 Found 4286 hits with Last Name = 'gao' and Initial = 'l'
Found 4286 hits with Last Name = 'gao' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H](+)8-OH-DPAT from human 5HT1A receptor expressed in human HeLa cells measured after 60 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50312599
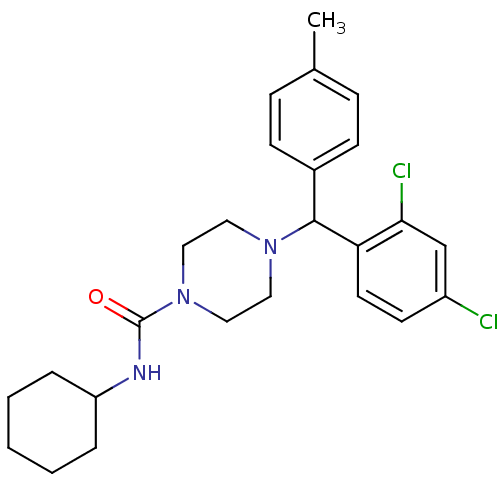
(CHEMBL1093735 | N-Cyclohexyl-4-[(2,4-dichloropheny...)Show SMILES Cc1ccc(cc1)C(N1CCN(CC1)C(=O)NC1CCCCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H31Cl2N3O/c1-18-7-9-19(10-8-18)24(22-12-11-20(26)17-23(22)27)29-13-15-30(16-14-29)25(31)28-21-5-3-2-4-6-21/h7-12,17,21,24H,2-6,13-16H2,1H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting |
Eur J Med Chem 46: 5310-6 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.030
BindingDB Entry DOI: 10.7270/Q22J6C85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5-HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127909
BindingDB Entry DOI: 10.7270/Q2R2154C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50355892
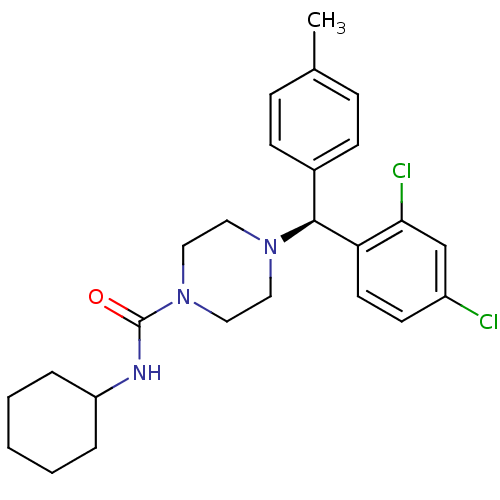
(CHEMBL1910035)Show SMILES Cc1ccc(cc1)[C@H](N1CCN(CC1)C(=O)NC1CCCCC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C25H31Cl2N3O/c1-18-7-9-19(10-8-18)24(22-12-11-20(26)17-23(22)27)29-13-15-30(16-14-29)25(31)28-21-5-3-2-4-6-21/h7-12,17,21,24H,2-6,13-16H2,1H3,(H,28,31)/t24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting |
Eur J Med Chem 46: 5310-6 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.030
BindingDB Entry DOI: 10.7270/Q22J6C85 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]p-Cl-DPDPE from rat delta opioid receptor expressed in rat C6 cells |
Bioorg Med Chem Lett 19: 4603-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.093
BindingDB Entry DOI: 10.7270/Q2WQ04RF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50001043
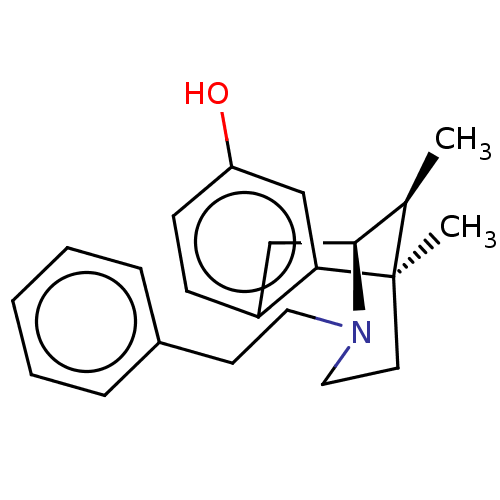
(6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CCc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H27NO/c1-16-21-14-18-8-9-19(24)15-20(18)22(16,2)11-13-23(21)12-10-17-6-4-3-5-7-17/h3-9,15-16,21,24H,10-14H2,1-2H3/t16-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in guinea pig brain membrane incubated for 2.5 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50434823
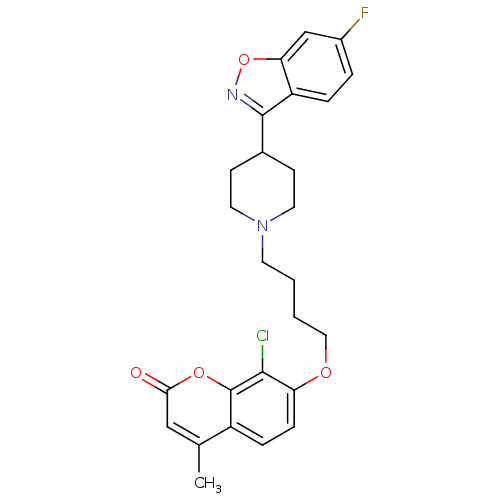
