

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50208836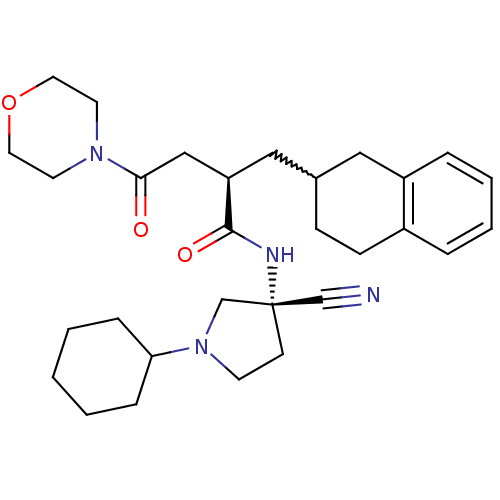 ((R)-N-((R)-3-cyano-1-cyclohexylpyrrolidin-3-yl)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121542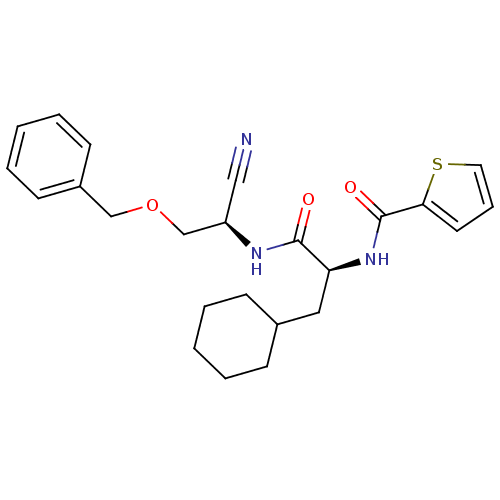 (CHEMBL155560 | Thiophene-2-carboxylic acid {1-[(be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121562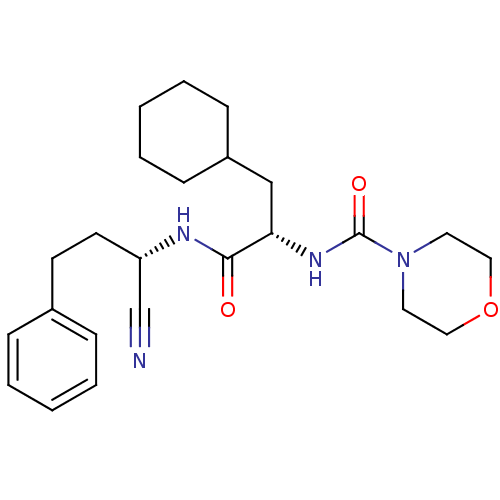 (CHEMBL153239 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121549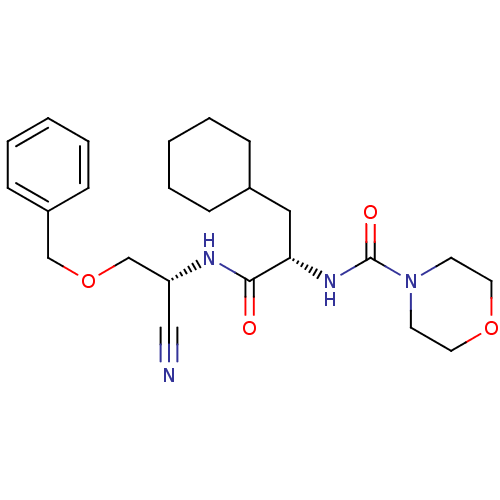 (CHEMBL347111 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121554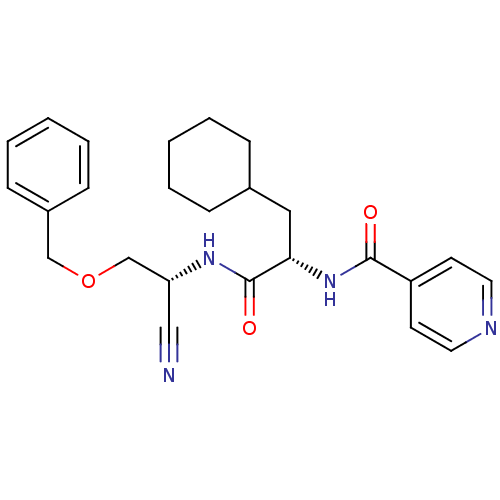 (CHEMBL356155 | N-{1-[(Benzyloxymethyl-cyano-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121575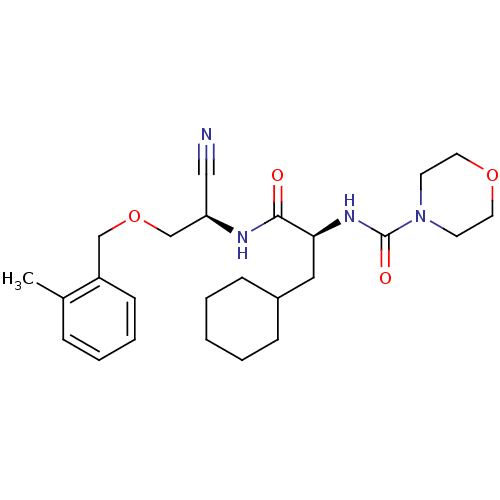 (CHEMBL356442 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121581 (CHEMBL150253 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121569 (CHEMBL153783 