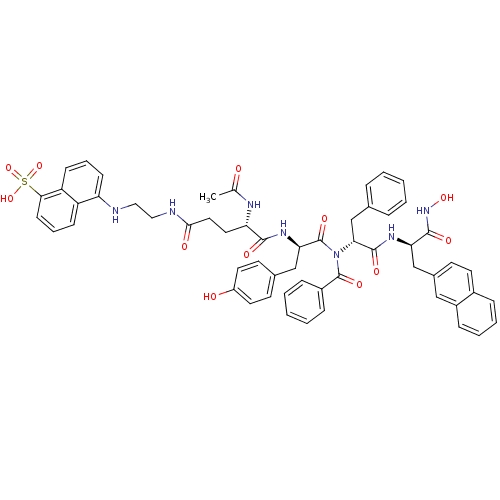Found 1058 hits with Last Name = 'field' and Initial = 't'
Found 1058 hits with Last Name = 'field' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50199865
((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(18-24-20-39-29-12-7-6-11-26(24)29)34(45)40-30(33(38)44)17-23-9-4-3-5-10-23/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199865

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(18-24-20-39-29-12-7-6-11-26(24)29)34(45)40-30(33(38)44)17-23-9-4-3-5-10-23/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50272031

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES CCCCCCC(N)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C44H57N7O6/c1-4-5-6-10-17-34(45)41(54)50-38(25-33-27(2)21-31(52)22-28(33)3)44(57)51-20-13-19-39(51)43(56)49-37(24-30-26-47-35-18-12-11-16-32(30)35)42(55)48-36(40(46)53)23-29-14-8-7-9-15-29/h7-9,11-12,14-16,18,21-22,26,34,36-39,47,52H,4-6,10,13,17,19-20,23-25,45H2,1-3H3,(H2,46,53)(H,48,55)(H,49,56)(H,50,54)/t34?,36-,37-,38-,39-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor expressed in CHOK1 cells after overnight incubation by scintillation proximity assay |
J Med Chem 55: 5859-67 (2012)
Article DOI: 10.1021/jm300418d
BindingDB Entry DOI: 10.7270/Q2PR7X3K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor expressed in CHOK1 cells after overnight incubation by scintillation proximity assay |
J Med Chem 55: 5859-67 (2012)
Article DOI: 10.1021/jm300418d
BindingDB Entry DOI: 10.7270/Q2PR7X3K |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191554
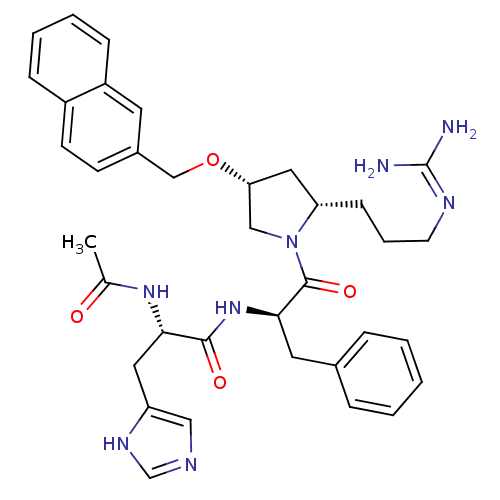
((S)-2-acetamido-N-((R)-1-((2S,4R)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:28.31,14.14,26.38,wD:4.3,(17.84,3.85,;16.5,3.08,;16.5,1.54,;15.17,3.85,;13.84,3.08,;12.5,3.85,;12.5,5.39,;11.25,6.28,;11.72,7.75,;13.26,7.75,;13.74,6.28,;13.84,1.54,;15.17,.77,;12.5,.77,;12.51,-.77,;11.17,-1.54,;9.84,-.77,;8.5,-1.55,;7.16,-.77,;7.17,.77,;8.49,1.54,;9.83,.78,;13.84,-1.54,;15.17,-.77,;13.84,-3.08,;12.59,-3.99,;13.07,-5.45,;14.61,-5.45,;15.09,-3.98,;16.43,-3.24,;17.75,-4.03,;19.1,-3.28,;20.42,-4.07,;21.77,-3.32,;23.09,-4.11,;21.79,-1.78,;12.17,-6.7,;12.8,-8.1,;12.03,-9.44,;12.8,-10.76,;12.04,-12.1,;10.49,-12.11,;9.73,-13.43,;8.2,-13.44,;7.43,-12.1,;8.2,-10.78,;9.73,-10.78,;10.49,-9.44,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31+,32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191569
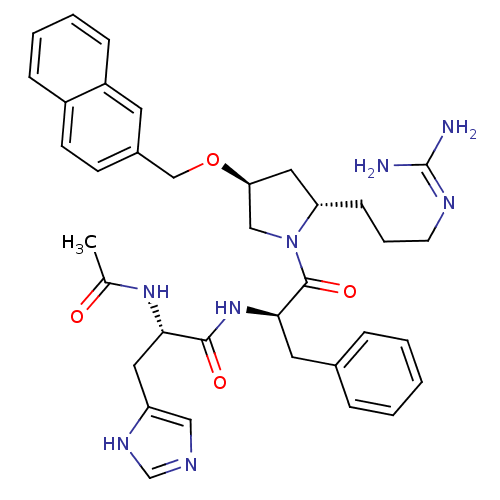
((S)-2-Acetamido-N-((R)-1-((2S,4S)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@H](C[C@@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:28.31,14.14,4.3,wD:26.38,(12.66,3.78,;13.99,3.01,;13.99,1.47,;15.32,3.78,;16.66,3.01,;17.99,3.78,;17.99,5.32,;16.73,6.23,;17.21,7.69,;18.75,7.7,;19.23,6.23,;16.66,1.47,;17.99,.7,;15.33,.7,;15.33,-.84,;14,-1.61,;12.66,-.84,;11.32,-1.61,;9.99,-.84,;9.99,.7,;11.31,1.47,;12.66,.71,;16.66,-1.61,;18,-.84,;16.66,-3.15,;15.42,-4.06,;15.9,-5.52,;17.44,-5.52,;17.91,-4.05,;19.26,-3.3,;20.58,-4.1,;21.92,-3.35,;23.24,-4.14,;24.59,-3.39,;25.91,-4.18,;24.62,-1.85,;14.99,-6.77,;15.62,-8.17,;14.85,-9.51,;15.63,-10.83,;14.87,-12.17,;13.32,-12.18,;12.56,-13.5,;11.03,-13.51,;10.25,-12.17,;11.02,-10.84,;12.55,-10.84,;13.31,-9.51,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31-,32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50427698
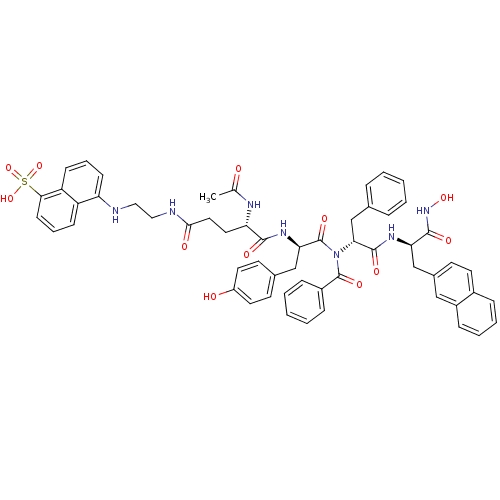
(CHEMBL2324206)Show SMILES CC(=O)N[C@@H](CCC(=O)NCCNc1cccc2c(cccc12)S(O)(=O)=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N([C@H](Cc1ccccc1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)NO)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C57H57N7O12S/c1-36(65)60-47(28-29-52(67)59-31-30-58-46-20-10-19-45-44(46)18-11-21-51(45)77(74,75)76)53(68)62-49(33-38-23-26-43(66)27-24-38)57(72)64(56(71)41-15-6-3-7-16-41)50(35-37-12-4-2-5-13-37)55(70)61-48(54(69)63-73)34-39-22-25-40-14-8-9-17-42(40)32-39/h2-27,32,47-50,58,66,73H,28-31,33-35H2,1H3,(H,59,67)(H,60,65)(H,61,70)(H,62,68)(H,63,69)(H,74,75,76)/t47-,48+,49+,50+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated fluorescein-Abeta-(1-40)-Lys-biotin degradation |
J Med Chem 56: 2246-55 (2013)
Article DOI: 10.1021/jm301280p
BindingDB Entry DOI: 10.7270/Q2DB836R |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SH-SY5Y cells |
Bioorg Med Chem 16: 4341-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.074
BindingDB Entry DOI: 10.7270/Q2MG7P8J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50272022