(CHEMBL2387229)Show SMILES Cc1cc(=O)oc2c(Cl)c(OCCCCN3CCC(CC3)c3noc4cc(F)ccc34)ccc12 Show InChI InChI=1S/C26H26ClFN2O4/c1-16-14-23(31)33-26-19(16)6-7-21(24(26)27)32-13-3-2-10-30-11-8-17(9-12-30)25-20-5-4-18(28)15-22(20)34-29-25/h4-7,14-15,17H,2-3,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5-HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127909
BindingDB Entry DOI: 10.7270/Q2R2154C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| DrugBank
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]raclopride from human D2 long receptor expressed in CHO cells measured after 60 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50208447
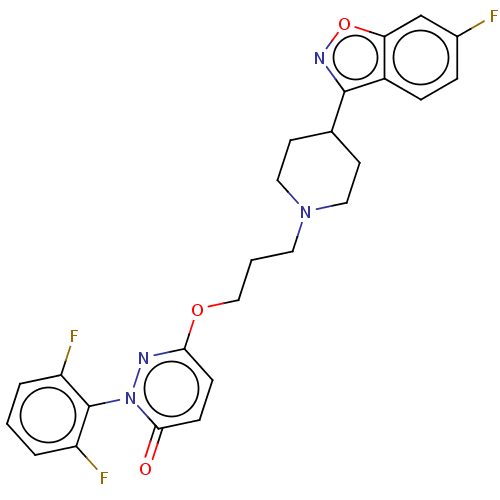
(CHEMBL3883955)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(=O)n(n2)-c2c(F)cccc2F)CC1 |(23.65,-6.56,;22.14,-6.23,;21.11,-7.37,;19.61,-7.04,;19.15,-5.58,;17.75,-4.96,;17.92,-3.43,;19.42,-3.11,;20.19,-4.45,;21.68,-4.77,;16.42,-5.72,;16.4,-7.26,;15.07,-8.02,;13.74,-7.24,;12.4,-8.01,;11.07,-7.23,;9.73,-8,;8.4,-7.22,;7.06,-7.99,;5.73,-7.21,;4.4,-7.99,;4.4,-9.53,;3.07,-10.3,;5.73,-10.29,;7.06,-9.53,;5.73,-11.83,;7.06,-12.6,;8.4,-11.82,;7.07,-14.14,;5.73,-14.91,;4.4,-14.14,;4.4,-12.6,;3.06,-11.83,;13.74,-5.71,;15.08,-4.94,)| Show InChI InChI=1S/C25H23F3N4O3/c26-17-5-6-18-21(15-17)35-30-24(18)16-9-12-31(13-10-16)11-2-14-34-22-7-8-23(33)32(29-22)25-19(27)3-1-4-20(25)28/h1,3-8,15-16H,2,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5-HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127909
BindingDB Entry DOI: 10.7270/Q2R2154C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50008984

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show InChI InChI=1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of sigma 1 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394084

(US9974785, Example 3)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C19H18ClF2N3O2S/c20-14-4-1-2-7-17(14)28(26,27)25-12-13(19(21)22)18-15(5-3-6-16(18)25)24-10-8-23-9-11-24/h1-7,12,19,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394083

(US9974785, Example 2)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H18F3N3O2S/c20-14-4-1-2-7-17(14)28(26,27)25-12-13(19(21)22)18-15(5-3-6-16(18)25)24-10-8-23-9-11-24/h1-7,12,19,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO-K1 cells measured after 20 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50208447

