 Found 1157 hits with Last Name = 'forrest' and Initial = 'mj'
Found 1157 hits with Last Name = 'forrest' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313984
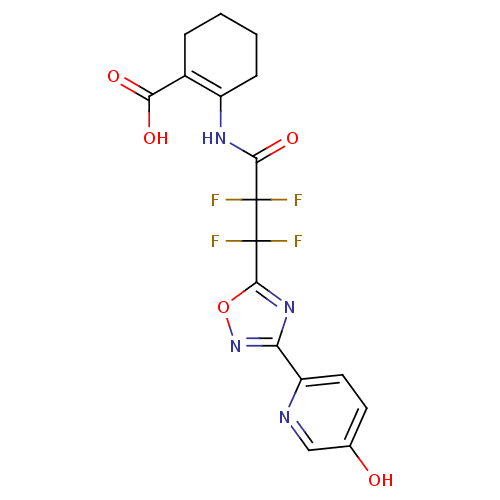
(2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C(F)(F)C(F)(F)c1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H14F4N4O5/c18-16(19,14(29)23-10-4-2-1-3-9(10)13(27)28)17(20,21)15-24-12(25-30-15)11-6-5-8(26)7-22-11/h5-7,26H,1-4H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313977
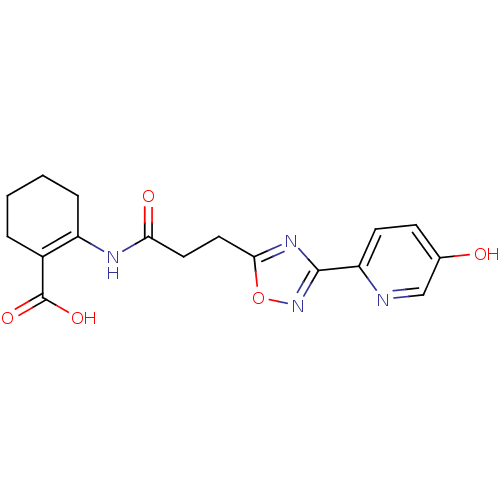
(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H18N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h5-6,9,22H,1-4,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23533
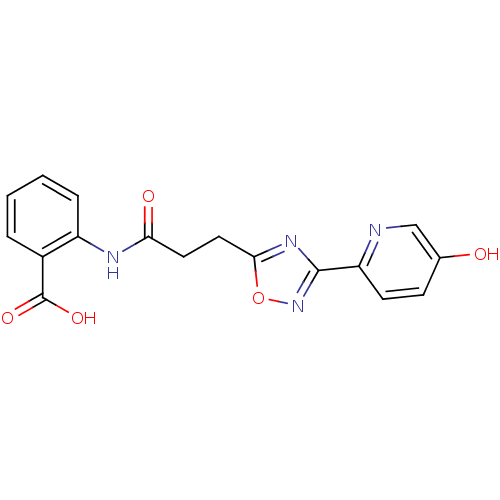
(2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nc(no1)-c1ccc(O)cn1 Show InChI InChI=1S/C17H14N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h1-6,9,22H,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313976
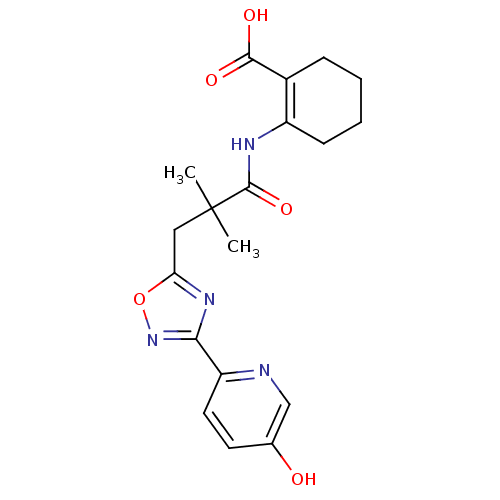
(2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES CC(C)(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:21| Show InChI InChI=1S/C19H22N4O5/c1-19(2,18(27)21-13-6-4-3-5-12(13)17(25)26)9-15-22-16(23-28-15)14-8-7-11(24)10-20-14/h7-8,10,24H,3-6,9H2,1-2H3,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313979
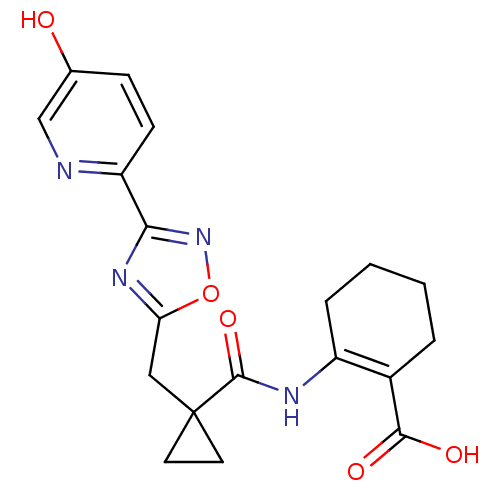