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES CCCCCCC(N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C42H53N7O6/c1-2-3-4-8-15-32(43)39(52)48-36(24-28-18-20-30(50)21-19-28)42(55)49-22-11-17-37(49)41(54)47-35(25-29-26-45-33-16-10-9-14-31(29)33)40(53)46-34(38(44)51)23-27-12-6-5-7-13-27/h5-7,9-10,12-14,16,18-21,26,32,34-37,45,50H,2-4,8,11,15,17,22-25,43H2,1H3,(H2,44,51)(H,46,53)(H,47,54)(H,48,52)/t32?,34-,35-,36-,37-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50192462

((S)-2-(2,3-bis(2-chlorobenzyloxy)phenyl)-2-hydroxy...)Show SMILES O[C@H](C(O)=O)c1cccc(OCc2ccccc2Cl)c1OCc1ccccc1Cl Show InChI InChI=1S/C22H18Cl2O5/c23-17-9-3-1-6-14(17)12-28-19-11-5-8-16(20(25)22(26)27)21(19)29-13-15-7-2-4-10-18(15)24/h1-11,20,25H,12-13H2,(H,26,27)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to ap2 |
J Med Chem 49: 5013-7 (2006)
Article DOI: 10.1021/jm060360i
BindingDB Entry DOI: 10.7270/Q2TQ6157 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50272022

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES CCCCCCC(N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C42H53N7O6/c1-2-3-4-8-15-32(43)39(52)48-36(24-28-18-20-30(50)21-19-28)42(55)49-22-11-17-37(49)41(54)47-35(25-29-26-45-33-16-10-9-14-31(29)33)40(53)46-34(38(44)51)23-27-12-6-5-7-13-27/h5-7,9-10,12-14,16,18-21,26,32,34-37,45,50H,2-4,8,11,15,17,22-25,43H2,1H3,(H2,44,51)(H,46,53)(H,47,54)(H,48,52)/t32?,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50272023

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES CCCCCC[C@@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C42H53N7O6/c1-2-3-4-8-15-32(43)39(52)48-36(24-28-18-20-30(50)21-19-28)42(55)49-22-11-17-37(49)41(54)47-35(25-29-26-45-33-16-10-9-14-31(29)33)40(53)46-34(38(44)51)23-27-12-6-5-7-13-27/h5-7,9-10,12-14,16,18-21,26,32,34-37,45,50H,2-4,8,11,15,17,22-25,43H2,1H3,(H2,44,51)(H,46,53)(H,47,54)(H,48,52)/t32-,34+,35+,36+,37+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein 5
(Homo sapiens (Human)) | BDBM50192463
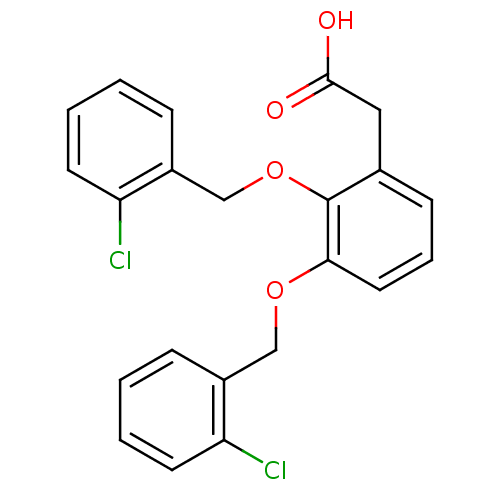
(2-(2,3-bis(2-chlorobenzyloxy)phenyl)acetic acid | ...)Show InChI InChI=1S/C22H18Cl2O4/c23-18-9-3-1-6-16(18)13-27-20-11-5-8-15(12-21(25)26)22(20)28-14-17-7-2-4-10-19(17)24/h1-11H,12-14H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human kFABP |
J Med Chem 49: 5013-7 (2006)
Article DOI: 10.1021/jm060360i
BindingDB Entry DOI: 10.7270/Q2TQ6157 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50427703

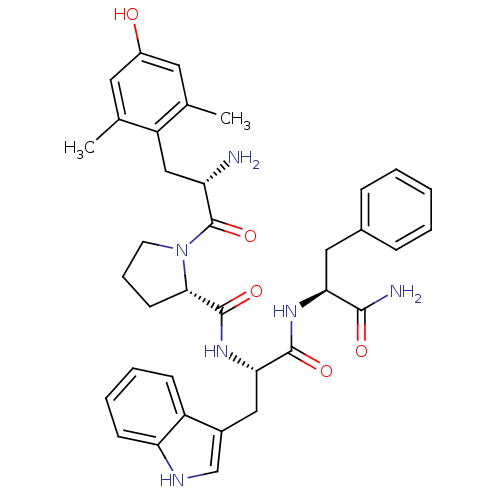
(CHEMBL2324220)Show SMILES NC(N)=NCCC[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccc2ccccc2c1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r,wU:7.7,45.48,11.11,wD:31.32,(15.26,-7.72,;16.62,-8.43,;17.92,-7.6,;16.69,-9.97,;15.39,-10.79,;15.46,-12.33,;14.16,-13.16,;14.24,-14.7,;12.94,-15.53,;11.61,-14.76,;11.61,-13.22,;10.27,-15.53,;10.27,-17.07,;8.94,-17.84,;7.6,-17.07,;8.94,-19.38,;7.6,-20.16,;8.97,-14.76,;8.94,-13.22,;7.58,-12.47,;7.55,-10.93,;8.87,-10.13,;8.84,-8.59,;10.15,-7.8,;11.51,-8.53,;11.55,-10.08,;10.22,-10.88,;10.26,-12.42,;15.6,-15.4,;15.66,-16.95,;16.9,-14.58,;18.26,-15.29,;18.34,-16.83,;19.7,-17.53,;21.15,-17.03,;22.08,-18.26,;21.2,-19.52,;21.54,-21.02,;20.42,-22.07,;18.95,-21.62,;18.6,-20.12,;19.73,-19.07,;19.56,-14.46,;19.49,-12.92,;20.92,-15.17,;22.22,-14.34,;22.16,-12.8,;23.45,-11.98,;23.38,-10.44,;22.02,-9.73,;24.68,-9.61,;23.58,-15.05,;24.88,-14.22,;23.66,-16.59,)| Show InChI InChI=1S/C37H45N9O8/c38-33(50)28(13-14-32(48)49)43-36(53)30(18-25-20-42-27-9-4-3-8-26(25)27)45-35(52)29(10-5-15-41-37(39)40)44-34(51)24(19-31(47)46-54)17-21-11-12-22-6-1-2-7-23(22)16-21/h1-4,6-9,11-12,16,20,24,28-30,42,54H,5,10,13-15,17-19H2,(H2,38,50)(H,43,53)(H,44,51)(H,45,52)(H,46,47)(H,48,49)(H4,39,40,41)/t24-,28+,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated FRET1 degradation |
J Med Chem 56: 2246-55 (2013)
Article DOI: 10.1021/jm301280p
BindingDB Entry DOI: 10.7270/Q2DB836R |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50272024
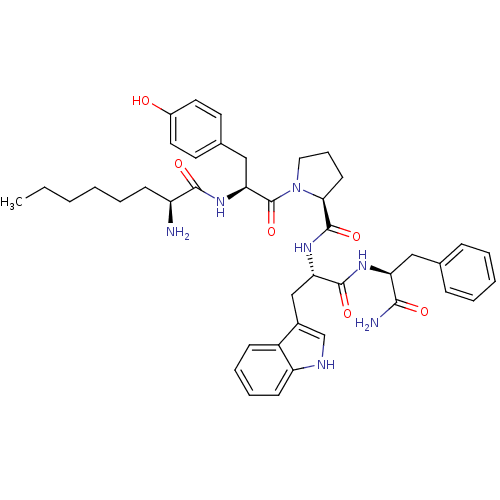
((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES CCCCCC[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C42H53N7O6/c1-2-3-4-8-15-32(43)39(52)48-36(24-28-18-20-30(50)21-19-28)42(55)49-22-11-17-37(49)41(54)47-35(25-29-26-45-33-16-10-9-14-31(29)33)40(53)46-34(38(44)51)23-27-12-6-5-7-13-27/h5-7,9-10,12-14,16,18-21,26,32,34-37,45,50H,2-4,8,11,15,17,22-25,43H2,1H3,(H2,44,51)(H,46,53)(H,47,54)(H,48,52)/t32-,34-,35-,36-,37-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191575

