

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0210 | -63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233601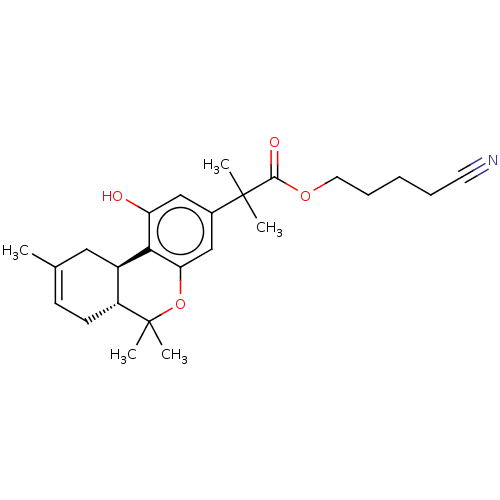 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,I543V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -57.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599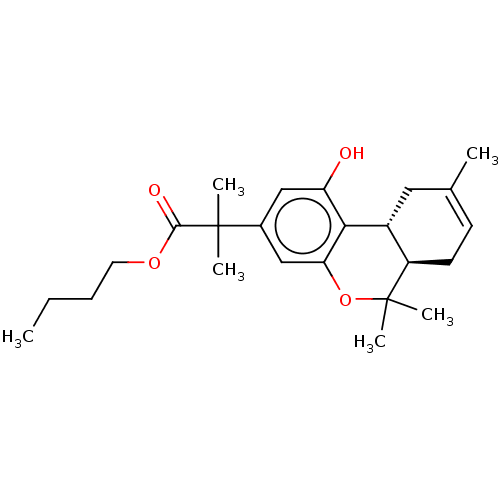 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160917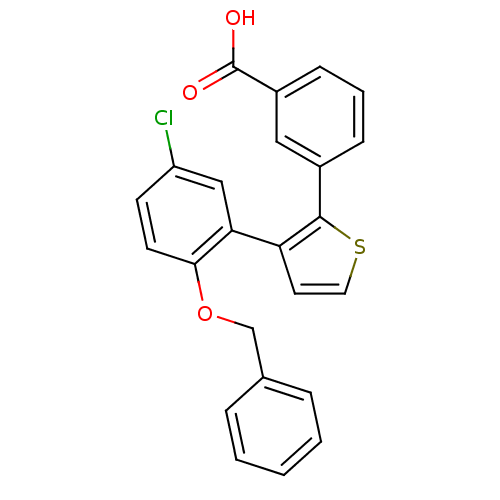 (3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPR | Bioorg Med Chem Lett 16: 2666-71 (2006) Article DOI: 10.1016/j.bmcl.2006.02.014 BindingDB Entry DOI: 10.7270/Q2J102RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233619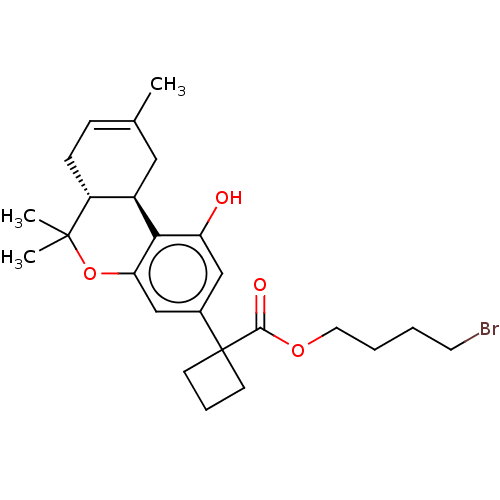 (CHEMBL4085404 | US10882838, Example 1.18) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233613 (CHEMBL4092136 | US10882838, Example 1.33) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233602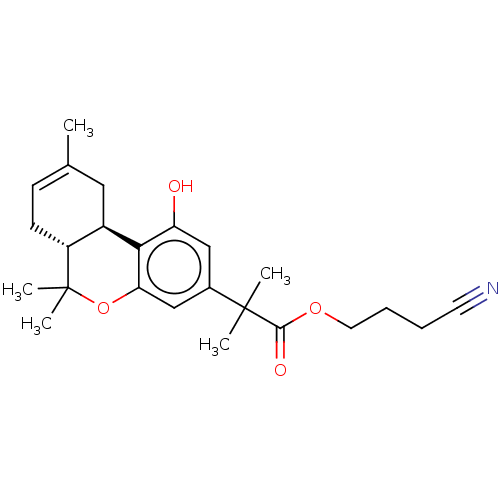 (CHEMBL4071389 | US10882838, Example 1.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,M535I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233622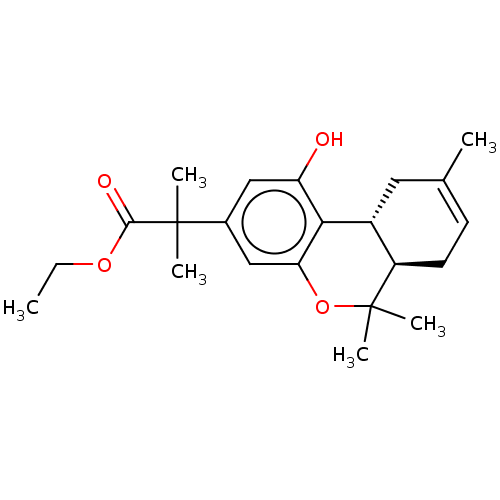 (CHEMBL4073011 | US10882838, Example 1.35) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,I573V,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233608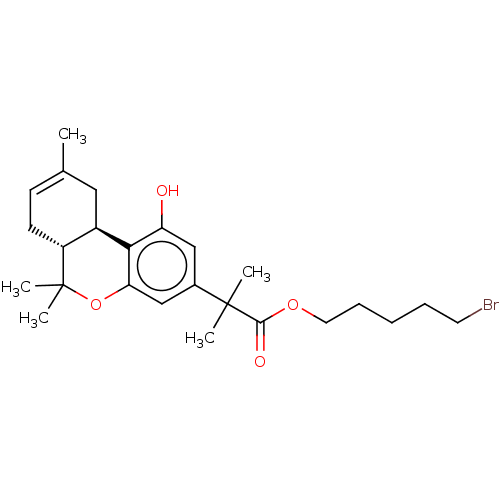 (CHEMBL4094603) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50495106 (CHEMBL3104358 | US10882838, Example 1.16) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233612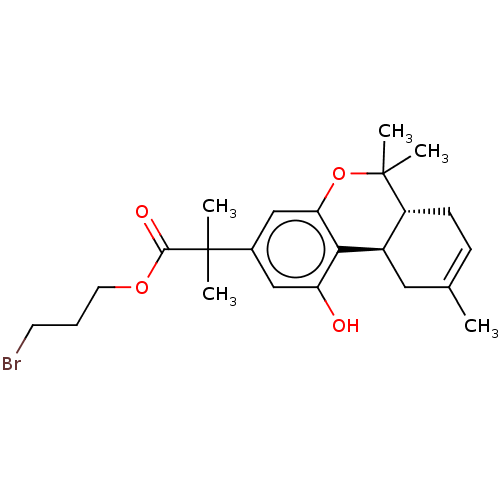 (CHEMBL4102348 | US10882838, Example 1.