 Found 45 hits with Last Name = 'furukawa' and Initial = 'k'
Found 45 hits with Last Name = 'furukawa' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50160807
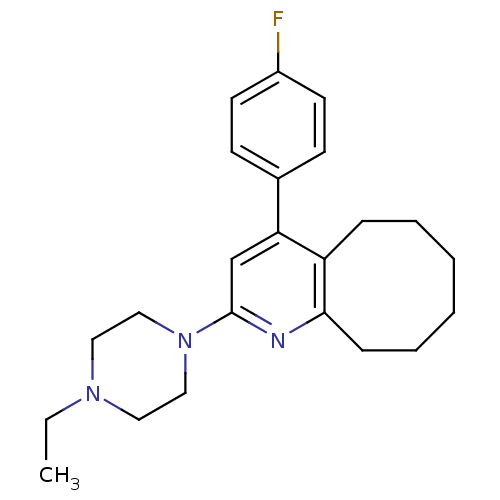
(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 8.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 20.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 26.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 56.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 59.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 875 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid type B receptor subunit 1
(Rattus norvegicus (Rat)) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50160807

(4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,...)Show InChI InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 158-65 (1993)
BindingDB Entry DOI: 10.7270/Q2TH8K60 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50499276

(CHEMBL3735861)Show SMILES COc1ccc2C(=O)\C(Oc2c1CN1CCN[C@@H](C)C1)=C\c1n[nH]c2ncccc12 |r| Show InChI InChI=1S/C22H23N5O3/c1-13-11-27(9-8-23-13)12-16-18(29-2)6-5-15-20(28)19(30-21(15)16)10-17-14-4-3-7-24-22(14)26-25-17/h3-7,10,13,23H,8-9,11-12H2,1-2H3,(H,24,25,26)/b19-10-/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50499277
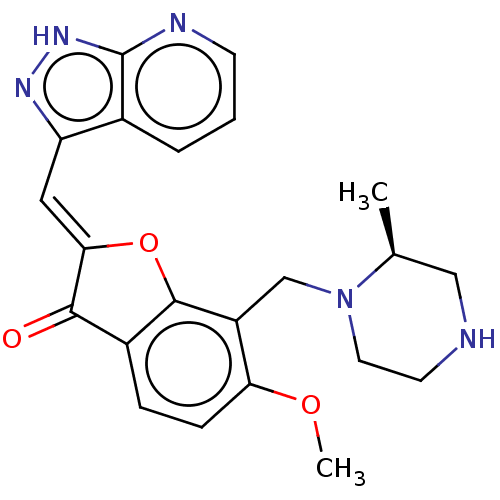
(CHEMBL3735633)Show SMILES COc1ccc2C(=O)\C(Oc2c1CN1CCNC[C@@H]1C)=C\c1n[nH]c2ncccc12 |r| Show InChI InChI=1S/C22H23N5O3/c1-13-11-23-8-9-27(13)12-16-18(29-2)6-5-15-20(28)19(30-21(15)16)10-17-14-4-3-7-24-22(14)26-25-17/h3-7,10,13,23H,8-9,11-12H2,1-2H3,(H,24,25,26)/b19-10-/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50391948