((S)-2-acetamido-N-((R)-1-((2R,4R)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:14.14,26.38,4.3,wD:28.31,(10.98,-16.28,;12.31,-17.05,;12.32,-18.59,;13.65,-16.27,;14.98,-17.04,;16.31,-16.27,;16.31,-14.73,;15.06,-13.83,;15.53,-12.36,;17.07,-12.36,;17.55,-13.82,;14.98,-18.58,;16.32,-19.35,;13.65,-19.35,;13.65,-20.89,;12.32,-21.67,;10.98,-20.9,;9.64,-21.67,;8.31,-20.9,;8.31,-19.35,;9.64,-18.58,;10.98,-19.35,;14.99,-21.66,;16.32,-20.89,;14.99,-23.2,;13.74,-24.11,;14.22,-25.57,;15.76,-25.57,;16.23,-24.11,;17.58,-23.36,;18.9,-24.15,;20.25,-23.4,;21.57,-24.19,;22.91,-23.44,;24.24,-24.24,;22.94,-21.9,;13.31,-26.82,;13.94,-28.23,;13.18,-29.56,;13.95,-30.89,;13.19,-32.22,;11.64,-32.23,;10.88,-33.55,;9.35,-33.56,;8.58,-32.23,;9.34,-30.9,;10.88,-30.9,;11.63,-29.56,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31-,32+,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199865

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(18-24-20-39-29-12-7-6-11-26(24)29)34(45)40-30(33(38)44)17-23-9-4-3-5-10-23/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein 5
(Homo sapiens (Human)) | BDBM50192465
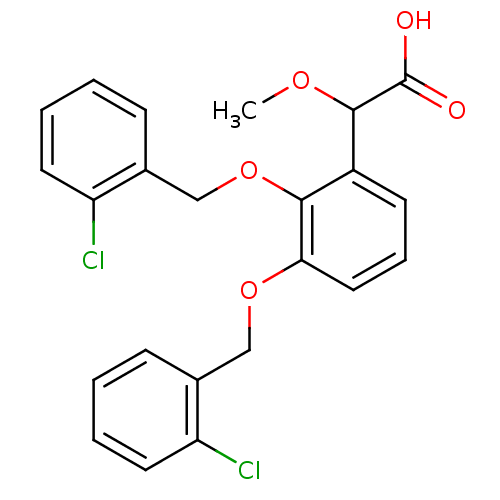
(2-(2,3-bis(2-chlorobenzyloxy)phenyl)-2-methoxyacet...)Show SMILES COC(C(O)=O)c1cccc(OCc2ccccc2Cl)c1OCc1ccccc1Cl Show InChI InChI=1S/C23H20Cl2O5/c1-28-22(23(26)27)17-9-6-12-20(29-13-15-7-2-4-10-18(15)24)21(17)30-14-16-8-3-5-11-19(16)25/h2-12,22H,13-14H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human kFABP |
J Med Chem 49: 5013-7 (2006)
Article DOI: 10.1021/jm060360i
BindingDB Entry DOI: 10.7270/Q2TQ6157 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50119368

((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...)Show SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(Cn3cncn3)(CC2)C2CCCCC2)cc1 Show InChI InChI=1S/C33H41ClN6O2/c34-28-12-10-24(11-13-28)18-30(38-31(41)29-19-25-6-4-5-7-26(25)20-36-29)32(42)39-16-14-33(15-17-39,21-40-23-35-22-37-40)27-8-2-1-3-9-27/h4-7,10-13,22-23,27,29-30,36H,1-3,8-9,14-21H2,(H,38,41)/t29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter& Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human Melanocortin 4 Receptor |
Bioorg Med Chem Lett 15: 2819-23 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.120
BindingDB Entry DOI: 10.7270/Q2F76DBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50191556
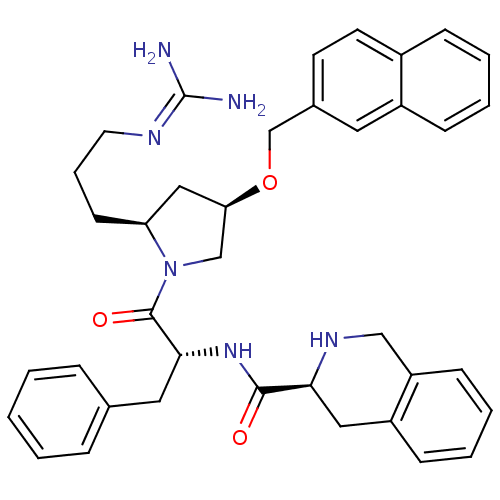
((S)-N-((R)-1-((2S,4R)-2-(3-guanidinopropyl)-4-(nap...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H]-1-[#6]-[#6@H](-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-c2ccccc2-[#6]-[#7]-1)-[#8]-[#6]-c1ccc2ccccc2c1 Show InChI InChI=1S/C38H44N6O3/c39-38(40)41-18-8-15-32-22-33(47-25-27-16-17-28-11-4-5-12-29(28)19-27)24-44(32)37(46)35(20-26-9-2-1-3-10-26)43-36(45)34-21-30-13-6-7-14-31(30)23-42-34/h1-7,9-14,16-17,19,32-35,42H,8,15,18,20-25H2,(H,43,45)(H4,39,40,41)/t32-,33+,34-,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50080456

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C28H38N6O6/c1-17(2)12-22(26(30)38)34-28(40)23(14-18-6-4-3-5-7-18)33-25(37)16-31-24(36)15-32-27(39)21(29)13-19-8-10-20(35)11-9-19/h3-11,17,21-23,35H,12-16,29H2,1-2H3,(H2,30,38)(H,31,36)(H,32,39)(H,33,37)(H,34,40)/t21-,22-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SH-SY5Y cells |
Bioorg Med Chem 16: 4341-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.074
BindingDB Entry DOI: 10.7270/Q2MG7P8J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50261483