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233605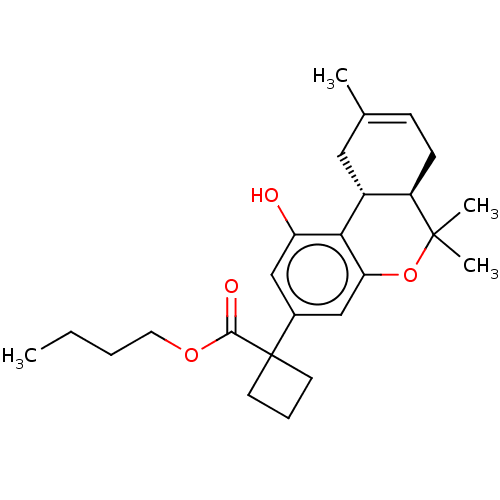 (CHEMBL3104362 | US10882838, Example 1.20) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233619 (CHEMBL4085404 | US10882838, Example 1.18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233605 (CHEMBL3104362 | US10882838, Example 1.20) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233601 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233602 (CHEMBL4071389 | US10882838, Example 1.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233612 (CHEMBL4102348 | US10882838, Example 1.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233609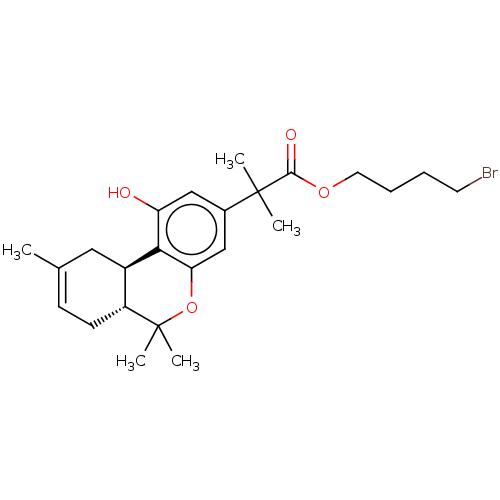 (CHEMBL4074070) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus (rat)) | BDBM50198230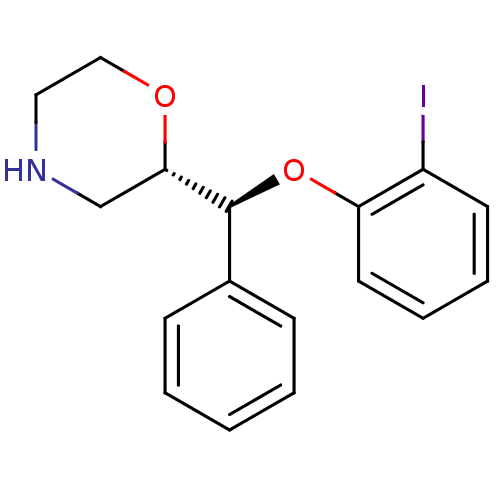 ((S)-2-((S)-(2-iodophenoxy)(phenyl)methyl)morpholin...) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NAT in Sprague-Dawley rat brain after 4 hrs by liquid scintillation analysis | Bioorg Med Chem Lett 19: 4996-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.064 BindingDB Entry DOI: 10.7270/Q22V2G5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233609 (CHEMBL4074070) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233601 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233604 (CHEMBL4063822 | US10882838, Example 1.19) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233613 (CHEMBL4092136 | US10882838, Example 1.33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233608 (CHEMBL4094603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233612 (CHEMBL4102348 | US10882838, Example 1.