(2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C1(Cc2nc(no2)-c2ccc(O)cn2)CC1 |t:3| Show InChI InChI=1S/C19H20N4O5/c24-11-5-6-14(20-10-11)16-22-15(28-23-16)9-19(7-8-19)18(27)21-13-4-2-1-3-12(13)17(25)26/h5-6,10,24H,1-4,7-9H2,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132563
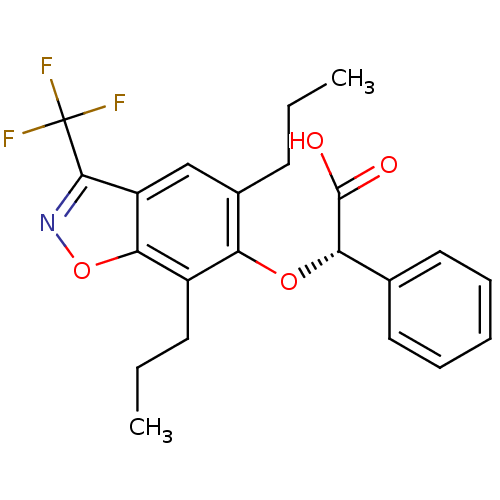
((S)-(5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxaz...)Show SMILES CCCc1cc2c(noc2c(CCC)c1O[C@H](C(O)=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H22F3NO4/c1-3-8-14-12-16-19(30-26-20(16)22(23,24)25)15(9-4-2)17(14)29-18(21(27)28)13-10-6-5-7-11-13/h5-7,10-12,18H,3-4,8-9H2,1-2H3,(H,27,28)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132574
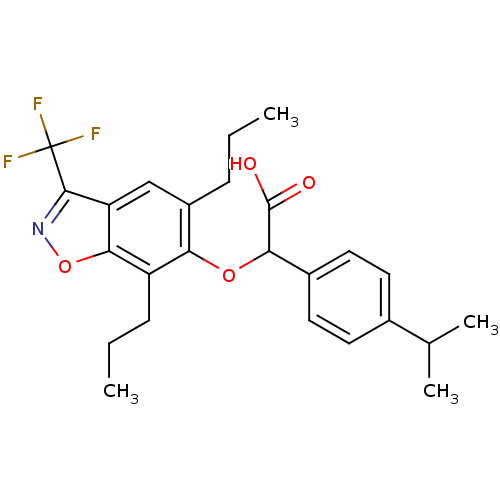
((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(C)C)C(F)(F)F Show InChI InChI=1S/C25H28F3NO4/c1-5-7-17-13-19-22(33-29-23(19)25(26,27)28)18(8-6-2)20(17)32-21(24(30)31)16-11-9-15(10-12-16)14(3)4/h9-14,21H,5-8H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132567
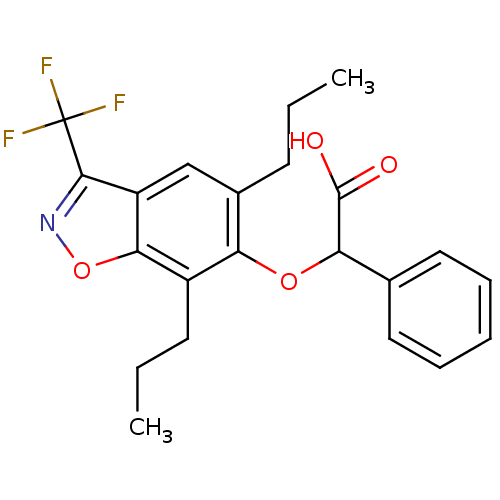
((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H22F3NO4/c1-3-8-14-12-16-19(30-26-20(16)22(23,24)25)15(9-4-2)17(14)29-18(21(27)28)13-10-6-5-7-11-13/h5-7,10-12,18H,3-4,8-9H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132564
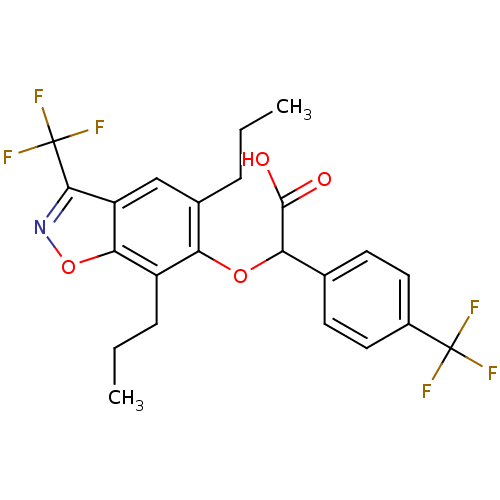
((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H21F6NO4/c1-3-5-13-11-16-19(34-30-20(16)23(27,28)29)15(6-4-2)17(13)33-18(21(31)32)12-7-9-14(10-8-12)22(24,25)26/h7-11,18H,3-6H2,1-2H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132561