(CHEMBL504973 | YGGWL-NH2)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C30H39N7O6/c1-17(2)11-24(28(32)41)37-30(43)25(13-19-14-33-23-6-4-3-5-21(19)23)36-27(40)16-34-26(39)15-35-29(42)22(31)12-18-7-9-20(38)10-8-18/h3-10,14,17,22,24-25,33,38H,11-13,15-16,31H2,1-2H3,(H2,32,41)(H,34,39)(H,35,42)(H,36,40)(H,37,43)/t22-,24-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SH-SY5Y cells |
Bioorg Med Chem 16: 4341-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.074
BindingDB Entry DOI: 10.7270/Q2MG7P8J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from kappa-opioid receptor expressed in CHOK1 cells after overnight incubation by scintillation proximity assay |
J Med Chem 55: 5859-67 (2012)
Article DOI: 10.1021/jm300418d
BindingDB Entry DOI: 10.7270/Q2PR7X3K |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50189018

((S)-2-{(R)-2-[(S)-2-acetylamino-3-(3H-imidazol-4-y...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O |wU:4.17,wD:30.40,41.51,19.28,(25,.39,;23.66,1.16,;22.33,.39,;22.33,-1.16,;20.99,1.16,;20.99,2.69,;22.33,3.47,;23.66,2.69,;24.99,3.46,;25,5,;26.33,5.78,;26.33,7.33,;24.97,8.09,;23.64,7.31,;23.65,5.77,;22.33,5,;19.66,.39,;18.33,1.16,;18.33,2.69,;16.99,.39,;16.99,-1.16,;18.33,-1.92,;18.33,-3.47,;19.66,-4.23,;19.66,-5.77,;20.99,-6.54,;18.33,-6.54,;15.66,1.16,;14.33,.39,;14.33,-1.16,;12.99,1.16,;12.99,2.69,;14.33,3.47,;15.66,2.69,;16.99,3.46,;16.99,5,;15.65,5.77,;14.32,4.99,;11.66,.39,;10.32,1.16,;10.32,2.69,;8.99,.39,;8.99,-1.16,;10.32,-1.93,;10.29,-3.46,;11.75,-3.97,;12.68,-2.74,;11.8,-1.47,;7.66,1.16,;6.32,.39,;4.99,1.16,;6.32,-1.16,)| Show InChI InChI=1S/C37H46N10O5/c1-23(48)44-32(20-28-21-41-22-43-28)36(52)47-31(18-24-9-4-3-5-10-24)35(51)45-29(13-8-16-42-37(38)39)34(50)46-30(33(49)40-2)19-25-14-15-26-11-6-7-12-27(26)17-25/h3-7,9-12,14-15,17,21-22,29-32H,8,13,16,18-20H2,1-2H3,(H,40,49)(H,41,43)(H,44,48)(H,45,51)(H,46,50)(H,47,52)(H4,38,39,42)/t29-,30-,31+,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191580

((2R,4R)-1-((R)-2-((S)-2-acetamido-3-(1H-imidazol-4...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@@H]1C(=O)NCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:28.31,14.14,26.40,wD:4.3,(-.65,-12.93,;-1.99,-13.7,;-1.98,-15.24,;-3.32,-12.93,;-4.65,-13.71,;-5.99,-12.94,;-5.99,-11.4,;-7.24,-10.5,;-6.77,-9.03,;-5.23,-9.03,;-4.75,-10.49,;-4.65,-15.25,;-3.32,-16.01,;-5.98,-16.02,;-5.98,-17.56,;-7.32,-18.33,;-8.65,-17.56,;-9.99,-18.33,;-11.32,-17.56,;-11.32,-16.02,;-10,-15.25,;-8.66,-16.01,;-4.65,-18.33,;-3.32,-17.55,;-4.65,-19.87,;-5.89,-20.77,;-5.42,-22.24,;-3.88,-22.24,;-3.4,-20.77,;-2.06,-20.02,;-2.03,-18.48,;-.73,-20.81,;.61,-20.06,;1.93,-20.86,;3.28,-20.11,;4.6,-20.9,;5.95,-20.15,;4.58,-22.44,;-6.32,-23.48,;-5.69,-24.89,;-6.46,-26.23,;-5.69,-27.55,;-6.45,-28.88,;-7.99,-28.89,;-8.76,-30.22,;-10.28,-30.22,;-11.06,-28.89,;-10.29,-27.56,;-8.76,-27.56,;-8,-26.23,)| Show InChI InChI=1S/C36H43N9O5/c1-23(46)43-30(17-28-19-39-22-42-28)33(47)44-31(16-24-7-3-2-4-8-24)35(49)45-20-29(18-32(45)34(48)40-13-14-41-36(37)38)50-21-25-11-12-26-9-5-6-10-27(26)15-25/h2-12,15,19,22,29-32H,13-14,16-18,20-21H2,1H3,(H,39,42)(H,40,48)(H,43,46)(H,44,47)(H4,37,38,41)/t29-,30+,31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50191565
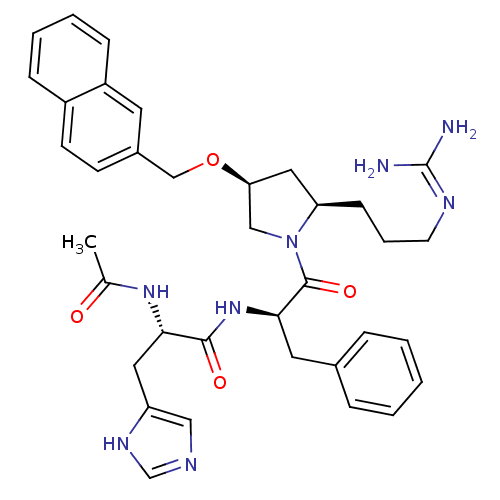
((S)-2-acetamido-N-((R)-1-((2R,4S)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@H](C[C@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:14.14,4.3,wD:28.31,26.38,(8.94,3.78,;10.28,3.01,;10.28,1.47,;11.61,3.78,;12.95,3.01,;14.28,3.78,;14.28,5.32,;13.02,6.23,;13.5,7.69,;15.04,7.7,;15.51,6.23,;12.95,1.47,;14.28,.7,;11.61,.7,;11.62,-.84,;10.28,-1.61,;8.95,-.84,;7.61,-1.61,;6.27,-.84,;6.28,.7,;7.6,1.47,;8.94,.71,;12.95,-1.61,;14.28,-.84,;12.95,-3.15,;11.7,-4.06,;12.18,-5.52,;13.72,-5.52,;14.2,-4.05,;15.54,-3.3,;16.86,-4.1,;18.21,-3.35,;19.53,-4.14,;20.88,-3.39,;22.2,-4.18,;20.9,-1.85,;11.28,-6.77,;11.91,-8.17,;11.14,-9.51,;11.91,-10.83,;11.15,-12.17,;9.6,-12.18,;8.84,-13.5,;7.31,-13.51,;6.54,-12.17,;7.31,-10.84,;8.84,-10.84,;9.6,-9.51,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31+,32+,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50272025

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES CCCCCCCCCCC(N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C46H61N7O6/c1-2-3-4-5-6-7-8-12-19-36(47)43(56)52-40(28-32-22-24-34(54)25-23-32)46(59)53-26-15-21-41(53)45(58)51-39(29-33-30-49-37-20-14-13-18-35(33)37)44(57)50-38(42(48)55)27-31-16-10-9-11-17-31/h9-11,13-14,16-18,20,22-25,30,36,38-41,49,54H,2-8,12,15,19,21,26-29,47H2,1H3,(H2,48,55)(H,50,57)(H,51,58)(H,52,56)/t36?,38-,39-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191551