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121543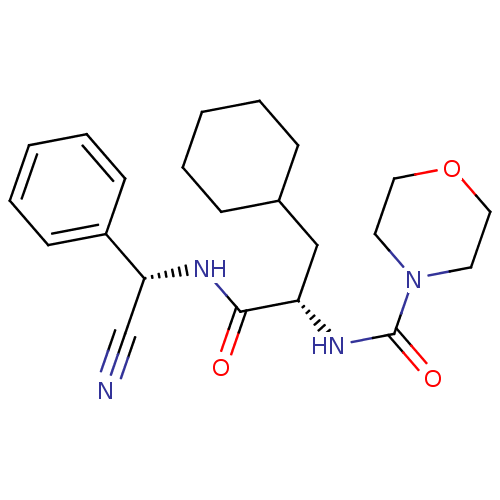 (CHEMBL435440 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121545 (CHEMBL149523 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121561 (CHEMBL356167 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121544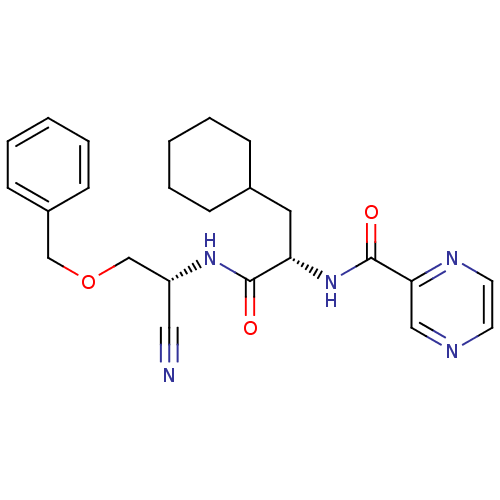 (CHEMBL153248 | Pyrazine-2-carboxylic acid {1-[(ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050527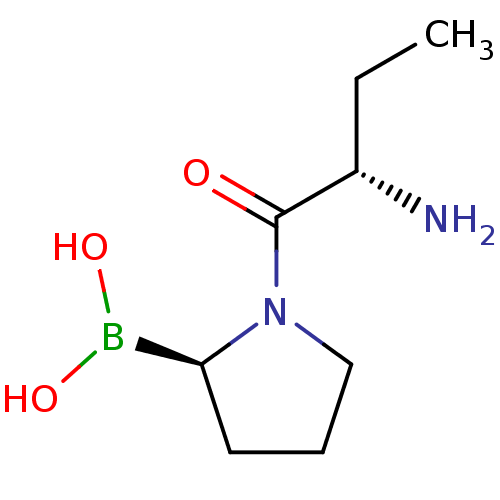 (Boronic acid derivative | CHEMBL66032 | US11096924...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121558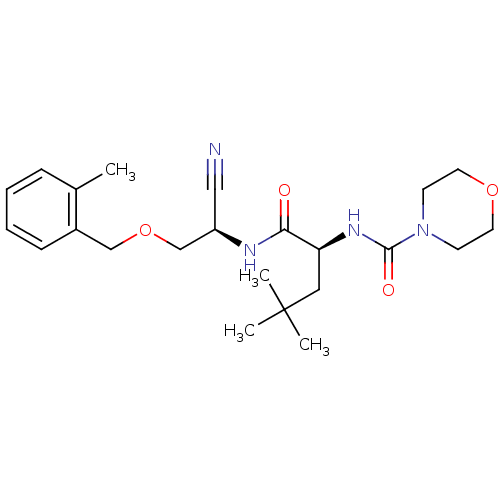 (CHEMBL152940 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208837 ((R)-N-((R)-3-cyano-1-cyclohexylpyrrolidin-3-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121572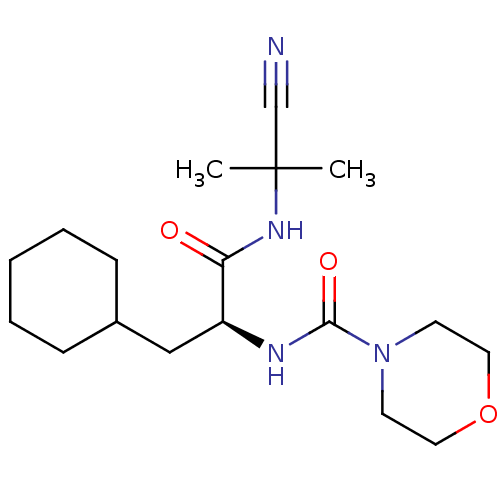 (CHEMBL150358 