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(OCc2ccccn2)cc1)C(F)(F)F Show InChI InChI=1S/C28H27F3N2O5/c1-3-7-18-15-22-25(38-33-26(22)28(29,30)31)21(8-4-2)23(18)37-24(27(34)35)17-10-12-20(13-11-17)36-16-19-9-5-6-14-32-19/h5-6,9-15,24H,3-4,7-8,16H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313981
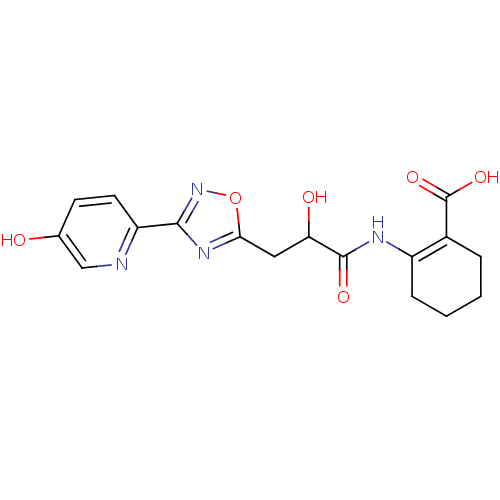
(CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...)Show SMILES OC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C17H18N4O6/c22-9-5-6-12(18-8-9)15-20-14(27-21-15)7-13(23)16(24)19-11-4-2-1-3-10(11)17(25)26/h5-6,8,13,22-23H,1-4,7H2,(H,19,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132564