(CHEMBL3883955)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(=O)n(n2)-c2c(F)cccc2F)CC1 |(23.65,-6.56,;22.14,-6.23,;21.11,-7.37,;19.61,-7.04,;19.15,-5.58,;17.75,-4.96,;17.92,-3.43,;19.42,-3.11,;20.19,-4.45,;21.68,-4.77,;16.42,-5.72,;16.4,-7.26,;15.07,-8.02,;13.74,-7.24,;12.4,-8.01,;11.07,-7.23,;9.73,-8,;8.4,-7.22,;7.06,-7.99,;5.73,-7.21,;4.4,-7.99,;4.4,-9.53,;3.07,-10.3,;5.73,-10.29,;7.06,-9.53,;5.73,-11.83,;7.06,-12.6,;8.4,-11.82,;7.07,-14.14,;5.73,-14.91,;4.4,-14.14,;4.4,-12.6,;3.06,-11.83,;13.74,-5.71,;15.08,-4.94,)| Show InChI InChI=1S/C25H23F3N4O3/c26-17-5-6-18-21(15-17)35-30-24(18)16-9-12-31(13-10-16)11-2-14-34-22-7-8-23(33)32(29-22)25-19(27)3-1-4-20(25)28/h1,3-8,15-16H,2,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5-HT6 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127909
BindingDB Entry DOI: 10.7270/Q2R2154C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394090
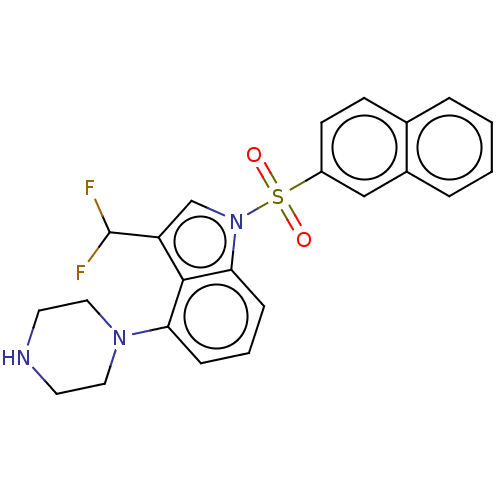
(US9974785, Example 10)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H21F2N3O2S/c24-23(25)19-15-28(21-7-3-6-20(22(19)21)27-12-10-26-11-13-27)31(29,30)18-9-8-16-4-1-2-5-17(16)14-18/h1-9,14-15,23,26H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394086

(US9974785, Example 5)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C19H18F3N3O2S/c20-13-3-1-4-14(11-13)28(26,27)25-12-15(19(21)22)18-16(5-2-6-17(18)25)24-9-7-23-8-10-24/h1-6,11-12,19,23H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM423299
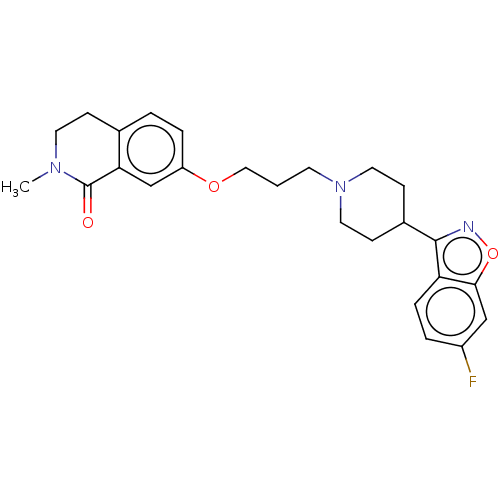
(US10501452, Compound 5)Show SMILES CN1CCc2ccc(OCCCN3CCC(CC3)c3noc4cc(F)ccc34)cc2C1=O Show InChI InChI=1S/C25H28FN3O3/c1-28-11-7-17-3-5-20(16-22(17)25(28)30)31-14-2-10-29-12-8-18(9-13-29)24-21-6-4-19(26)15-23(21)32-27-24/h3-6,15-16,18H,2,7-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50355742

(CHEMBL1911273)Show SMILES CCN(C)C(=O)c1ccc(cc1)-c1ccc2[C@@H](c3ccccc3Oc2n1)C(C)(C)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C28H27N5O3S/c1-5-33(4)25(34)18-12-10-17(11-13-18)21-15-14-20-23(19-8-6-7-9-22(19)36-24(20)30-21)28(2,3)26(35)31-27-32-29-16-37-27/h6-16,23H,5H2,1-4H3,(H,31,32,35)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay |
J Med Chem 54: 7318-33 (2011)
Article DOI: 10.1021/jm200879j
BindingDB Entry DOI: 10.7270/Q2W0969M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394104
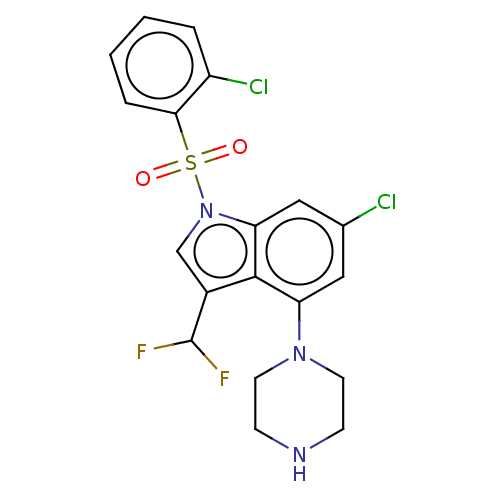