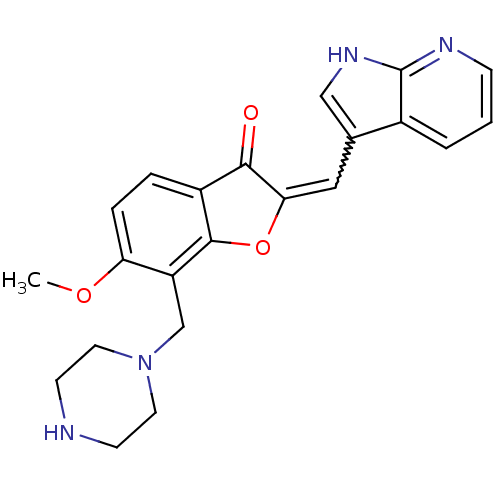
(CHEMBL2147767)Show SMILES COc1ccc2C(=O)C(Oc2c1CN1CCNCC1)=Cc1c[nH]c2ncccc12 |w:19.22| Show InChI InChI=1S/C22H22N4O3/c1-28-18-5-4-16-20(27)19(11-14-12-25-22-15(14)3-2-6-24-22)29-21(16)17(18)13-26-9-7-23-8-10-26/h2-6,11-12,23H,7-10,13H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50499281
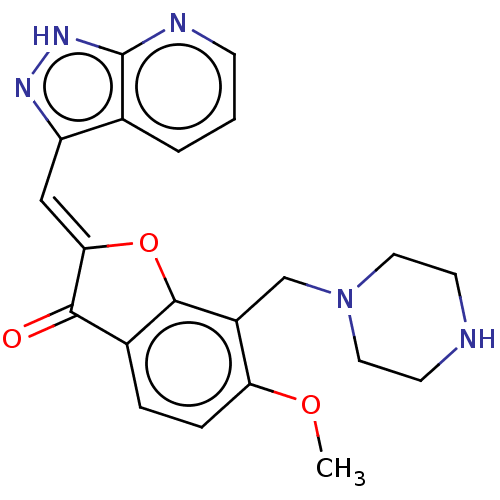
(CHEMBL3735816)Show SMILES COc1ccc2C(=O)\C(Oc2c1CN1CCNCC1)=C\c1n[nH]c2ncccc12 Show InChI InChI=1S/C21H21N5O3/c1-28-17-5-4-14-19(27)18(11-16-13-3-2-6-23-21(13)25-24-16)29-20(14)15(17)12-26-9-7-22-8-10-26/h2-6,11,22H,7-10,12H2,1H3,(H,23,24,25)/b18-11- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50499279

(CHEMBL3735890)Show SMILES COc1ccc2C(=O)\C(Oc2c1C(C)N1CCNCC1)=C\c1n[nH]c2ncccc12 Show InChI InChI=1S/C22H23N5O3/c1-13(27-10-8-23-9-11-27)19-17(29-2)6-5-15-20(28)18(30-21(15)19)12-16-14-4-3-7-24-22(14)26-25-16/h3-7,12-13,23H,8-11H2,1-2H3,(H,24,25,26)/b18-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50499279

(CHEMBL3735890)Show SMILES COc1ccc2C(=O)\C(Oc2c1C(C)N1CCNCC1)=C\c1n[nH]c2ncccc12 Show InChI InChI=1S/C22H23N5O3/c1-13(27-10-8-23-9-11-27)19-17(29-2)6-5-15-20(28)18(30-21(15)19)12-16-14-4-3-7-24-22(14)26-25-16/h3-7,12-13,23H,8-11H2,1-2H3,(H,24,25,26)/b18-12- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
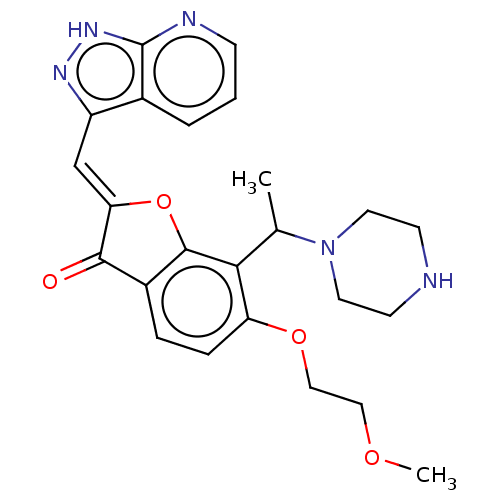
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50499282
(CHEMBL3735657)Show SMILES COCCOc1ccc2C(=O)\C(Oc2c1C(C)N1CCNCC1)=C\c1n[nH]c2ncccc12 Show InChI InChI=1S/C24H27N5O4/c1-15(29-10-8-25-9-11-29)21-19(32-13-12-31-2)6-5-17-22(30)20(33-23(17)21)14-18-16-4-3-7-26-24(16)28-27-18/h3-7,14-15,25H,8-13H2,1-2H3,(H,26,27,28)/b20-14- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