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H21F6NO4/c1-3-5-13-11-16-19(34-30-20(16)23(27,28)29)15(6-4-2)17(13)33-18(21(31)32)12-7-9-14(10-8-12)22(24,25)26/h7-11,18H,3-6H2,1-2H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132565
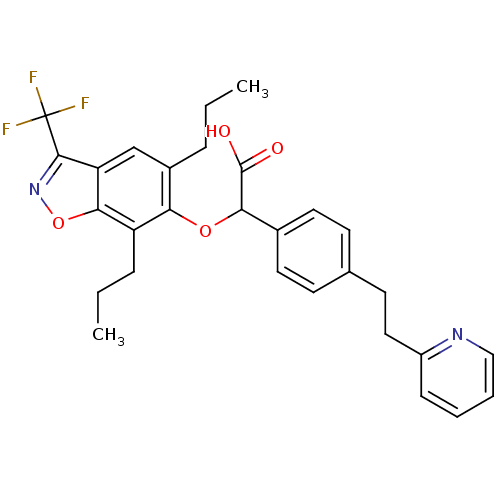
((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(CCc2ccccn2)cc1)C(F)(F)F Show InChI InChI=1S/C29H29F3N2O4/c1-3-7-20-17-23-26(38-34-27(23)29(30,31)32)22(8-4-2)24(20)37-25(28(35)36)19-13-10-18(11-14-19)12-15-21-9-5-6-16-33-21/h5-6,9-11,13-14,16-17,25H,3-4,7-8,12,15H2,1-2H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132576
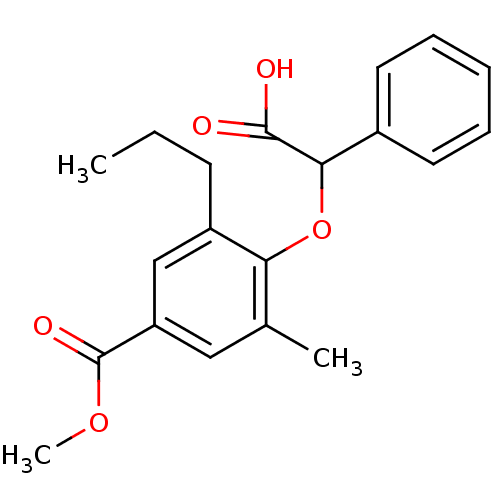
(4-(Carboxy-phenyl-methoxy)-3-methyl-5-propyl-benzo...)Show InChI InChI=1S/C20H22O5/c1-4-8-15-12-16(20(23)24-3)11-13(2)17(15)25-18(19(21)22)14-9-6-5-7-10-14/h5-7,9-12,18H,4,8H2,1-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313982
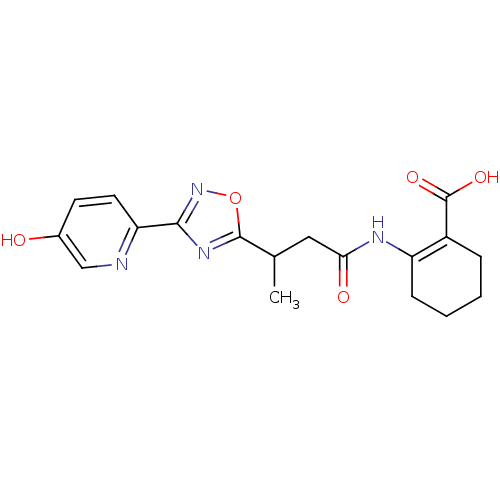
(CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...)Show SMILES CC(CC(=O)NC1=C(CCCC1)C(O)=O)c1nc(no1)-c1ccc(O)cn1 |t:6| Show InChI InChI=1S/C18H20N4O5/c1-10(8-15(24)20-13-5-3-2-4-12(13)18(25)26)17-21-16(22-27-17)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132574