(US9974785, Example 25)Show SMILES FC(F)c1cn(c2cc(Cl)cc(N3CCNCC3)c12)S(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C19H17Cl2F2N3O2S/c20-12-9-15(25-7-5-24-6-8-25)18-13(19(22)23)11-26(16(18)10-12)29(27,28)17-4-2-1-3-14(17)21/h1-4,9-11,19,24H,5-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394087
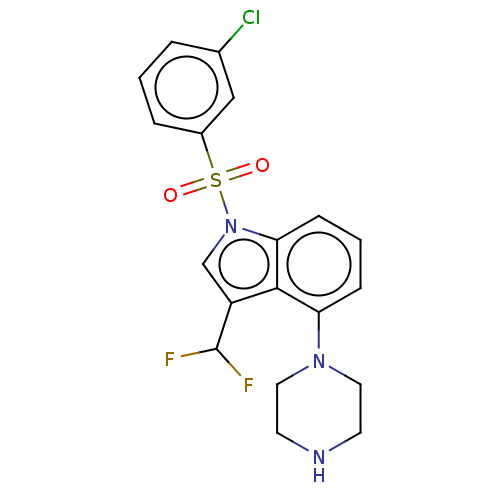
(US9974785, Example 6)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H18ClF2N3O2S/c20-13-3-1-4-14(11-13)28(26,27)25-12-15(19(21)22)18-16(5-2-6-17(18)25)24-9-7-23-8-10-24/h1-6,11-12,19,23H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394112
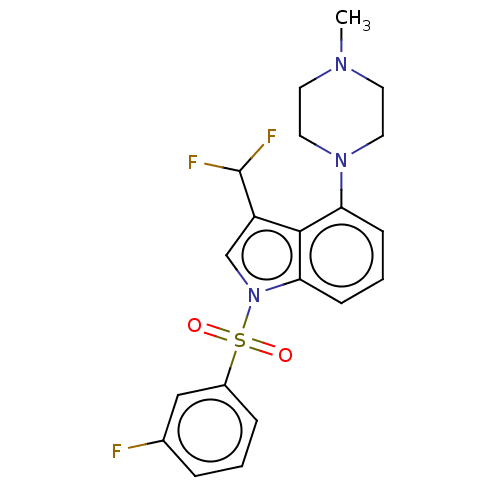
(US9974785, Example 42)Show SMILES CN1CCN(CC1)c1cccc2n(cc(C(F)F)c12)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C20H20F3N3O2S/c1-24-8-10-25(11-9-24)17-6-3-7-18-19(17)16(20(22)23)13-26(18)29(27,28)15-5-2-4-14(21)12-15/h2-7,12-13,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50048342
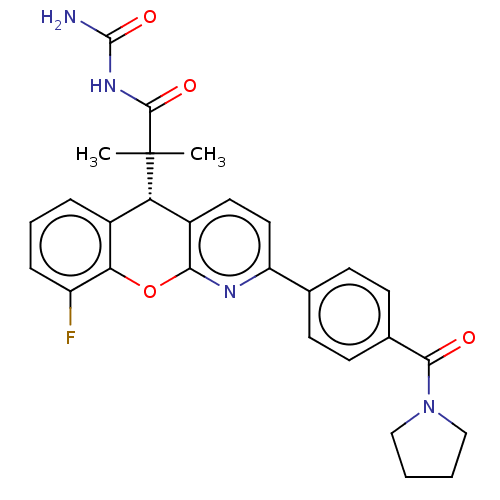
(CHEMBL3315064 | US9593113, Example 30)Show SMILES CC(C)([C@@H]1c2ccc(nc2Oc2c(F)cccc12)-c1ccc(cc1)C(=O)N1CCCC1)C(=O)NC(N)=O |r| Show InChI InChI=1S/C28H27FN4O4/c1-28(2,26(35)32-27(30)36)22-18-6-5-7-20(29)23(18)37-24-19(22)12-13-21(31-24)16-8-10-17(11-9-16)25(34)33-14-3-4-15-33/h5-13,22H,3-4,14-15H2,1-2H3,(H3,30,32,35,36)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 3268-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.010
BindingDB Entry DOI: 10.7270/Q21N82SN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50048348

(CHEMBL3315070)Show SMILES CC(C)Oc1ccc(cn1)-c1ccc2[C@H](c3cccc(F)c3Oc2n1)C(C)(C)C(=O)NC(N)=O |r| Show InChI InChI=1S/C25H25FN4O4/c1-13(2)33-19-11-8-14(12-28-19)18-10-9-16-20(25(3,4)23(31)30-24(27)32)15-6-5-7-17(26)21(15)34-22(16)29-18/h5-13,20H,1-4H3,(H3,27,30,31,32)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 3268-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.010
BindingDB Entry DOI: 10.7270/Q21N82SN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50469497
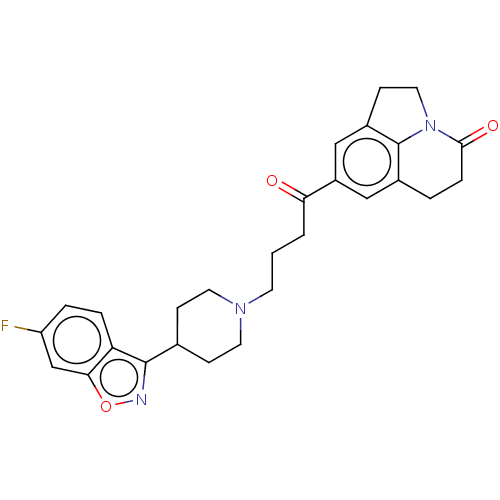
(CHEMBL4289498)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCC(=O)c2cc3CCN4c3c(CCC4=O)c2)CC1 Show InChI InChI=1S/C27H28FN3O3/c28-21-4-5-22-24(16-21)34-29-26(22)17-7-11-30(12-8-17)10-1-2-23(32)20-14-18-3-6-25(33)31-13-9-19(15-20)27(18)31/h4-5,14-17H,1-3,6-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5-HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127909