((2R,4R)-1-((R)-2-((S)-2-amino-3-(1H-imidazol-4-yl)...)Show SMILES N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@@H]1C(=O)NCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:25.28,11.11,23.37,wD:1.0,(15.6,-11.59,;14.27,-12.36,;12.94,-11.59,;12.94,-10.05,;11.68,-9.15,;12.16,-7.69,;13.7,-7.69,;14.17,-9.15,;14.27,-13.9,;15.61,-14.67,;12.94,-14.67,;12.94,-16.21,;11.61,-16.99,;10.27,-16.22,;8.93,-16.99,;7.6,-16.22,;7.6,-14.67,;8.93,-13.9,;10.27,-14.67,;14.28,-16.98,;15.61,-16.21,;14.28,-18.52,;13.03,-19.43,;13.51,-20.89,;15.05,-20.89,;15.52,-19.43,;16.87,-18.68,;16.89,-17.14,;18.19,-19.47,;19.54,-18.72,;20.86,-19.51,;22.2,-18.76,;23.52,-19.56,;24.87,-18.81,;23.5,-21.1,;12.6,-22.14,;13.23,-23.55,;12.47,-24.88,;13.24,-26.21,;12.48,-27.54,;10.93,-27.55,;10.17,-28.87,;8.64,-28.88,;7.87,-27.55,;8.63,-26.22,;10.17,-26.22,;10.92,-24.88,)| Show InChI InChI=1S/C34H41N9O4/c35-28(16-26-18-38-21-41-26)31(44)42-29(15-22-6-2-1-3-7-22)33(46)43-19-27(17-30(43)32(45)39-12-13-40-34(36)37)47-20-23-10-11-24-8-4-5-9-25(24)14-23/h1-11,14,18,21,27-30H,12-13,15-17,19-20,35H2,(H,38,41)(H,39,45)(H,42,44)(H4,36,37,40)/t27-,28+,29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50393705
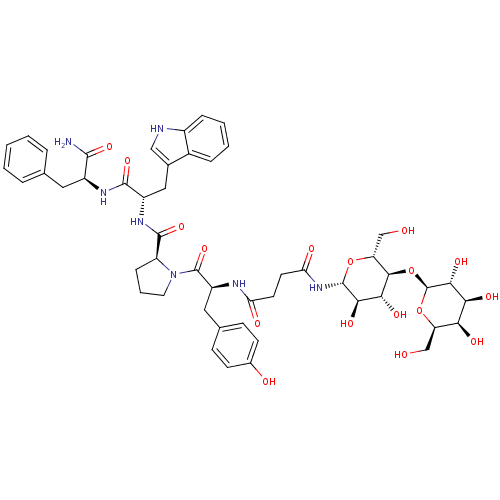
(CHEMBL2158990)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCC(=O)N[C@@H]1O[C@H](CO)[C@@H](O[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C50H63N7O17/c51-45(68)31(19-25-7-2-1-3-8-25)54-46(69)32(21-27-22-52-30-10-5-4-9-29(27)30)55-47(70)34-11-6-18-57(34)49(71)33(20-26-12-14-28(60)15-13-26)53-37(61)16-17-38(62)56-48-42(66)41(65)44(36(24-59)72-48)74-50-43(67)40(64)39(63)35(23-58)73-50/h1-5,7-10,12-15,22,31-36,39-44,48,50,52,58-60,63-67H,6,11,16-21,23-24H2,(H2,51,68)(H,53,61)(H,54,69)(H,55,70)(H,56,62)/t31-,32-,33-,34-,35+,36+,39-,40-,41+,42+,43+,44+,48+,50-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor expressed in CHOK1 cells after overnight incubation by scintillation proximity assay |
J Med Chem 55: 5859-67 (2012)
Article DOI: 10.1021/jm300418d
BindingDB Entry DOI: 10.7270/Q2PR7X3K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50272025

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES CCCCCCCCCCC(N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C46H61N7O6/c1-2-3-4-5-6-7-8-12-19-36(47)43(56)52-40(28-32-22-24-34(54)25-23-32)46(59)53-26-15-21-41(53)45(58)51-39(29-33-30-49-37-20-14-13-18-35(33)37)44(57)50-38(42(48)55)27-31-16-10-9-11-17-31/h9-11,13-14,16-18,20,22-25,30,36,38-41,49,54H,2-8,12,15,19,21,26-29,47H2,1H3,(H2,48,55)(H,50,57)(H,51,58)(H,52,56)/t36?,38-,39-,40-,41-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50192463

(2-(2,3-bis(2-chlorobenzyloxy)phenyl)acetic acid | ...)Show InChI InChI=1S/C22H18Cl2O4/c23-18-9-3-1-6-16(18)13-27-20-11-5-8-15(12-21(25)26)22(20)28-14-17-7-2-4-10-19(17)24/h1-11H,12-14H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to ap2 |
J Med Chem 49: 5013-7 (2006)
Article DOI: 10.1021/jm060360i
BindingDB Entry DOI: 10.7270/Q2TQ6157 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50191554

((S)-2-acetamido-N-((R)-1-((2S,4R)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:28.31,14.14,26.38,wD:4.3,(17.84,3.85,;16.5,3.08,;16.5,1.54,;15.17,3.85,;13.84,3.08,;12.5,3.85,;12.5,5.39,;11.25,6.28,;11.72,7.75,;13.26,7.75,;13.74,6.28,;13.84,1.54,;15.17,.77,;12.5,.77,;12.51,-.77,;11.17,-1.54,;9.84,-.77,;8.5,-1.55,;7.16,-.77,;7.17,.77,;8.49,1.54,;9.83,.78,;13.84,-1.54,;15.17,-.77,;13.84,-3.08,;12.59,-3.99,;13.07,-5.45,;14.61,-5.45,;15.09,-3.98,;16.43,-3.24,;17.75,-4.03,;19.1,-3.28,;20.42,-4.07,;21.77,-3.32,;23.09,-4.11,;21.79,-1.78,;12.17,-6.7,;12.8,-8.1,;12.03,-9.44,;12.8,-10.76,;12.04,-12.1,;10.49,-12.11,;9.73,-13.43,;8.2,-13.44,;7.43,-12.1,;8.2,-10.78,;9.73,-10.78,;10.49,-9.44,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31+,32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50191553
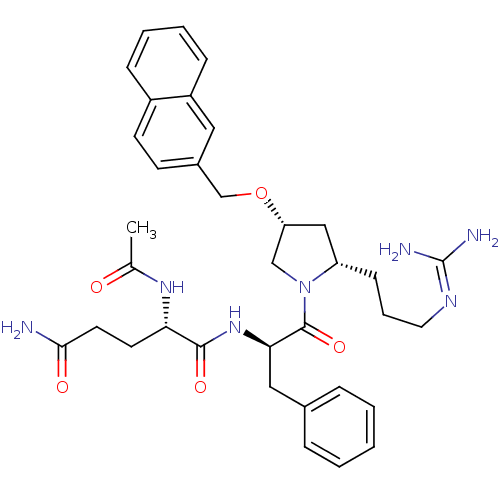
((S)-2-acetamido-N1-((R)-1-((2S,4R)-2-(3-guanidinop...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6@@H](-[#6]-[#6@@H]-1-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#8]-[#6]-c1ccc2ccccc2c1 Show InChI InChI=1S/C35H45N7O5/c1-23(43)40-30(15-16-32(36)44)33(45)41-31(19-24-8-3-2-4-9-24)34(46)42-21-29(20-28(42)12-7-17-39-35(37)38)47-22-25-13-14-26-10-5-6-11-27(26)18-25/h2-6,8-11,13-14,18,28-31H,7,12,15-17,19-22H2,1H3,(H2,36,44)(H,40,43)(H,41,45)(H4,37,38,39)/t28-,29+,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191577

