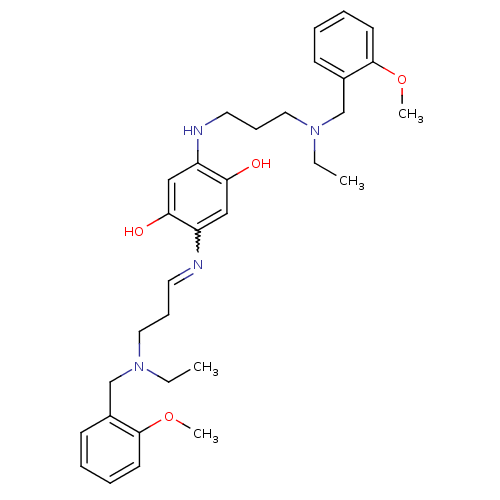
(2,5-bis{3-[ethyl(2-methoxybenzyl)amino]propylamino...)Show SMILES CCN(CCCNc1cc(O)c(cc1O)N=CCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:15.15| Show InChI InChI=1S/C32H44N4O4/c1-5-35(23-25-13-7-9-15-31(25)39-3)19-11-17-33-27-21-30(38)28(22-29(27)37)34-18-12-20-36(6-2)24-26-14-8-10-16-32(26)40-4/h7-10,13-17,21-22,34,37-38H,5-6,11-12,18-20,23-24H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221909

(2,5-bis{5-[ethyl(2-methoxybenzyl)amino]pentylamino...)Show SMILES CCN(CCCCCNc1cc(O)c(cc1O)N=CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:17.17| Show InChI InChI=1S/C36H52N4O4/c1-5-39(27-29-17-9-11-19-35(29)43-3)23-15-7-13-21-37-31-25-34(42)32(26-33(31)41)38-22-14-8-16-24-40(6-2)28-30-18-10-12-20-36(30)44-4/h9-12,17-21,25-26,38,41-42H,5-8,13-16,22-24,27-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221918

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(Br)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1Br)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54Br2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221908

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(Cl)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1Cl)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54Cl2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028691