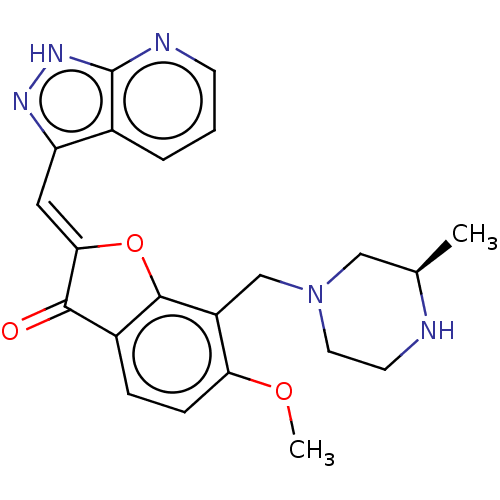
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50499275
(CHEMBL3736117)Show SMILES COc1ccc2C(=O)\C(Oc2c1CN1CCN[C@H](C)C1)=C\c1n[nH]c2ncccc12 |r| Show InChI InChI=1S/C22H23N5O3/c1-13-11-27(9-8-23-13)12-16-18(29-2)6-5-15-20(28)19(30-21(15)16)10-17-14-4-3-7-24-22(14)26-25-17/h3-7,10,13,23H,8-9,11-12H2,1-2H3,(H,24,25,26)/b19-10-/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50499279

(CHEMBL3735890)Show SMILES COc1ccc2C(=O)\C(Oc2c1C(C)N1CCNCC1)=C\c1n[nH]c2ncccc12 Show InChI InChI=1S/C22H23N5O3/c1-13(27-10-8-23-9-11-27)19-17(29-2)6-5-15-20(28)18(30-21(15)19)12-16-14-4-3-7-24-22(14)26-25-16/h3-7,12-13,23H,8-11H2,1-2H3,(H,24,25,26)/b18-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
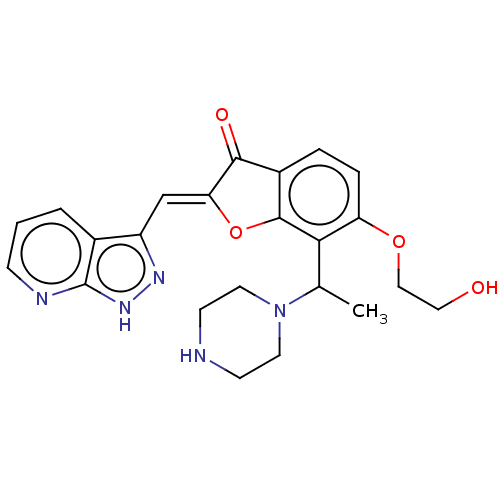
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50499280
(CHEMBL3735412)Show SMILES CC(N1CCNCC1)c1c2O\C(=C/c3n[nH]c4ncccc34)C(=O)c2ccc1OCCO Show InChI InChI=1S/C23H25N5O4/c1-14(28-9-7-24-8-10-28)20-18(31-12-11-29)5-4-16-21(30)19(32-22(16)20)13-17-15-3-2-6-25-23(15)27-26-17/h2-6,13-14,24,29H,7-12H2,1H3,(H,25,26,27)/b19-13- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method |
J Med Chem 61: 5047-5053 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00484
BindingDB Entry DOI: 10.7270/Q2NK3HN7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Death-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50499279

(CHEMBL3735890)Show SMILES COc1ccc2C(=O)\C(Oc2c1C(C)N1CCNCC1)=C\c1n[nH]c2ncccc12 Show InChI InChI=1S/C22H23N5O3/c1-13(27-10-8-23-9-11-27)19-17(29-2)6-5-15-20(28)18(30-21(15)19)12-16-14-4-3-7-24-22(14)26-25-16/h3-7,12-13,23H,8-11H2,1-2H3,(H,24,25,26)/b18-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of DAPK1 (unknown origin) by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