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(C)C)C(F)(F)F Show InChI InChI=1S/C25H28F3NO4/c1-5-7-17-13-19-22(33-29-23(19)25(26,27)28)18(8-6-2)20(17)32-21(24(30)31)16-11-9-15(10-12-16)14(3)4/h9-14,21H,5-8H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132567

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H22F3NO4/c1-3-8-14-12-16-19(30-26-20(16)22(23,24)25)15(9-4-2)17(14)29-18(21(27)28)13-10-6-5-7-11-13/h5-7,10-12,18H,3-4,8-9H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132568
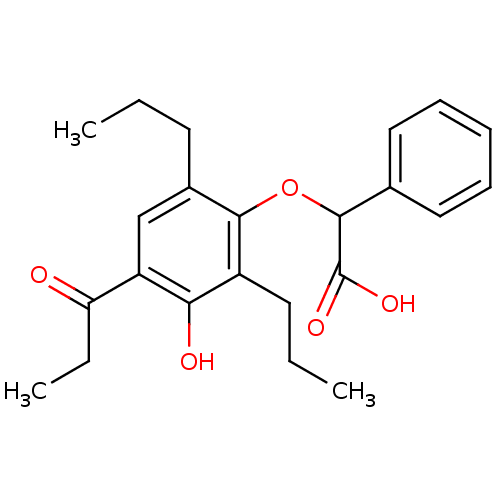
((3-Hydroxy-4-propionyl-2,6-dipropyl-phenoxy)-pheny...)Show SMILES CCCc1cc(C(=O)CC)c(O)c(CCC)c1OC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C23H28O5/c1-4-10-16-14-18(19(24)6-3)20(25)17(11-5-2)21(16)28-22(23(26)27)15-12-8-7-9-13-15/h7-9,12-14,22,25H,4-6,10-11H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132568

((3-Hydroxy-4-propionyl-2,6-dipropyl-phenoxy)-pheny...)Show SMILES CCCc1cc(C(=O)CC)c(O)c(CCC)c1OC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C23H28O5/c1-4-10-16-14-18(19(24)6-3)20(25)17(11-5-2)21(16)28-22(23(26)27)15-12-8-7-9-13-15/h7-9,12-14,22,25H,4-6,10-11H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132571
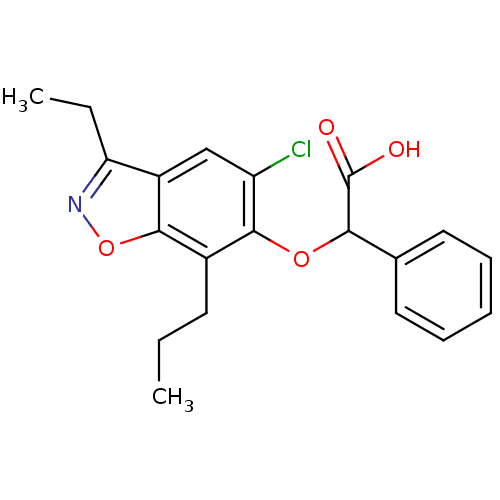
((5-Chloro-3-ethyl-7-propyl-benzo[d]isoxazol-6-ylox...)Show SMILES CCCc1c(OC(C(O)=O)c2ccccc2)c(Cl)cc2c(CC)noc12 Show InChI InChI=1S/C20H20ClNO4/c1-3-8-13-18-14(16(4-2)22-26-18)11-15(21)19(13)25-17(20(23)24)12-9-6-5-7-10-12/h5-7,9-11,17H,3-4,8H2,1-2H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50132574

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(C)C)C(F)(F)F Show InChI InChI=1S/C25H28F3NO4/c1-5-7-17-13-19-22(33-29-23(19)25(26,27)28)18(8-6-2)20(17)32-21(24(30)31)16-11-9-15(10-12-16)14(3)4/h9-14,21H,5-8H2,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50132561