(CHEMBL3356951)Show SMILES ClC1=C(NCCNc2c3CCCCc3nc3cc(Cl)ccc23)C(=O)c2ccccc2C1=O |c:1| Show InChI InChI=1S/C25H21Cl2N3O2/c26-14-9-10-18-20(13-14)30-19-8-4-3-7-17(19)22(18)28-11-12-29-23-21(27)24(31)15-5-1-2-6-16(15)25(23)32/h1-2,5-6,9-10,13,29H,3-4,7-8,11-12H2,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221926
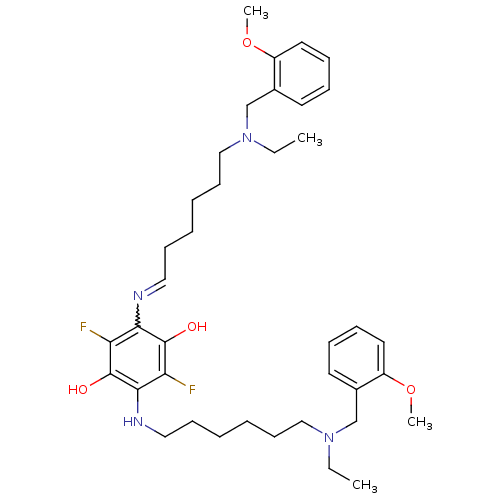
(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(F)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1F)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54F2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028682
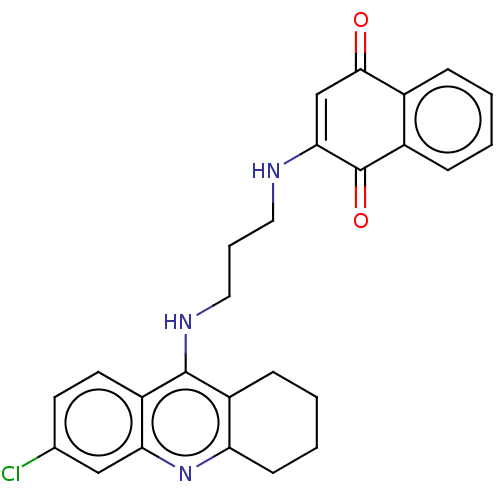
(CHEMBL3356533)Show SMILES Clc1ccc2c(NCCCNC3=CC(=O)c4ccccc4C3=O)c3CCCCc3nc2c1 |t:11| Show InChI InChI=1S/C26H24ClN3O2/c27-16-10-11-20-22(14-16)30-21-9-4-3-8-19(21)25(20)29-13-5-12-28-23-15-24(31)17-6-1-2-7-18(17)26(23)32/h1-2,6-7,10-11,14-15,28H,3-5,8-9,12-13H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50028679
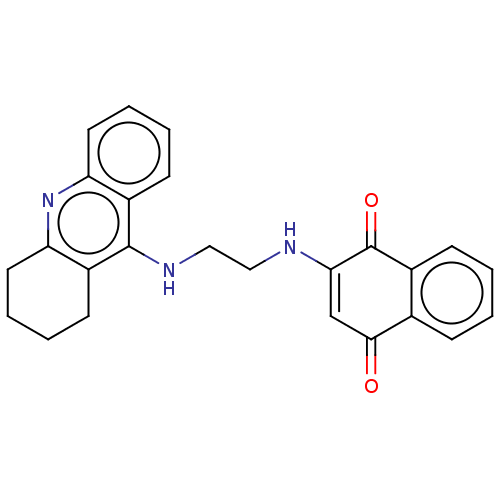
(CHEMBL3356530)Show SMILES O=C1C=C(NCCNc2c3CCCCc3nc3ccccc23)C(=O)c2ccccc12 |t:2| Show InChI InChI=1S/C25H23N3O2/c29-23-15-22(25(30)17-8-2-1-7-16(17)23)26-13-14-27-24-18-9-3-5-11-20(18)28-21-12-6-4-10-19(21)24/h1-3,5,7-9,11,15,26H,4,6,10,12-14H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221929
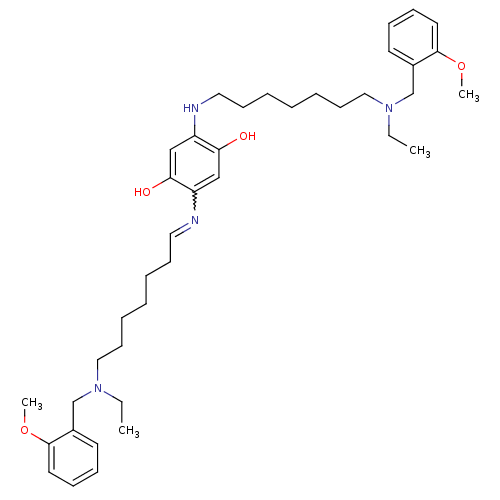
(2,5-bis{7-[ethyl(2-methoxybenzyl)amino]heptylamino...)Show SMILES CCN(CCCCCCCNc1cc(O)c(cc1O)N=CCCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:19.19| Show InChI InChI=1S/C40H60N4O4/c1-5-43(31-33-21-13-15-23-39(33)47-3)27-19-11-7-9-17-25-41-35-29-38(46)36(30-37(35)45)42-26-18-10-8-12-20-28-44(6-2)32-34-22-14-16-24-40(34)48-4/h13-16,21-25,29-30,42,45-46H,5-12,17-20,26-28,31-32H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028692

(CHEMBL3356952)Show SMILES ClC1=C(NCCCNc2c3CCCCc3nc3cc(Cl)ccc23)C(=O)c2ccccc2C1=O |c:1| Show InChI InChI=1S/C26H23Cl2N3O2/c27-15-10-11-19-21(14-15)31-20-9-4-3-8-18(20)23(19)29-12-5-13-30-24-22(28)25(32)16-6-1-2-7-17(16)26(24)33/h1-2,6-7,10-11,14,30H,3-5,8-9,12-13H2,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028686
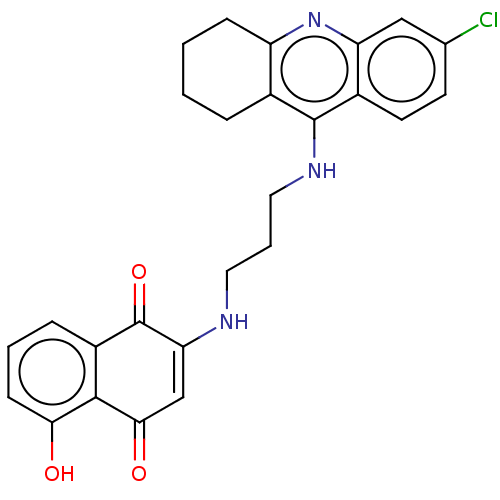
(CHEMBL3356537)Show SMILES Oc1cccc2C(=O)C(NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)=CC(=O)c12 |c:31| Show InChI InChI=1S/C26H24ClN3O3/c27-15-9-10-17-20(13-15)30-19-7-2-1-5-16(19)25(17)29-12-4-11-28-21-14-23(32)24-18(26(21)33)6-3-8-22(24)31/h3,6,8-10,13-14,28,31H,1-2,4-5,7,11-12H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221922
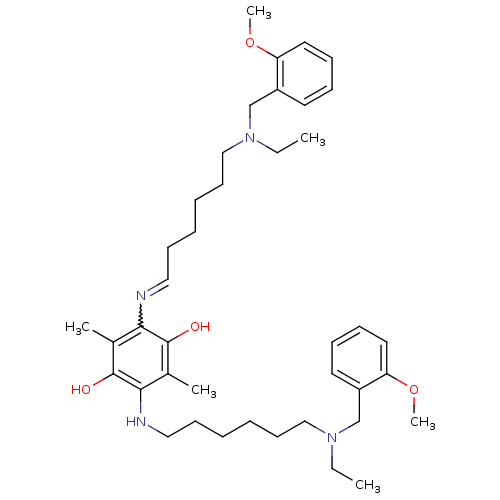
(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(C)c(O)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(C)c1O)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C40H60N4O4/c1-7-43(29-33-21-13-15-23-35(33)47-5)27-19-11-9-17-25-41-37-31(3)40(46)38(32(4)39(37)45)42-26-18-10-12-20-28-44(8-2)30-34-22-14-16-24-36(34)48-6/h13-16,21-25,42,45-46H,7-12,17-20,26-30H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10512