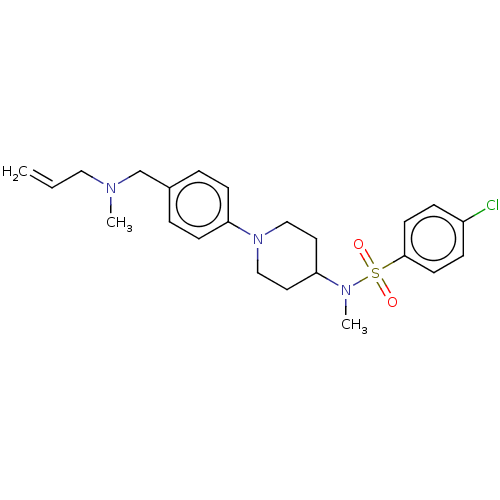
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50456340
(CHEMBL4207987)Show SMILES Cl.Cl.CN(CC=C)Cc1ccc(cc1)N1CCC(CC1)N(C)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H30ClN3O2S/c1-4-15-25(2)18-19-5-9-22(10-6-19)27-16-13-21(14-17-27)26(3)30(28,29)23-11-7-20(24)8-12-23/h4-12,21H,1,13-18H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method |
J Med Chem 61: 5047-5053 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00484
BindingDB Entry DOI: 10.7270/Q2NK3HN7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50499278
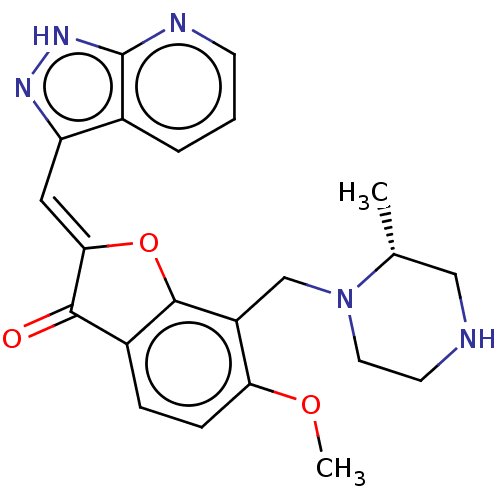
(CHEMBL3735628)Show SMILES COc1ccc2C(=O)\C(Oc2c1CN1CCNC[C@H]1C)=C\c1n[nH]c2ncccc12 |r| Show InChI InChI=1S/C22H23N5O3/c1-13-11-23-8-9-27(13)12-16-18(29-2)6-5-15-20(28)19(30-21(15)16)10-17-14-4-3-7-24-22(14)26-25-17/h3-7,10,13,23H,8-9,11-12H2,1-2H3,(H,24,25,26)/b19-10-/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50391938
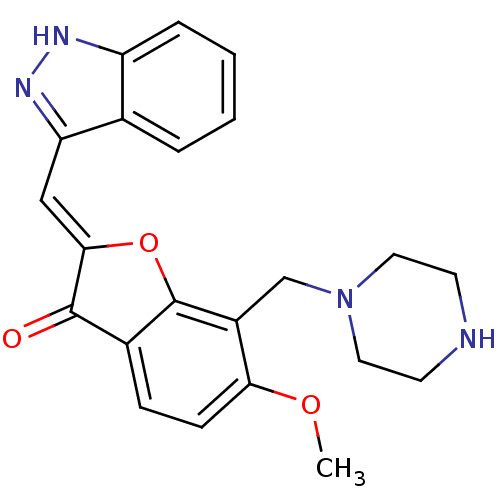
(CHEMBL2147845)Show SMILES COc1ccc2C(=O)\C(Oc2c1CN1CCNCC1)=C\c1n[nH]c2ccccc12 Show InChI InChI=1S/C22H22N4O3/c1-28-19-7-6-15-21(27)20(12-18-14-4-2-3-5-17(14)24-25-18)29-22(15)16(19)13-26-10-8-23-9-11-26/h2-7,12,23H,8-11,13H2,1H3,(H,24,25)/b20-12- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50391949
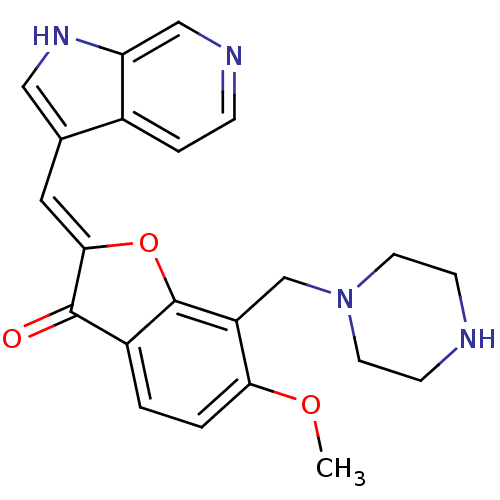