BindingDB Entry DOI: 10.7270/Q2R2154C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50469504

(CHEMBL4293999)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCCC(=O)c2cc3CCN4c3c(CCC4=O)c2)CC1 Show InChI InChI=1S/C28H30FN3O3/c29-22-5-6-23-25(17-22)35-30-27(23)18-8-12-31(13-9-18)11-2-1-3-24(33)21-15-19-4-7-26(34)32-14-10-20(16-21)28(19)32/h5-6,15-18H,1-4,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348415
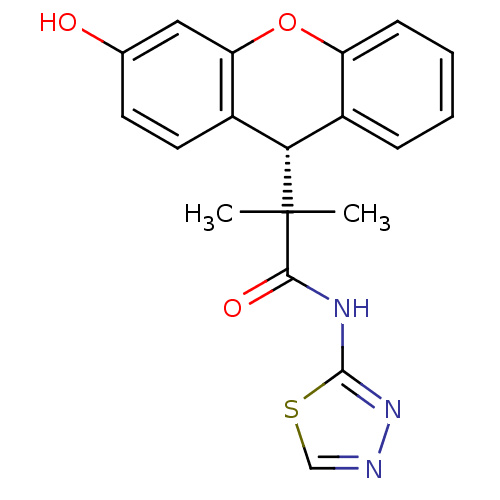
(CHEMBL1800985)Show SMILES CC(C)([C@H]1c2ccccc2Oc2cc(O)ccc12)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C19H17N3O3S/c1-19(2,17(24)21-18-22-20-10-26-18)16-12-5-3-4-6-14(12)25-15-9-11(23)7-8-13(15)16/h3-10,16,23H,1-2H3,(H,21,22,24)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay |
J Med Chem 54: 7318-33 (2011)
Article DOI: 10.1021/jm200879j
BindingDB Entry DOI: 10.7270/Q2W0969M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327904

(2-amino-4-methyl-6-(1H-pyrazol-3-yl)-8-(tetrahydro...)Show SMILES Cc1nc(N)nc2n(C3CCOCC3)c(=O)c(nc12)-c1cc[nH]n1 Show InChI InChI=1S/C15H17N7O2/c1-8-11-13(20-15(16)18-8)22(9-3-6-24-7-4-9)14(23)12(19-11)10-2-5-17-21-10/h2,5,9H,3-4,6-7H2,1H3,(H,17,21)(H2,16,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327907
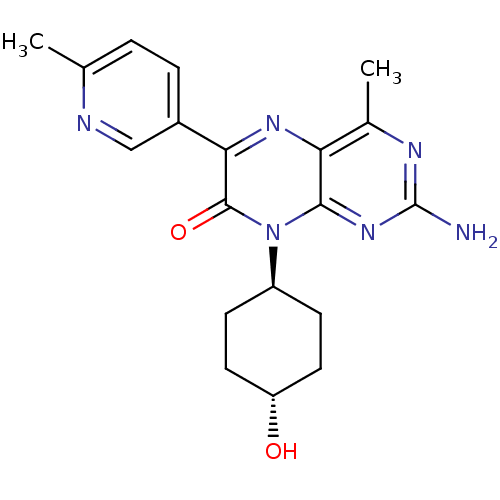
(CHEMBL1257295 | trans-2-amino-8-(4-hydroxycyclohex...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:21.23,wD:18.19,(6.09,-29.48,;4.76,-30.26,;4.77,-31.8,;3.44,-32.58,;2.1,-31.81,;2.09,-30.28,;3.42,-29.5,;.78,-32.59,;-.55,-31.83,;-1.88,-32.6,;-3.22,-31.84,;-3.22,-30.3,;-4.54,-32.61,;-4.55,-34.15,;-5.88,-34.92,;-3.21,-34.92,;-1.88,-34.14,;-.54,-34.91,;-.54,-36.45,;-1.87,-37.22,;-1.87,-38.76,;-.54,-39.53,;-.54,-41.07,;.79,-38.76,;.8,-37.22,;.79,-34.14,;2.13,-34.9,)| Show InChI InChI=1S/C19H22N6O2/c1-10-3-4-12(9-21-10)16-18(27)25(13-5-7-14(26)8-6-13)17-15(23-16)11(2)22-19(20)24-17/h3-4,9,13-14,26H,5-8H2,1-2H3,(H2,20,22,24)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50249150
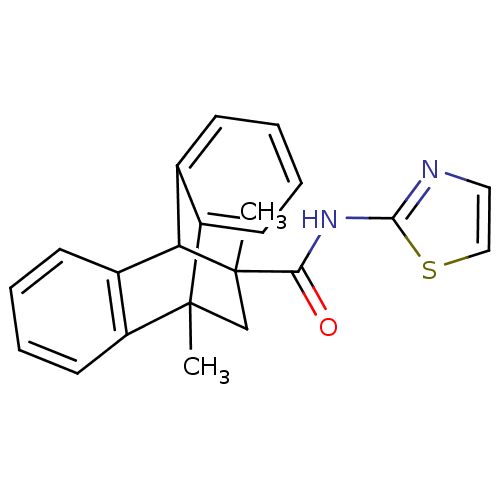