(CHEMBL194823 | N-{3-[(6-chloro-1,2,3,4-tetrahydroa...)Show SMILES Clc1ccc2c(NCCCNC(=O)CCCCC3CCSS3)c3CCCCc3nc2c1 Show InChI InChI=1S/C24H32ClN3OS2/c25-17-10-11-20-22(16-17)28-21-8-3-2-7-19(21)24(20)27-14-5-13-26-23(29)9-4-1-6-18-12-15-30-31-18/h10-11,16,18H,1-9,12-15H2,(H,26,29)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE by Ellman's method |
J Med Chem 52: 7883-6 (2009)
Article DOI: 10.1021/jm901123n
BindingDB Entry DOI: 10.7270/Q2571C2D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221905
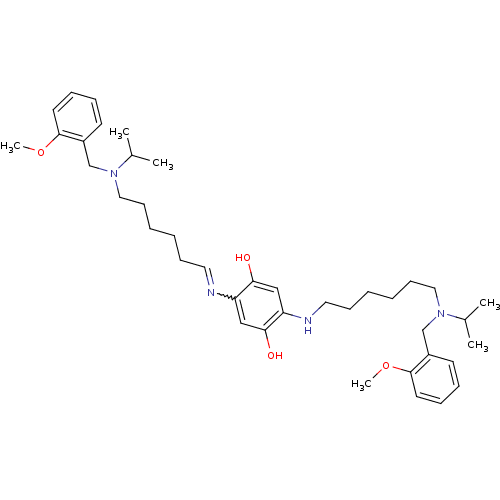
(2,5-bis{6-[isopropyl(2-methoxybenzyl)amino]hexylam...)Show SMILES COc1ccccc1CN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(Cc1ccccc1OC)C(C)C)C(C)C |w:25.26| Show InChI InChI=1S/C40H60N4O4/c1-31(2)43(29-33-19-11-13-21-39(33)47-5)25-17-9-7-15-23-41-35-27-38(46)36(28-37(35)45)42-24-16-8-10-18-26-44(32(3)4)30-34-20-12-14-22-40(34)48-6/h11-14,19-23,27-28,31-32,42,45-46H,7-10,15-18,24-26,29-30H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221910
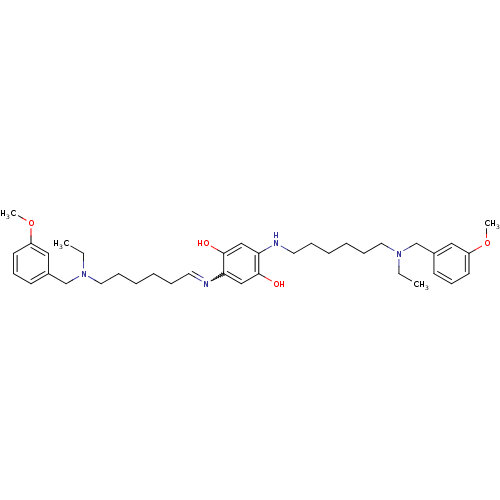
(2,5-bis{6-[ethyl(3-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1cccc(OC)c1)Cc1cccc(OC)c1 |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-17-15-19-33(25-31)45-3)23-13-9-7-11-21-39-35-27-38(44)36(28-37(35)43)40-22-12-8-10-14-24-42(6-2)30-32-18-16-20-34(26-32)46-4/h15-21,25-28,40,43-44H,5-14,22-24,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221921