(CHEMBL2147768)Show SMILES COc1ccc2C(=O)\C(Oc2c1CN1CCNCC1)=C\c1c[nH]c2cnccc12 Show InChI InChI=1S/C22H22N4O3/c1-28-19-3-2-16-21(27)20(10-14-11-25-18-12-24-5-4-15(14)18)29-22(16)17(19)13-26-8-6-23-7-9-26/h2-5,10-12,23,25H,6-9,13H2,1H3/b20-10- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Trypanothione synthetase
(Trypanosoma cruzi) | BDBM50152094
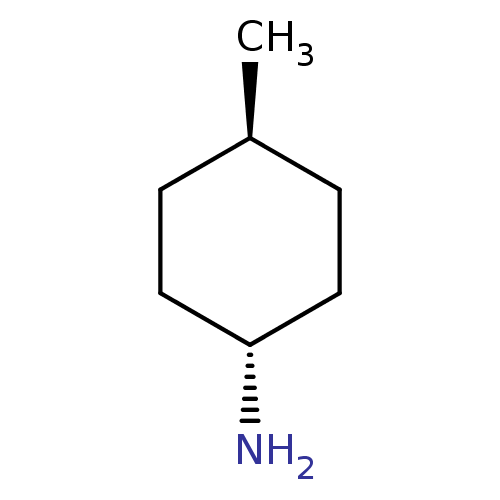
(CHEMBL3780789)Show SMILES C[C@H]1CC[C@H](N)CC1 |r,wU:1.0,wD:4.4,(-2.4,1.39,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;2.4,-1.39,;,-1.54,;-1.33,-.77,)| Show InChI InChI=1S/C7H15N/c1-6-2-4-7(8)5-3-6/h6-7H,2-5,8H2,1H3/t6-,7- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi strain CL Brener N-terminal poly-His tagged spermidine synthase expressed in Escherichia coli BL21(DE3) using 50 uM p... |
J Med Chem 59: 2261-6 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01769
BindingDB Entry DOI: 10.7270/Q2CV4KM8 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50456341
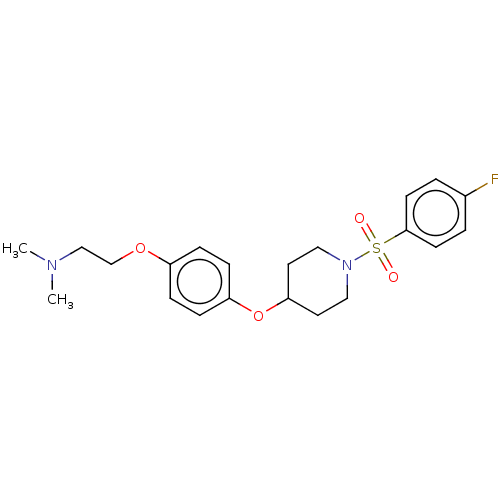
(CHEMBL4212932)Show SMILES Cl.CN(C)CCOc1ccc(OC2CCN(CC2)S(=O)(=O)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H27FN2O4S/c1-23(2)15-16-27-18-5-7-19(8-6-18)28-20-11-13-24(14-12-20)29(25,26)21-9-3-17(22)4-10-21/h3-10,20H,11-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method |
J Med Chem 61: 5047-5053 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00484
BindingDB Entry DOI: 10.7270/Q2NK3HN7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50391944
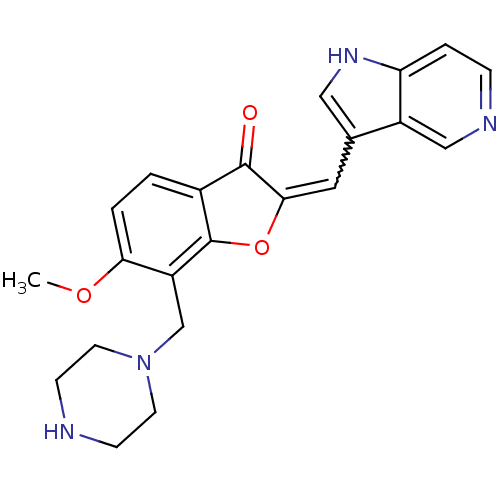
(CHEMBL2147769)Show SMILES COc1ccc2C(=O)C(Oc2c1CN1CCNCC1)=Cc1c[nH]c2ccncc12 |w:19.22| Show InChI InChI=1S/C22H22N4O3/c1-28-19-3-2-15-21(27)20(10-14-11-25-18-4-5-24-12-16(14)18)29-22(15)17(19)13-26-8-6-23-7-9-26/h2-5,10-12,23,25H,6-9,13H2,1H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50456342