((S)-N-((R)-1-((2R,4R)-2-(3-guanidinopropyl)-4-(nap...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#6]-[#6@H](-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-1-[#6]-c2ccccc2-[#6]-[#7]-1)-[#8]-[#6]-c1ccc2ccccc2c1 Show InChI InChI=1S/C38H44N6O3/c39-38(40)41-18-8-15-32-22-33(47-25-27-16-17-28-11-4-5-12-29(28)19-27)24-44(32)37(46)35(20-26-9-2-1-3-10-26)43-36(45)34-21-30-13-6-7-14-31(30)23-42-34/h1-7,9-14,16-17,19,32-35,42H,8,15,18,20-25H2,(H,43,45)(H4,39,40,41)/t32-,33-,34?,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50191563

((S)-2-acetamido-N-((R)-1-((2S,4R)-2-(3-guanidinopr...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6@@H](-[#6]-[#6@@H]-1-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#8]-[#6]-c1ccc2ccccc2c1 Show InChI InChI=1S/C39H46N6O5/c1-26(46)43-35(21-28-14-17-33(47)18-15-28)37(48)44-36(22-27-8-3-2-4-9-27)38(49)45-24-34(23-32(45)12-7-19-42-39(40)41)50-25-29-13-16-30-10-5-6-11-31(30)20-29/h2-6,8-11,13-18,20,32,34-36,47H,7,12,19,21-25H2,1H3,(H,43,46)(H,44,48)(H4,40,41,42)/t32-,34+,35-,36+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50427703

(CHEMBL2324220)Show SMILES NC(N)=NCCC[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccc2ccccc2c1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r,wU:7.7,45.48,11.11,wD:31.32,(15.26,-7.72,;16.62,-8.43,;17.92,-7.6,;16.69,-9.97,;15.39,-10.79,;15.46,-12.33,;14.16,-13.16,;14.24,-14.7,;12.94,-15.53,;11.61,-14.76,;11.61,-13.22,;10.27,-15.53,;10.27,-17.07,;8.94,-17.84,;7.6,-17.07,;8.94,-19.38,;7.6,-20.16,;8.97,-14.76,;8.94,-13.22,;7.58,-12.47,;7.55,-10.93,;8.87,-10.13,;8.84,-8.59,;10.15,-7.8,;11.51,-8.53,;11.55,-10.08,;10.22,-10.88,;10.26,-12.42,;15.6,-15.4,;15.66,-16.95,;16.9,-14.58,;18.26,-15.29,;18.34,-16.83,;19.7,-17.53,;21.15,-17.03,;22.08,-18.26,;21.2,-19.52,;21.54,-21.02,;20.42,-22.07,;18.95,-21.62,;18.6,-20.12,;19.73,-19.07,;19.56,-14.46,;19.49,-12.92,;20.92,-15.17,;22.22,-14.34,;22.16,-12.8,;23.45,-11.98,;23.38,-10.44,;22.02,-9.73,;24.68,-9.61,;23.58,-15.05,;24.88,-14.22,;23.66,-16.59,)| Show InChI InChI=1S/C37H45N9O8/c38-33(50)28(13-14-32(48)49)43-36(53)30(18-25-20-42-27-9-4-3-8-26(25)27)45-35(52)29(10-5-15-41-37(39)40)44-34(51)24(19-31(47)46-54)17-21-11-12-22-6-1-2-7-23(22)16-21/h1-4,6-9,11-12,16,20,24,28-30,42,54H,5,10,13-15,17-19H2,(H2,38,50)(H,43,53)(H,44,51)(H,45,52)(H,46,47)(H,48,49)(H4,39,40,41)/t24-,28+,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated fluorescein-Abeta-(1-40)-Lys-biotin degradation |
J Med Chem 56: 2246-55 (2013)
Article DOI: 10.1021/jm301280p
BindingDB Entry DOI: 10.7270/Q2DB836R |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50427697