(2,5-bis{4-[ethyl(2-methoxybenzyl)amino]butylamino}...)Show SMILES CCN(CCCCNc1cc(O)c(cc1O)N=CCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:16.16| Show InChI InChI=1S/C34H48N4O4/c1-5-37(25-27-15-7-9-17-33(27)41-3)21-13-11-19-35-29-23-32(40)30(24-31(29)39)36-20-12-14-22-38(6-2)26-28-16-8-10-18-34(28)42-4/h7-10,15-19,23-24,36,39-40H,5-6,11-14,20-22,25-26H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028679

(CHEMBL3356530)Show SMILES O=C1C=C(NCCNc2c3CCCCc3nc3ccccc23)C(=O)c2ccccc12 |t:2| Show InChI InChI=1S/C25H23N3O2/c29-23-15-22(25(30)17-8-2-1-7-16(17)23)26-13-14-27-24-18-9-3-5-11-20(18)28-21-12-6-4-10-19(21)24/h1-3,5,7-9,11,15,26H,4,6,10,12-14H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50028684

(CHEMBL3356535)Show SMILES Oc1cccc2C(=O)C(NCCCNc3c4CCCCc4nc4ccccc34)=CC(=O)c12 |c:30| Show InChI InChI=1S/C26H25N3O3/c30-22-12-5-9-18-24(22)23(31)15-21(26(18)32)27-13-6-14-28-25-16-7-1-3-10-19(16)29-20-11-4-2-8-17(20)25/h1,3,5,7,9-10,12,15,27,30H,2,4,6,8,11,13-14H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221932
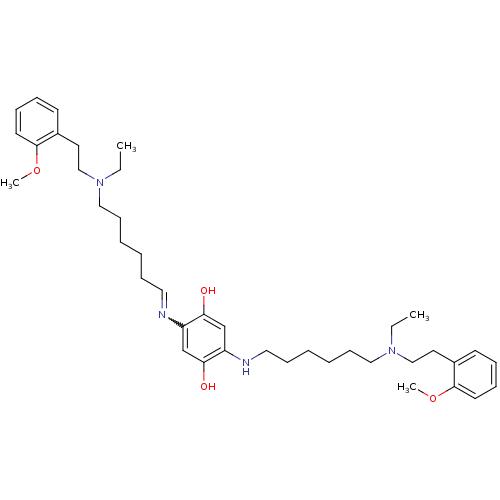
(2,5-bis(6-{ethyl[2-(2-methoxyphenyl)ethyl]amino}he...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)CCc1ccccc1OC)CCc1ccccc1OC |w:18.18| Show InChI InChI=1S/C40H60N4O4/c1-5-43(29-23-33-19-11-13-21-39(33)47-3)27-17-9-7-15-25-41-35-31-38(46)36(32-37(35)45)42-26-16-8-10-18-28-44(6-2)30-24-34-20-12-14-22-40(34)48-4/h11-14,19-22,25,31-32,42,45-46H,5-10,15-18,23-24,26-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50028680