(8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...)Show SMILES CC1(CC2(C)c3ccccc3C1c1ccccc21)C(=O)Nc1nccs1 |TLB:0:1:5.10:12.17,9:10:2.1:12.17,13:12:5.10:2.1,THB:18:1:5.10:12.17,(17.67,-16.57,;19.01,-17.35,;19.52,-18.11,;19.53,-20.31,;19.51,-21.84,;17.66,-20.83,;16.31,-21.6,;14.98,-20.83,;14.98,-19.28,;16.31,-18.51,;17.62,-19.43,;19.01,-18.76,;20.55,-19.66,;21.82,-18.92,;23.1,-19.65,;23.11,-21.14,;21.82,-21.88,;20.54,-21.14,;19.77,-16,;18.98,-14.68,;21.31,-15.99,;22.07,-14.64,;23.61,-14.46,;23.91,-12.95,;22.57,-12.19,;21.43,-13.24,)| Show InChI InChI=1S/C22H20N2OS/c1-21-13-22(2,19(25)24-20-23-11-12-26-20)18(14-7-3-5-9-16(14)21)15-8-4-6-10-17(15)21/h3-12,18H,13H2,1-2H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay |
J Med Chem 54: 7318-33 (2011)
Article DOI: 10.1021/jm200879j
BindingDB Entry DOI: 10.7270/Q2W0969M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50355749

(CHEMBL1911150)Show SMILES CCc1ccc(cc1)-c1ccc2[C@@H](c3ccccc3Oc2n1)C(C)(C)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C26H24N4O2S/c1-4-16-9-11-17(12-10-16)20-14-13-19-22(18-7-5-6-8-21(18)32-23(19)28-20)26(2,3)24(31)29-25-30-27-15-33-25/h5-15,22H,4H2,1-3H3,(H,29,30,31)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay |
J Med Chem 54: 7318-33 (2011)
Article DOI: 10.1021/jm200879j
BindingDB Entry DOI: 10.7270/Q2W0969M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50355891
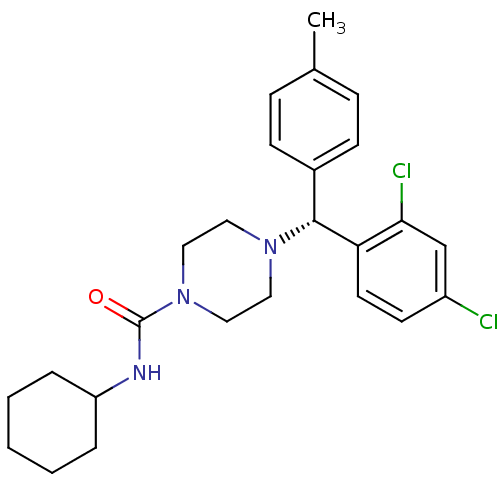
(CHEMBL1910034)Show SMILES Cc1ccc(cc1)[C@@H](N1CCN(CC1)C(=O)NC1CCCCC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C25H31Cl2N3O/c1-18-7-9-19(10-8-18)24(22-12-11-20(26)17-23(22)27)29-13-15-30(16-14-29)25(31)28-21-5-3-2-4-6-21/h7-12,17,21,24H,2-6,13-16H2,1H3,(H,28,31)/t24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting |
Eur J Med Chem 46: 5310-6 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.030
BindingDB Entry DOI: 10.7270/Q22J6C85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394105
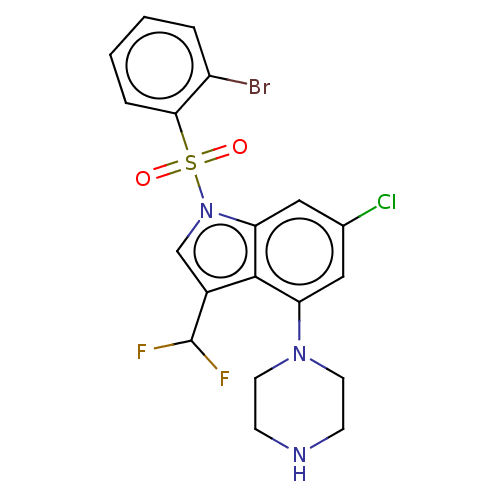
(US9974785, Example 26)Show SMILES FC(F)c1cn(c2cc(Cl)cc(N3CCNCC3)c12)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C19H17BrClF2N3O2S/c20-14-3-1-2-4-17(14)29(27,28)26-11-13(19(22)23)18-15(9-12(21)10-16(18)26)25-7-5-24-6-8-25/h1-4,9-11,19,24H,5-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394085

(US9974785, Example 4)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C19H18BrF2N3O2S/c20-14-4-1-2-7-17(14)28(26,27)25-12-13(19(21)22)18-15(5-3-6-16(18)25)24-10-8-23-9-11-24/h1-7,12,19,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348401
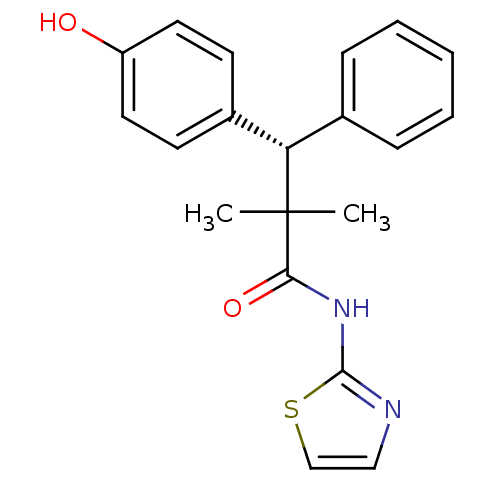