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121548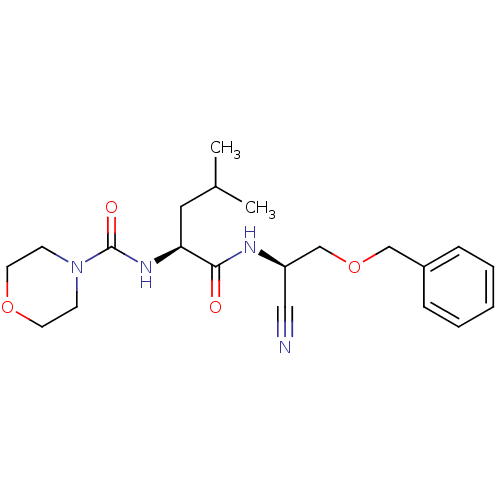 (CHEMBL153813 | MORPHOLINE-4-CARBOXYLIC ACID [1S-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121541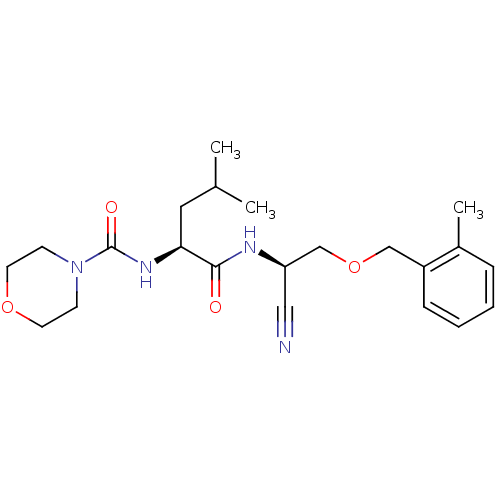 (CHEMBL346448 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050521 ((2-Dihydroxyborane-pyrrolidin-1-yl)-pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121576 (CHEMBL153018 | Furan-2-carboxylic acid {1-[(benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050517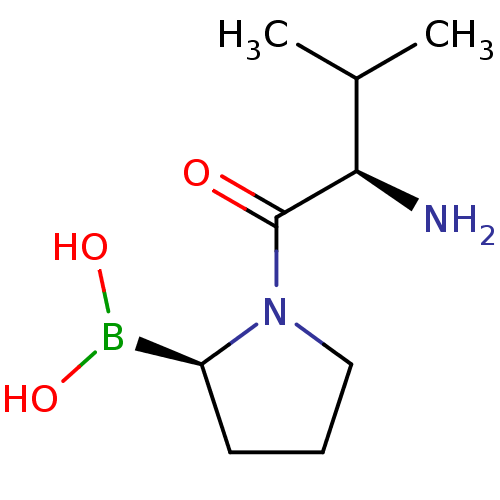 (Boronic acid derivative | CHEMBL305170 | N-alkyl G...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208855 ((R)-N-((R)-3-cyano-1-(cyclopropylmethyl)pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208856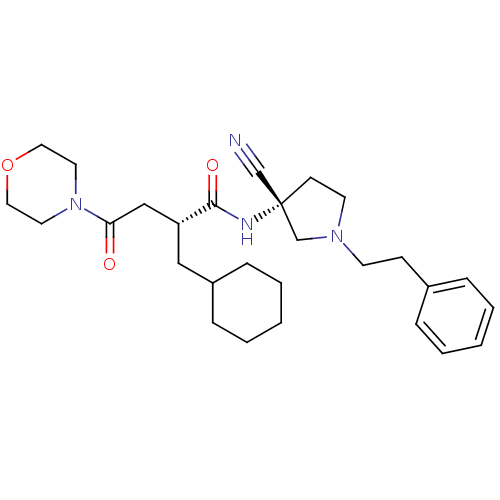 ((R)-N-((R)-3-cyano-1-phenethylpyrrolidin-3-yl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208846 ((R)-N-((R)-3-cyano-1-cyclohexylpyrrolidin-3-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208858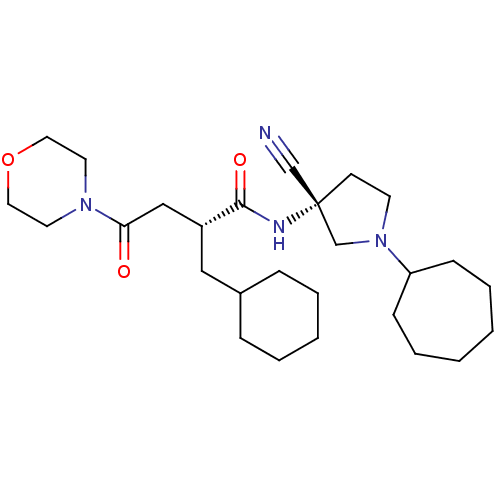 ((R)-N-((R)-3-cyano-1-cycloheptylpyrrolidin-3-yl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121570 (CHEMBL348679 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50369128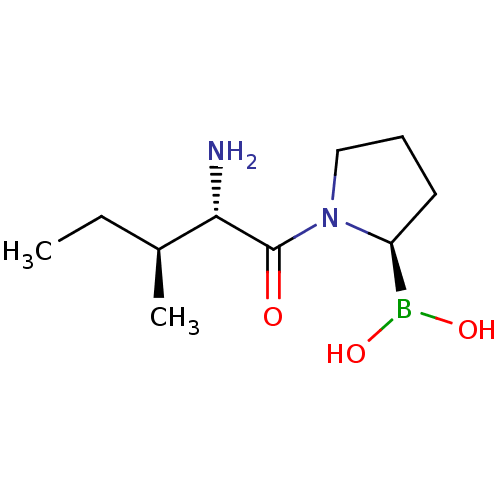 (CHEMBL1790483 | US11096924, DASH-inhibitors 4316 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121557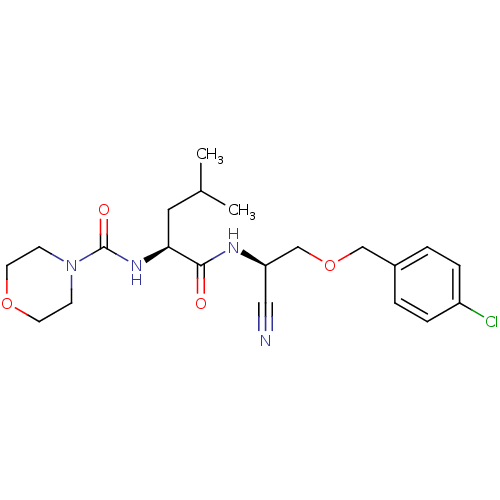 (CHEMBL151642 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208852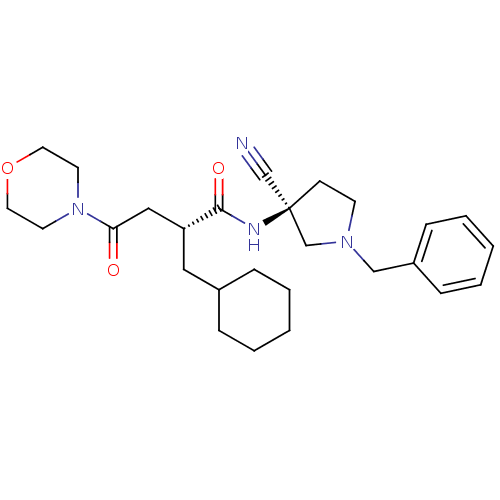 ((R)-N-((S)-1-benzyl-3-cyanopyrrolidin-3-yl)-2-(cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050514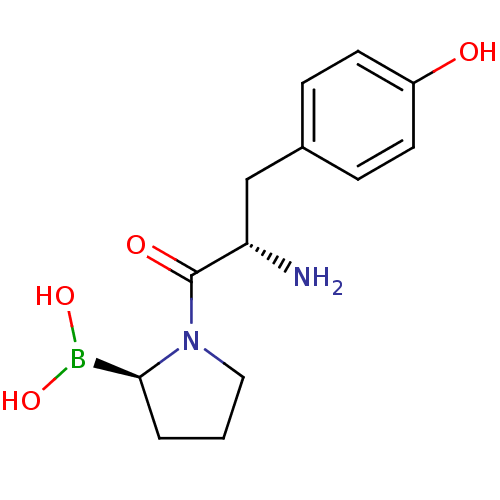 (Boronic acid derivative | CHEMBL304007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208839 ((R)-N-((R)-2-(benzyloxy)-1-cyanoethyl)-2-(cyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208849 ((R)-N-((R)-3-cyano-1-isobutylpyrrolidin-3-yl)-2-(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121551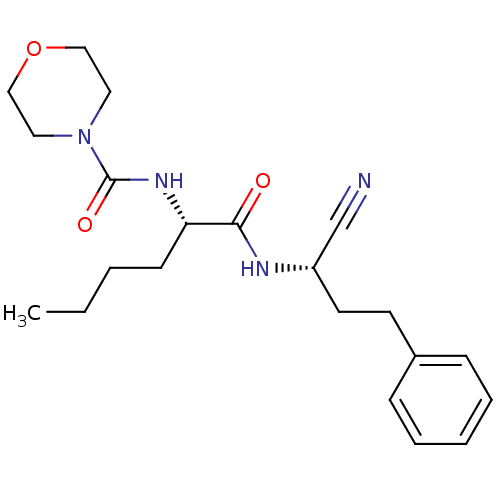 (CHEMBL150574 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208853 ((R)-N-((S)-3-cyano-1-(cyclohexylmethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208838 ((R)-N-((R)-3-cyano-1-cyclohexylpyrrolidin-3-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050528 (Boronic acid derivative | CHEMBL63698 | US11096924...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208848 ((R)-N-((R)-3-cyano-1-cyclohexylpyrrolidin-3-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121564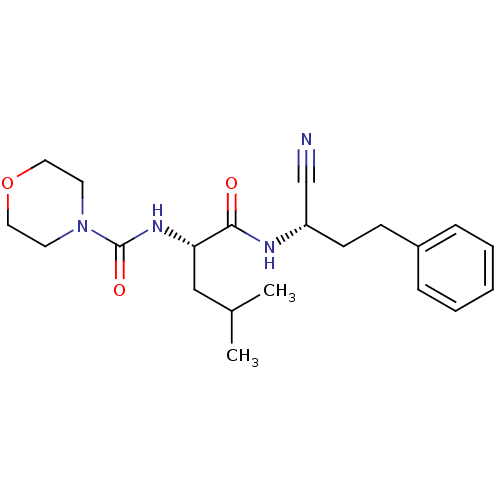 (CHEMBL153678 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121546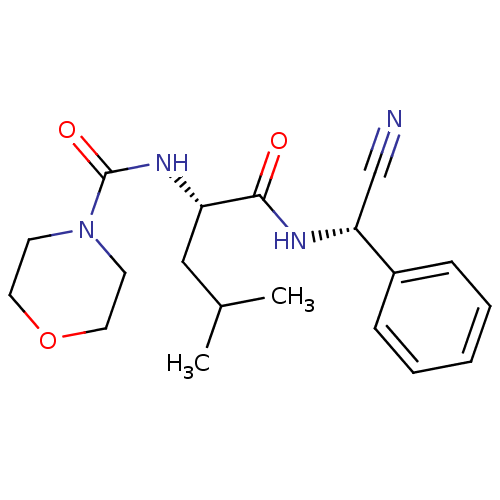 (CHEMBL347745 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121566 (CHEMBL153319 