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(OCc2ccccn2)cc1)C(F)(F)F Show InChI InChI=1S/C28H27F3N2O5/c1-3-7-18-15-22-25(38-33-26(22)28(29,30)31)21(8-4-2)23(18)37-24(27(34)35)17-10-12-20(13-11-17)36-16-19-9-5-6-14-32-19/h5-6,9-15,24H,3-4,7-8,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50174201
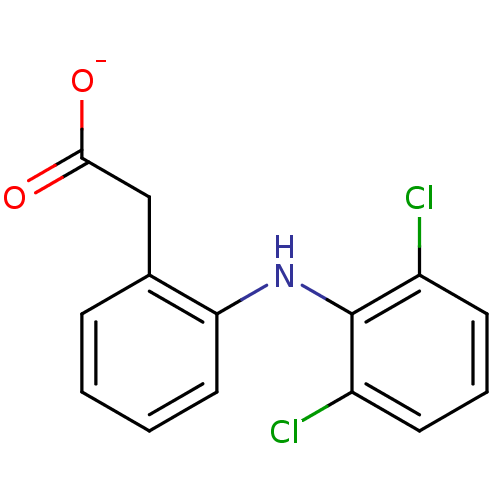
(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132575
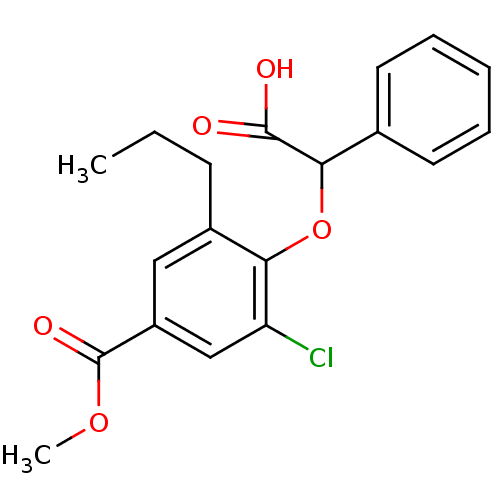
(4-(Carboxy-phenyl-methoxy)-3-chloro-5-propyl-benzo...)Show InChI InChI=1S/C19H19ClO5/c1-3-7-13-10-14(19(23)24-2)11-15(20)16(13)25-17(18(21)22)12-8-5-4-6-9-12/h4-6,8-11,17H,3,7H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132570
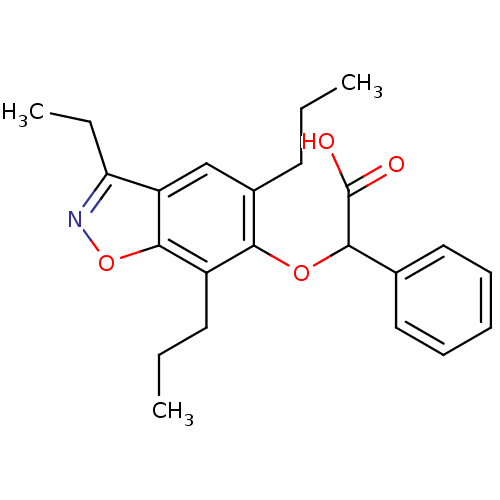
((3-Ethyl-5,7-dipropyl-benzo[d]isoxazol-6-yloxy)-ph...)Show SMILES CCCc1cc2c(CC)noc2c(CCC)c1OC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C23H27NO4/c1-4-10-16-14-18-19(6-3)24-28-22(18)17(11-5-2)20(16)27-21(23(25)26)15-12-8-7-9-13-15/h7-9,12-14,21H,4-6,10-11H2,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132572
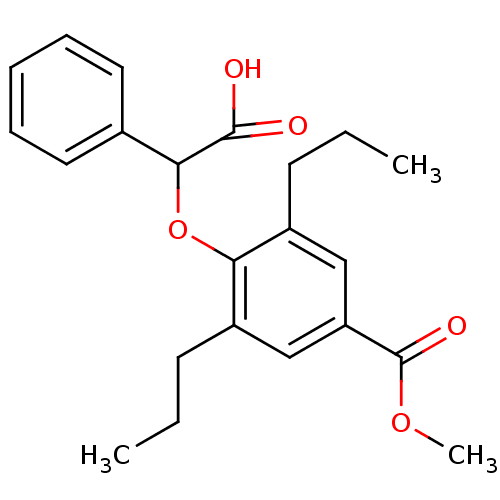
(4-(Carboxy-phenyl-methoxy)-3,5-dipropyl-benzoic ac...)Show SMILES CCCc1cc(cc(CCC)c1OC(C(O)=O)c1ccccc1)C(=O)OC Show InChI InChI=1S/C22H26O5/c1-4-9-16-13-18(22(25)26-3)14-17(10-5-2)19(16)27-20(21(23)24)15-11-7-6-8-12-15/h6-8,11-14,20H,4-5,9-10H2,1-3H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132561