(CHEMBL3356531)Show SMILES O=C1C=C(NCCCNc2c3CCCCc3nc3ccccc23)C(=O)c2ccccc12 |t:2| Show InChI InChI=1S/C26H25N3O2/c30-24-16-23(26(31)18-9-2-1-8-17(18)24)27-14-7-15-28-25-19-10-3-5-12-21(19)29-22-13-6-4-11-20(22)25/h1-3,5,8-10,12,16,27H,4,6-7,11,13-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028683
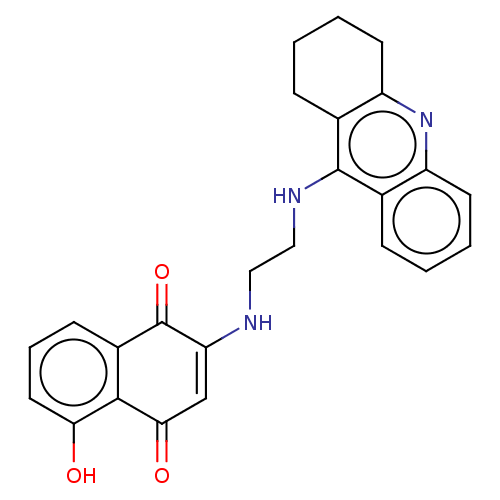
(CHEMBL3356534)Show SMILES Oc1cccc2C(=O)C(NCCNc3c4CCCCc4nc4ccccc34)=CC(=O)c12 |c:29| Show InChI InChI=1S/C25H23N3O3/c29-21-11-5-8-17-23(21)22(30)14-20(25(17)31)26-12-13-27-24-15-6-1-3-9-18(15)28-19-10-4-2-7-16(19)24/h1,3,5-6,8-9,11,14,26,29H,2,4,7,10,12-13H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50028694
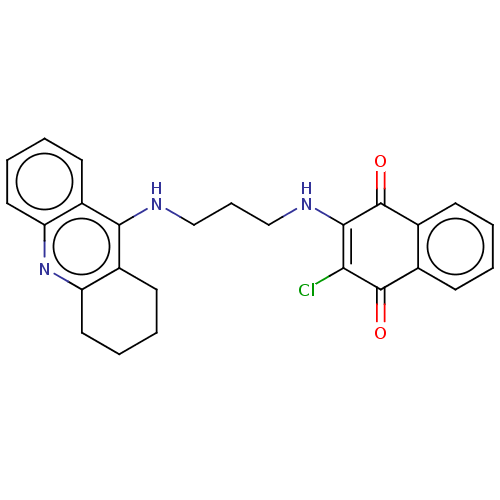
(CHEMBL3356954)Show SMILES ClC1=C(NCCCNc2c3CCCCc3nc3ccccc23)C(=O)c2ccccc2C1=O |c:1| Show InChI InChI=1S/C26H24ClN3O2/c27-22-24(26(32)17-9-2-1-8-16(17)25(22)31)29-15-7-14-28-23-18-10-3-5-12-20(18)30-21-13-6-4-11-19(21)23/h1-3,5,8-10,12,29H,4,6-7,11,13-15H2,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50145903

(CHEMBL3763728)Show SMILES O\C(\C=C\c1ccc(OCc2ccccc2)cc1)=C/C(=O)/C=C/c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C33H28O4/c34-30(17-11-26-13-19-32(20-14-26)36-24-28-7-3-1-4-8-28)23-31(35)18-12-27-15-21-33(22-16-27)37-25-29-9-5-2-6-10-29/h1-23,34H,24-25H2/b17-11+,18-12+,30-23- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum - University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using M-2420 as substrate preincubated for 1 hr followed by substrate addition incubated for 15 mins by FRET as... |
J Med Chem 59: 531-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00894
BindingDB Entry DOI: 10.7270/Q27P9183 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50028683

(CHEMBL3356534)Show SMILES Oc1cccc2C(=O)C(NCCNc3c4CCCCc4nc4ccccc34)=CC(=O)c12 |c:29| Show InChI InChI=1S/C25H23N3O3/c29-21-11-5-8-17-23(21)22(30)14-20(25(17)31)26-12-13-27-24-15-6-1-3-9-18(15)28-19-10-4-2-7-16(19)24/h1,3,5-6,8-9,11,14,26,29H,2,4,7,10,12-13H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman method |
J Med Chem 57: 8576-89 (2014)
Article DOI: 10.1021/jm5010804
BindingDB Entry DOI: 10.7270/Q25H7HVD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221920
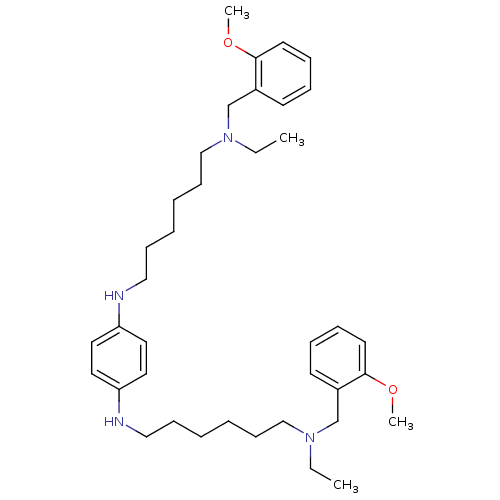
(CHEMBL230434 | N,N'-bis{6-[ethyl(2-methoxybenzyl)a...)Show SMILES CCN(CCCCCCNc1ccc(NCCCCCCN(CC)Cc2ccccc2OC)cc1)Cc1ccccc1OC Show InChI InChI=1S/C38H58N4O2/c1-5-41(31-33-19-11-13-21-37(33)43-3)29-17-9-7-15-27-39-35-23-25-36(26-24-35)40-28-16-8-10-18-30-42(6-2)32-34-20-12-14-22-38(34)44-4/h11-14,19-26,39-40H,5-10,15-18,27-32H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of binding to human integrin receptor alpha V beta3 |
Eur J Med Chem 141: 197-210 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.022
BindingDB Entry DOI: 10.7270/Q27083ZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data