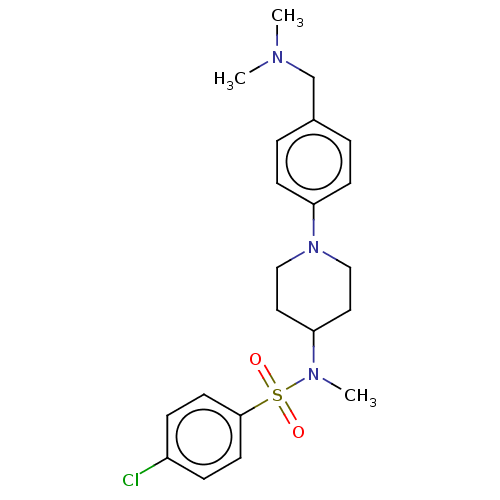
(CHEMBL4210513)Show SMILES Cl.Cl.CN(C)Cc1ccc(cc1)N1CCC(CC1)N(C)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H28ClN3O2S/c1-23(2)16-17-4-8-20(9-5-17)25-14-12-19(13-15-25)24(3)28(26,27)21-10-6-18(22)7-11-21/h4-11,19H,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method |
J Med Chem 61: 5047-5053 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00484
BindingDB Entry DOI: 10.7270/Q2NK3HN7 |
More data for this
Ligand-Target Pair | |
Trypanothione synthetase
(Trypanosoma cruzi) | BDBM50152094

(CHEMBL3780789)Show SMILES C[C@H]1CC[C@H](N)CC1 |r,wU:1.0,wD:4.4,(-2.4,1.39,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;2.4,-1.39,;,-1.54,;-1.33,-.77,)| Show InChI InChI=1S/C7H15N/c1-6-2-4-7(8)5-3-6/h6-7H,2-5,8H2,1H3/t6-,7- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi strain CL Brener N-terminal poly-His tagged spermidine synthase expressed in Escherichia coli BL21(DE3) using 0.5 mM ... |
J Med Chem 59: 2261-6 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01769
BindingDB Entry DOI: 10.7270/Q2CV4KM8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50391938

(CHEMBL2147845)Show SMILES COc1ccc2C(=O)\C(Oc2c1CN1CCNCC1)=C\c1n[nH]c2ccccc12 Show InChI InChI=1S/C22H22N4O3/c1-28-19-7-6-15-21(27)20(12-18-14-4-2-3-5-17(14)24-25-18)29-22(15)16(19)13-26-10-8-23-9-11-26/h2-7,12,23H,8-11,13H2,1H3,(H,24,25)/b20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM2 (unknown origin) by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Spermidine synthase
(Sus scrofa) | BDBM50152094