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121578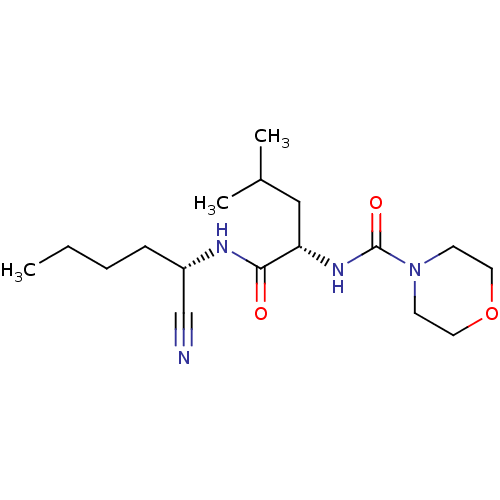 (CHEMBL435245 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050520 (Boronic acid derivative | CHEMBL63406 | US11096924...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208844 ((R)-N-((R)-2-(3-chlorobenzyloxy)-1-cyanoethyl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050524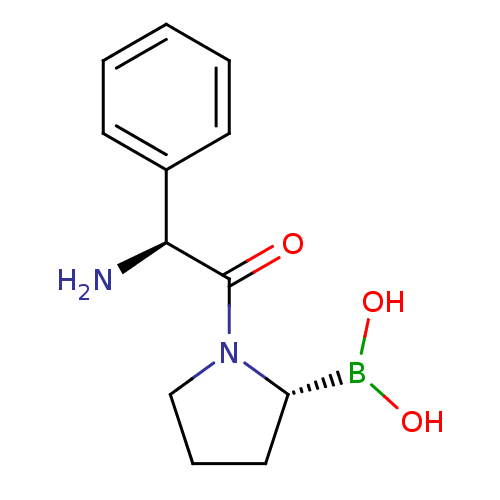 (Boronic acid derivative | CHEMBL291428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208842 ((R)-N-((R)-3-cyano-1-cyclohexylpyrrolidin-3-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50208840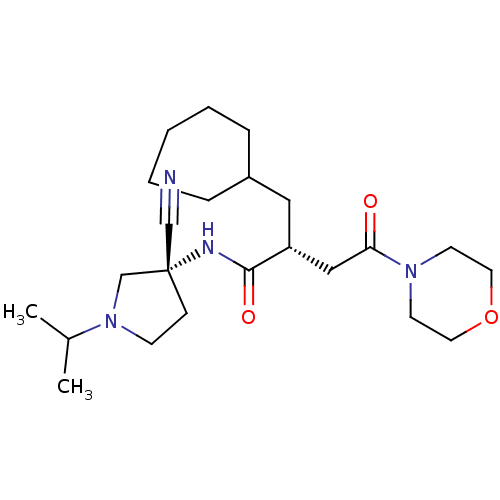 ((R)-N-((R)-3-cyano-1-isopropylpyrrolidin-3-yl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 2465-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.046 BindingDB Entry DOI: 10.7270/Q24X57G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 140 total ) | Next | Last >> |