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(OCc2ccccn2)cc1)C(F)(F)F Show InChI InChI=1S/C28H27F3N2O5/c1-3-7-18-15-22-25(38-33-26(22)28(29,30)31)21(8-4-2)23(18)37-24(27(34)35)17-10-12-20(13-11-17)36-16-19-9-5-6-14-32-19/h5-6,9-15,24H,3-4,7-8,16H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132565

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(CCc2ccccn2)cc1)C(F)(F)F Show InChI InChI=1S/C29H29F3N2O4/c1-3-7-20-17-23-26(38-34-27(23)29(30,31)32)22(8-4-2)24(20)37-25(28(35)36)19-13-10-18(11-14-19)12-15-21-9-5-6-16-33-21/h5-6,9-11,13-14,16-17,25H,3-4,7-8,12,15H2,1-2H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50132564

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H21F6NO4/c1-3-5-13-11-16-19(34-30-20(16)23(27,28)29)15(6-4-2)17(13)33-18(21(31)32)12-7-9-14(10-8-12)22(24,25)26/h7-11,18H,3-6H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132570

((3-Ethyl-5,7-dipropyl-benzo[d]isoxazol-6-yloxy)-ph...)Show SMILES CCCc1cc2c(CC)noc2c(CCC)c1OC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C23H27NO4/c1-4-10-16-14-18-19(6-3)24-28-22(18)17(11-5-2)20(16)27-21(23(25)26)15-12-8-7-9-13-15/h7-9,12-14,21H,4-6,10-11H2,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50132574

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(C)C)C(F)(F)F Show InChI InChI=1S/C25H28F3NO4/c1-5-7-17-13-19-22(33-29-23(19)25(26,27)28)18(8-6-2)20(17)32-21(24(30)31)16-11-9-15(10-12-16)14(3)4/h9-14,21H,5-8H2,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor gamma (PPAR gamma) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132578

(CHEMBL109161 | Phenyl-(7-propyl-3-trifluoromethyl-...)Show SMILES CCCc1c(OC(C(O)=O)c2ccccc2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C19H16F3NO4/c1-2-6-12-14(26-15(18(24)25)11-7-4-3-5-8-11)10-9-13-16(12)27-23-17(13)19(20,21)22/h3-5,7-10,15H,2,6H2,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM24566
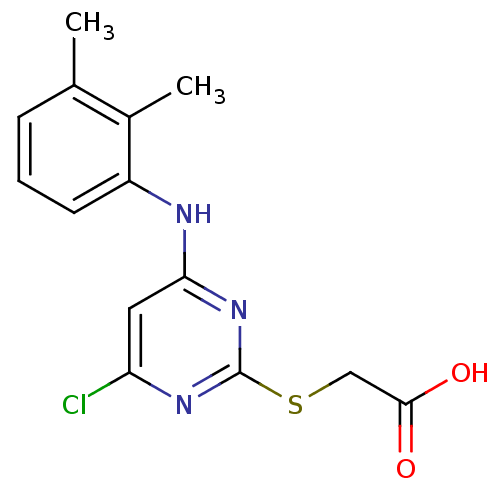
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (murinePPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data