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233609 (CHEMBL4074070) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233613 (CHEMBL4092136 | US10882838, Example 1.33) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233603 (CHEMBL4098489 | US10882838, Example 1.28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233608 (CHEMBL4094603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM518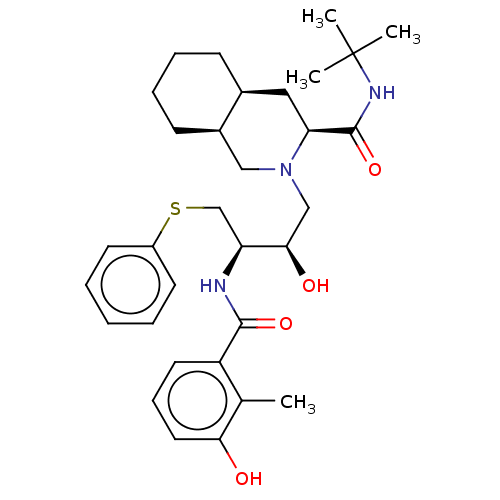 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20 | -53.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233600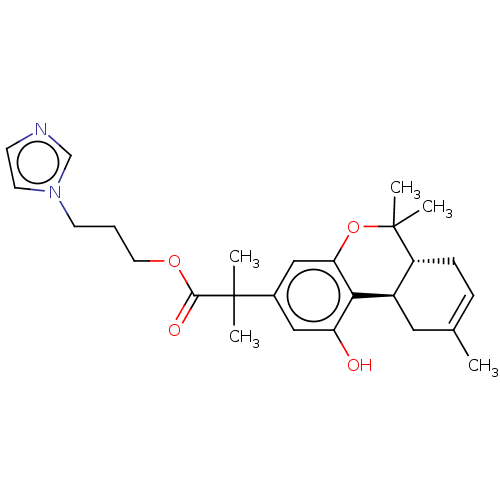 (CHEMBL4098428 | US10882838, Example 1.10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233602 (CHEMBL4071389 | US10882838, Example 1.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233604 (CHEMBL4063822 | US10882838, Example 1.19) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233622 (CHEMBL4073011 | US10882838, Example 1.35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50495107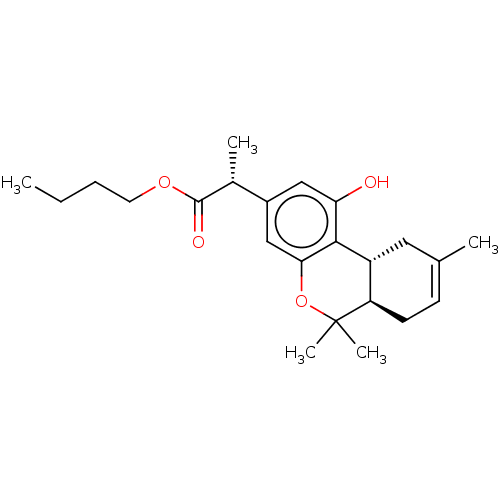 (CHEMBL3104356 | US10882838, Example 1.14) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233599 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cell membranes by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233599 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233604 (CHEMBL4063822 | US10882838, Example 1.19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233600 (CHEMBL4098428 | US10882838, Example 1.10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233603 (CHEMBL4098489 | US10882838, Example 1.28) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233599 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3315 total ) | Next | Last >> |