(CHEMBL1801012)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)cc1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C20H20N2O2S/c1-20(2,18(24)22-19-21-12-13-25-19)17(14-6-4-3-5-7-14)15-8-10-16(23)11-9-15/h3-13,17,23H,1-2H3,(H,21,22,24)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay |
J Med Chem 54: 7318-33 (2011)
Article DOI: 10.1021/jm200879j
BindingDB Entry DOI: 10.7270/Q2W0969M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50355746
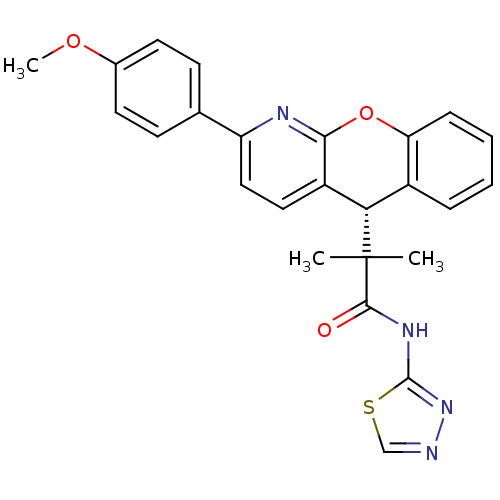
(CHEMBL1911149)Show SMILES COc1ccc(cc1)-c1ccc2[C@H](c3ccccc3Oc2n1)C(C)(C)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C25H22N4O3S/c1-25(2,23(30)28-24-29-26-14-33-24)21-17-6-4-5-7-20(17)32-22-18(21)12-13-19(27-22)15-8-10-16(31-3)11-9-15/h4-14,21H,1-3H3,(H,28,29,30)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay |
J Med Chem 54: 7318-33 (2011)
Article DOI: 10.1021/jm200879j
BindingDB Entry DOI: 10.7270/Q2W0969M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 3268-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.010
BindingDB Entry DOI: 10.7270/Q21N82SN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50048344
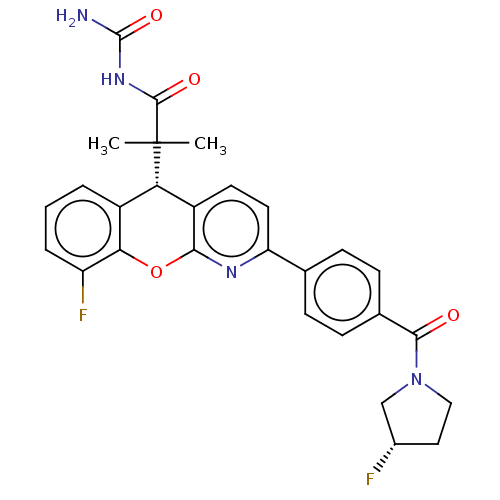
(CHEMBL3315066)Show SMILES CC(C)([C@@H]1c2ccc(nc2Oc2c(F)cccc12)-c1ccc(cc1)C(=O)N1CC[C@H](F)C1)C(=O)NC(N)=O |r| Show InChI InChI=1S/C28H26F2N4O4/c1-28(2,26(36)33-27(31)37)22-18-4-3-5-20(30)23(18)38-24-19(22)10-11-21(32-24)15-6-8-16(9-7-15)25(35)34-13-12-17(29)14-34/h3-11,17,22H,12-14H2,1-2H3,(H3,31,33,36,37)/t17-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 3268-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.010
BindingDB Entry DOI: 10.7270/Q21N82SN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394108

(US9974785, Example 29)Show SMILES FC(F)c1cn(c2cc(Cl)cc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Br)c1 Show InChI InChI=1S/C19H17BrClF2N3O2S/c20-12-2-1-3-14(8-12)29(27,28)26-11-15(19(22)23)18-16(9-13(21)10-17(18)26)25-6-4-24-5-7-25/h1-3,8-11,19,24H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564197

(CHEMBL4776178)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccc(F)cc2)CC1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50355716
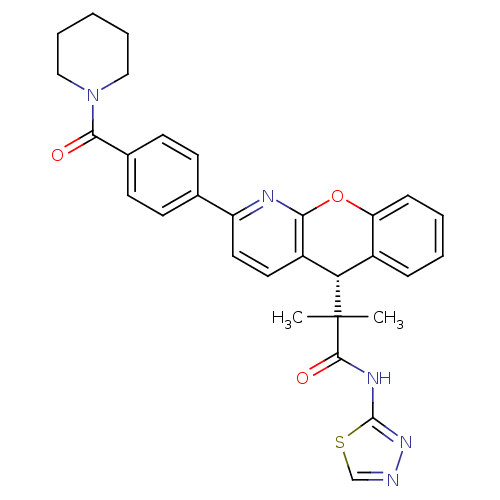