(CHEMBL2324207)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H](CC(O)=O)NC(=O)CC[C@@H](NC(=O)[C@@H](CCCN=C(N)N)NC1=O)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)NO |r,wU:8.8,39.56,71.75,wD:29.31,19.20,4.3,2.2,43.45,57.59,82.86,(26.91,-23.81,;25.59,-23.02,;25.62,-21.49,;26.96,-20.73,;24.29,-20.69,;24.33,-19.15,;25.67,-18.41,;26.99,-19.19,;25.69,-16.86,;24.37,-16.07,;23.02,-16.83,;21.71,-16.03,;20.37,-16.78,;20.34,-18.32,;21.66,-19.11,;23,-18.36,;27.04,-16.12,;28.35,-16.9,;28.34,-18.45,;29.7,-16.16,;31.02,-16.95,;32.36,-16.2,;33.6,-17.12,;34.86,-16.23,;34.4,-14.77,;32.94,-14.55,;29.73,-14.62,;28.4,-13.82,;27.06,-14.58,;28.43,-12.29,;29.77,-11.54,;29.8,-10,;31.15,-9.25,;28.48,-9.21,;27.11,-11.5,;16.32,-19.03,;15,-18.24,;16.3,-20.57,;17.62,-21.36,;17.6,-22.9,;18.91,-23.69,;20.27,-22.94,;20.28,-21.4,;21.58,-23.73,;21.56,-25.27,;20.22,-26.02,;20.19,-27.56,;18.85,-28.31,;18.83,-29.84,;20.14,-30.63,;17.47,-30.59,;22.93,-22.98,;22.95,-21.44,;21.63,-20.65,;16.25,-23.65,;16.23,-25.19,;14.93,-22.86,;13.59,-23.6,;13.6,-25.16,;12.25,-25.91,;10.87,-25.31,;9.83,-26.44,;10.57,-27.78,;10.08,-29.24,;11.09,-30.39,;12.6,-30.1,;13.11,-28.64,;12.09,-27.48,;12.26,-22.81,;12.28,-21.28,;10.92,-23.57,;9.6,-22.77,;9.63,-21.23,;8.3,-20.44,;8.33,-18.9,;7.01,-18.11,;7.03,-16.58,;8.37,-15.83,;5.7,-15.78,;8.25,-23.52,;8.23,-25.06,;6.93,-22.73,;5.59,-23.48,;5.57,-25.02,;4.22,-25.77,;2.91,-24.98,;1.55,-25.73,;1.53,-27.27,;.19,-28.02,;.16,-29.56,;1.48,-30.34,;2.84,-29.6,;2.86,-28.05,;4.2,-27.31,;4.26,-22.69,;4.29,-21.15,;2.92,-23.44,;1.6,-22.65,)| Show InChI InChI=1S/C66H85N19O13/c1-3-36(2)55-64(97)79-46(20-12-26-73-66(69)70)56(89)78-47(23-24-53(86)76-52(32-54(87)88)61(94)83-51(31-42-34-71-35-75-42)60(93)80-48(62(95)84-55)28-37-13-5-4-6-14-37)58(91)82-50(30-41-33-74-44-18-10-9-17-43(41)44)59(92)77-45(19-11-25-72-65(67)68)57(90)81-49(63(96)85-98)29-38-21-22-39-15-7-8-16-40(39)27-38/h4-10,13-18,21-22,27,33-36,45-52,55,74,98H,3,11-12,19-20,23-26,28-32H2,1-2H3,(H,71,75)(H,76,86)(H,77,92)(H,78,89)(H,79,97)(H,80,93)(H,81,90)(H,82,91)(H,83,94)(H,84,95)(H,85,96)(H,87,88)(H4,67,68,72)(H4,69,70,73)/t36-,45-,46-,47-,48-,49-,50-,51-,52-,55-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated FRET1 degradation |
J Med Chem 56: 2246-55 (2013)
Article DOI: 10.1021/jm301280p
BindingDB Entry DOI: 10.7270/Q2DB836R |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50427696
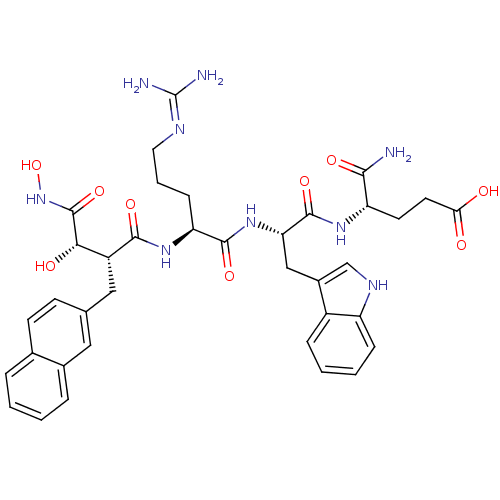
(CHEMBL2324201)Show SMILES NC(N)=NCCC[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r,wU:7.7,46.49,11.10,wD:32.33,23.25,(13.82,-27.05,;15.18,-27.77,;16.48,-26.94,;15.24,-29.3,;13.95,-30.13,;14.02,-31.67,;12.72,-32.49,;12.79,-34.03,;11.5,-34.86,;10.17,-34.09,;10.17,-32.55,;8.83,-34.87,;7.53,-34.09,;7.5,-32.55,;6.14,-31.8,;6.11,-30.26,;7.43,-29.47,;7.4,-27.93,;8.71,-27.13,;10.07,-27.87,;10.11,-29.41,;8.78,-30.21,;8.82,-31.75,;8.83,-36.41,;10.16,-37.18,;7.49,-37.17,;6.16,-36.41,;7.49,-38.72,;6.16,-39.49,;14.16,-34.74,;14.22,-36.28,;15.45,-33.91,;16.82,-34.62,;16.89,-36.16,;18.25,-36.87,;19.71,-36.36,;20.64,-37.59,;19.75,-38.85,;20.1,-40.35,;18.98,-41.4,;17.51,-40.96,;17.16,-39.45,;18.28,-38.41,;18.12,-33.79,;18.04,-32.26,;19.48,-34.51,;20.78,-33.67,;20.71,-32.14,;22.01,-31.31,;21.94,-29.77,;20.57,-29.06,;23.24,-28.94,;22.14,-34.38,;23.44,-33.56,;22.22,-35.92,)| Show InChI InChI=1S/C37H45N9O9/c38-32(50)27(13-14-30(47)48)43-35(53)29(18-23-19-42-26-9-4-3-8-24(23)26)45-34(52)28(10-5-15-41-37(39)40)44-33(51)25(31(49)36(54)46-55)17-20-11-12-21-6-1-2-7-22(21)16-20/h1-4,6-9,11-12,16,19,25,27-29,31,42,49,55H,5,10,13-15,17-18H2,(H2,38,50)(H,43,53)(H,44,51)(H,45,52)(H,46,54)(H,47,48)(H4,39,40,41)/t25-,27+,28+,29+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated FRET1 degradation |
J Med Chem 56: 2246-55 (2013)
Article DOI: 10.1021/jm301280p
BindingDB Entry DOI: 10.7270/Q2DB836R |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50191554

((S)-2-acetamido-N-((R)-1-((2S,4R)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:28.31,14.14,26.38,wD:4.3,(17.84,3.85,;16.5,3.08,;16.5,1.54,;15.17,3.85,;13.84,3.08,;12.5,3.85,;12.5,5.39,;11.25,6.28,;11.72,7.75,;13.26,7.75,;13.74,6.28,;13.84,1.54,;15.17,.77,;12.5,.77,;12.51,-.77,;11.17,-1.54,;9.84,-.77,;8.5,-1.55,;7.16,-.77,;7.17,.77,;8.49,1.54,;9.83,.78,;13.84,-1.54,;15.17,-.77,;13.84,-3.08,;12.59,-3.99,;13.07,-5.45,;14.61,-5.45,;15.09,-3.98,;16.43,-3.24,;17.75,-4.03,;19.1,-3.28,;20.42,-4.07,;21.77,-3.32,;23.09,-4.11,;21.79,-1.78,;12.17,-6.7,;12.8,-8.1,;12.03,-9.44,;12.8,-10.76,;12.04,-12.1,;10.49,-12.11,;9.73,-13.43,;8.2,-13.44,;7.43,-12.1,;8.2,-10.78,;9.73,-10.78,;10.49,-9.44,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31+,32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC3R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189018

((S)-2-{(R)-2-[(S)-2-acetylamino-3-(3H-imidazol-4-y...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O |wU:4.17,wD:30.40,41.51,19.28,(25,.39,;23.66,1.16,;22.33,.39,;22.33,-1.16,;20.99,1.16,;20.99,2.69,;22.33,3.47,;23.66,2.69,;24.99,3.46,;25,5,;26.33,5.78,;26.33,7.33,;24.97,8.09,;23.64,7.31,;23.65,5.77,;22.33,5,;19.66,.39,;18.33,1.16,;18.33,2.69,;16.99,.39,;16.99,-1.16,;18.33,-1.92,;18.33,-3.47,;19.66,-4.23,;19.66,-5.77,;20.99,-6.54,;18.33,-6.54,;15.66,1.16,;14.33,.39,;14.33,-1.16,;12.99,1.16,;12.99,2.69,;14.33,3.47,;15.66,2.69,;16.99,3.46,;16.99,5,;15.65,5.77,;14.32,4.99,;11.66,.39,;10.32,1.16,;10.32,2.69,;8.99,.39,;8.99,-1.16,;10.32,-1.93,;10.29,-3.46,;11.75,-3.97,;12.68,-2.74,;11.8,-1.47,;7.66,1.16,;6.32,.39,;4.99,1.16,;6.32,-1.16,)| Show InChI InChI=1S/C37H46N10O5/c1-23(48)44-32(20-28-21-41-22-43-28)36(52)47-31(18-24-9-4-3-5-10-24)35(51)45-29(13-8-16-42-37(38)39)34(50)46-30(33(49)40-2)19-25-14-15-26-11-6-7-12-27(26)17-25/h3-7,9-12,14-15,17,21-22,29-32H,8,13,16,18-20H2,1-2H3,(H,40,49)(H,41,43)(H,44,48)(H,45,51)(H,46,50)(H,47,52)(H4,38,39,42)/t29-,30-,31+,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50167675
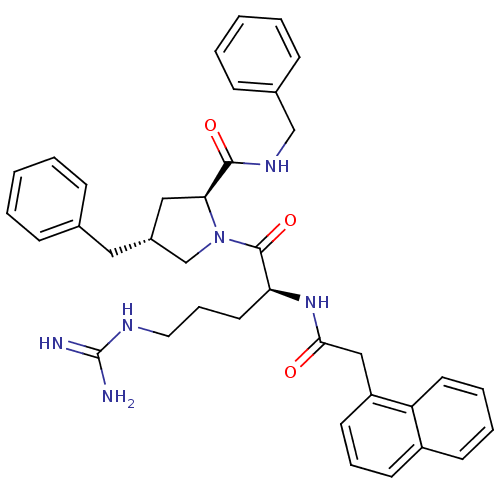
((2S,4R)-4-Benzyl-1-[(S)-5-guanidino-2-(2-naphthale...)Show SMILES NC(=N)NCCC[C@H](NC(=O)Cc1cccc2ccccc12)C(=O)N1C[C@H](Cc2ccccc2)C[C@H]1C(=O)NCc1ccccc1 Show InChI InChI=1S/C37H42N6O3/c38-37(39)40-20-10-19-32(42-34(44)23-30-17-9-16-29-15-7-8-18-31(29)30)36(46)43-25-28(21-26-11-3-1-4-12-26)22-33(43)35(45)41-24-27-13-5-2-6-14-27/h1-9,11-18,28,32-33H,10,19-25H2,(H,41,45)(H,42,44)(H4,38,39,40)/t28-,32+,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter& Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human Melanocortin 4 Receptor |
Bioorg Med Chem Lett 15: 2819-23 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.120
BindingDB Entry DOI: 10.7270/Q2F76DBS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50191576