(CHEMBL3780789)Show SMILES C[C@H]1CC[C@H](N)CC1 |r,wU:1.0,wD:4.4,(-2.4,1.39,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;2.4,-1.39,;,-1.54,;-1.33,-.77,)| Show InChI InChI=1S/C7H15N/c1-6-2-4-7(8)5-3-6/h6-7H,2-5,8H2,1H3/t6-,7- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of porcine spermidine synthase assessed as production of labeled 5'-methylthioadenosine using putrescine as substrate by enzymatic assay i... |
J Med Chem 59: 2261-6 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01769
BindingDB Entry DOI: 10.7270/Q2CV4KM8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50391945
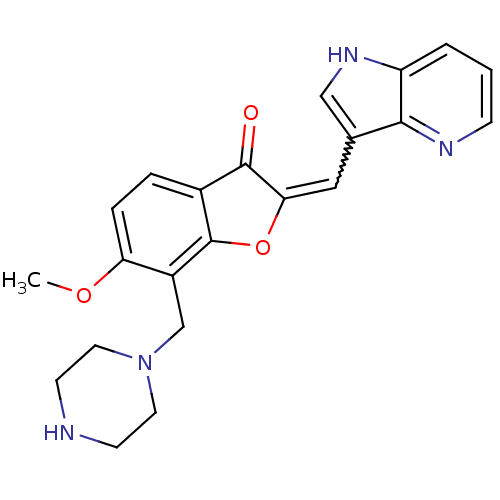
(CHEMBL2147770)Show SMILES COc1ccc2C(=O)C(Oc2c1CN1CCNCC1)=Cc1c[nH]c2cccnc12 |w:19.22| Show InChI InChI=1S/C22H22N4O3/c1-28-18-5-4-15-21(27)19(11-14-12-25-17-3-2-6-24-20(14)17)29-22(15)16(18)13-26-9-7-23-8-10-26/h2-6,11-12,23,25H,7-10,13H2,1H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using FL-Peptide 1 (5-FAM-AKRRRLSSLRA-COOH) substrate incubated for 2 hrs by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 25: 5687-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.098
BindingDB Entry DOI: 10.7270/Q2JD50SX |
More data for this
Ligand-Target Pair | |
Trypanothione synthetase
(Trypanosoma cruzi) | BDBM50152095
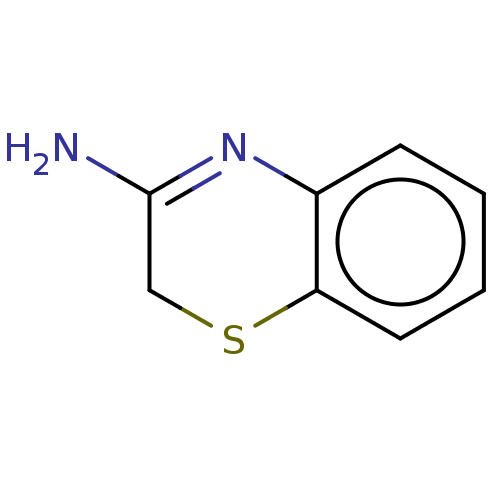
(CHEMBL3779908)Show InChI InChI=1S/C8H8N2S/c9-8-5-11-7-4-2-1-3-6(7)10-8/h1-4H,5H2,(H2,9,10) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi strain CL Brener N-terminal poly-His tagged spermidine synthase expressed in Escherichia coli BL21(DE3) using 50 uM p... |
J Med Chem 59: 2261-6 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01769
BindingDB Entry DOI: 10.7270/Q2CV4KM8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data