((S)-N-((R)-1-((2S,4R)-2-(3-guanidinopropyl)-4-(nap...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H]-1-[#6]-[#6@H](-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#6]-[#7]-1)-[#8]-[#6]-c1ccc2ccccc2c1 Show InChI InChI=1S/C34H44N6O3/c35-34(36)38-18-8-13-28-21-29(43-23-25-15-16-26-11-4-5-12-27(26)19-25)22-40(28)33(42)31(20-24-9-2-1-3-10-24)39-32(41)30-14-6-7-17-37-30/h1-5,9-12,15-16,19,28-31,37H,6-8,13-14,17-18,20-23H2,(H,39,41)(H4,35,36,38)/t28-,29+,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein 5
(Homo sapiens (Human)) | BDBM50192462

((S)-2-(2,3-bis(2-chlorobenzyloxy)phenyl)-2-hydroxy...)Show SMILES O[C@H](C(O)=O)c1cccc(OCc2ccccc2Cl)c1OCc1ccccc1Cl Show InChI InChI=1S/C22H18Cl2O5/c23-17-9-3-1-6-14(17)12-28-19-11-5-8-16(20(25)22(26)27)21(19)29-13-15-7-2-4-10-18(15)24/h1-11,20,25H,12-13H2,(H,26,27)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human kFABP |
J Med Chem 49: 5013-7 (2006)
Article DOI: 10.1021/jm060360i
BindingDB Entry DOI: 10.7270/Q2TQ6157 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50034910
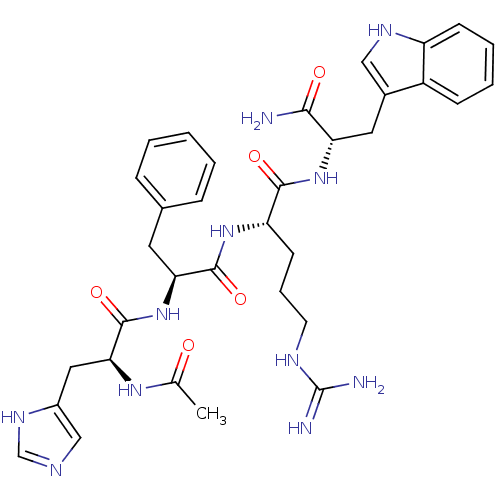
((S)-2-{(S)-2-[(S)-2-acetylamino-3-(3H-imidazol-4-y...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C34H43N11O5/c1-20(46)42-29(16-23-18-38-19-41-23)33(50)45-28(14-21-8-3-2-4-9-21)32(49)43-26(12-7-13-39-34(36)37)31(48)44-27(30(35)47)15-22-17-40-25-11-6-5-10-24(22)25/h2-6,8-11,17-19,26-29,40H,7,12-16H2,1H3,(H2,35,47)(H,38,41)(H,42,46)(H,43,49)(H,44,48)(H,45,50)(H4,36,37,39)/t26-,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191553

((S)-2-acetamido-N1-((R)-1-((2S,4R)-2-(3-guanidinop...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6@@H](-[#6]-[#6@@H]-1-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#8]-[#6]-c1ccc2ccccc2c1 Show InChI InChI=1S/C35H45N7O5/c1-23(43)40-30(15-16-32(36)44)33(45)41-31(19-24-8-3-2-4-9-24)34(46)42-21-29(20-28(42)12-7-17-39-35(37)38)47-22-25-13-14-26-10-5-6-11-27(26)18-25/h2-6,8-11,13-14,18,28-31H,7,12,15-17,19-22H2,1H3,(H2,36,44)(H,40,43)(H,41,45)(H4,37,38,39)/t28-,29+,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50261526

(Ac-YGGFL-NH2 | CHEMBL500212)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C30H40N6O7/c1-18(2)13-23(28(31)41)36-30(43)25(14-20-7-5-4-6-8-20)35-27(40)17-32-26(39)16-33-29(42)24(34-19(3)37)15-21-9-11-22(38)12-10-21/h4-12,18,23-25,38H,13-17H2,1-3H3,(H2,31,41)(H,32,39)(H,33,42)(H,34,37)(H,35,40)(H,36,43)/t23-,24-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SH-SY5Y cells |
Bioorg Med Chem 16: 4341-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.074
BindingDB Entry DOI: 10.7270/Q2MG7P8J |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50427703

(CHEMBL2324220)Show SMILES NC(N)=NCCC[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccc2ccccc2c1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r,wU:7.7,45.48,11.11,wD:31.32,(15.26,-7.72,;16.62,-8.43,;17.92,-7.6,;16.69,-9.97,;15.39,-10.79,;15.46,-12.33,;14.16,-13.16,;14.24,-14.7,;12.94,-15.53,;11.61,-14.76,;11.61,-13.22,;10.27,-15.53,;10.27,-17.07,;8.94,-17.84,;7.6,-17.07,;8.94,-19.38,;7.6,-20.16,;8.97,-14.76,;8.94,-13.22,;7.58,-12.47,;7.55,-10.93,;8.87,-10.13,;8.84,-8.59,;10.15,-7.8,;11.51,-8.53,;11.55,-10.08,;10.22,-10.88,;10.26,-12.42,;15.6,-15.4,;15.66,-16.95,;16.9,-14.58,;18.26,-15.29,;18.34,-16.83,;19.7,-17.53,;21.15,-17.03,;22.08,-18.26,;21.2,-19.52,;21.54,-21.02,;20.42,-22.07,;18.95,-21.62,;18.6,-20.12,;19.73,-19.07,;19.56,-14.46,;19.49,-12.92,;20.92,-15.17,;22.22,-14.34,;22.16,-12.8,;23.45,-11.98,;23.38,-10.44,;22.02,-9.73,;24.68,-9.61,;23.58,-15.05,;24.88,-14.22,;23.66,-16.59,)| Show InChI InChI=1S/C37H45N9O8/c38-33(50)28(13-14-32(48)49)43-36(53)30(18-25-20-42-27-9-4-3-8-26(25)27)45-35(52)29(10-5-15-41-37(39)40)44-34(51)24(19-31(47)46-54)17-21-11-12-22-6-1-2-7-23(22)16-21/h1-4,6-9,11-12,16,20,24,28-30,42,54H,5,10,13-15,17-19H2,(H2,38,50)(H,43,53)(H,44,51)(H,45,52)(H,46,47)(H,48,49)(H4,39,40,41)/t24-,28+,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated insulin degradation |
J Med Chem 56: 2246-55 (2013)
Article DOI: 10.1021/jm301280p
BindingDB Entry DOI: 10.7270/Q2DB836R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data