(CHEMBL1911276)Show SMILES CC(C)([C@H]1c2ccccc2Oc2nc(ccc12)-c1ccc(cc1)C(=O)N1CCCCC1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C30H29N5O3S/c1-30(2,28(37)33-29-34-31-18-39-29)25-21-8-4-5-9-24(21)38-26-22(25)14-15-23(32-26)19-10-12-20(13-11-19)27(36)35-16-6-3-7-17-35/h4-5,8-15,18,25H,3,6-7,16-17H2,1-2H3,(H,33,34,37)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay |
J Med Chem 54: 7318-33 (2011)
Article DOI: 10.1021/jm200879j
BindingDB Entry DOI: 10.7270/Q2W0969M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394107
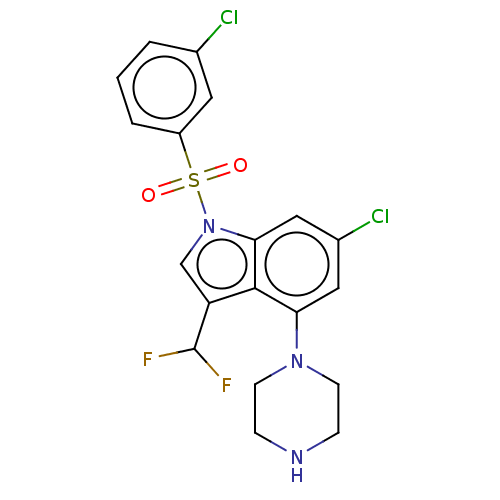
(US9974785, Example 28)Show SMILES FC(F)c1cn(c2cc(Cl)cc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H17Cl2F2N3O2S/c20-12-2-1-3-14(8-12)29(27,28)26-11-15(19(22)23)18-16(9-13(21)10-17(18)26)25-6-4-24-5-7-25/h1-3,8-11,19,24H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50048341
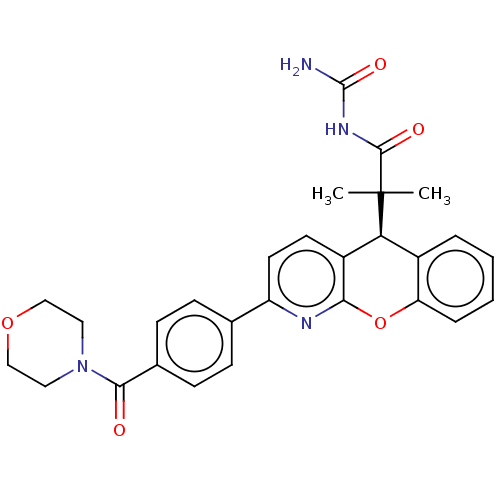
(CHEMBL3315063 | US9593113, Example 28)Show SMILES CC(C)([C@H]1c2ccccc2Oc2nc(ccc12)-c1ccc(cc1)C(=O)N1CCOCC1)C(=O)NC(N)=O |r| Show InChI InChI=1S/C28H28N4O5/c1-28(2,26(34)31-27(29)35)23-19-5-3-4-6-22(19)37-24-20(23)11-12-21(30-24)17-7-9-18(10-8-17)25(33)32-13-15-36-16-14-32/h3-12,23H,13-16H2,1-2H3,(H3,29,31,34,35)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 3268-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.010
BindingDB Entry DOI: 10.7270/Q21N82SN |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564176
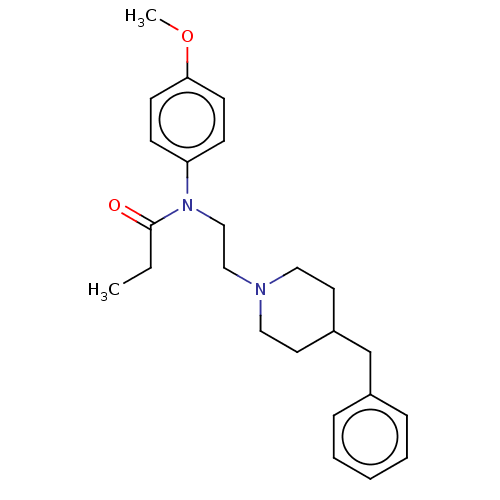
(CHEMBL4797982)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccccc2)CC1)c1ccc(OC)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50570443
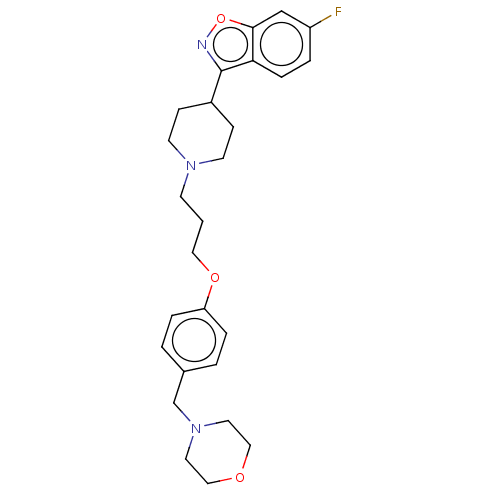
(CHEMBL4870716)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(CN3CCOCC3)cc2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to dopamine D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127909
BindingDB Entry DOI: 10.7270/Q2R2154C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19190
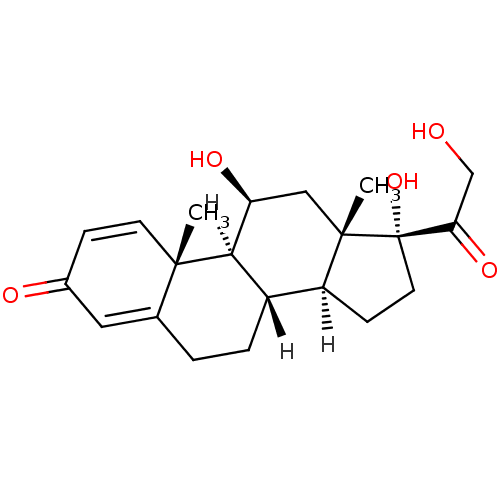
((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...)Show SMILES [H][C@@]12CC[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |r,c:27,t:23| Show InChI InChI=1S/C21H28O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-16,18,22,24,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,16-,18+,19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay |
J Med Chem 54: 7318-33 (2011)
Article DOI: 10.1021/jm200879j
BindingDB Entry DOI: 10.7270/Q2W0969M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394111
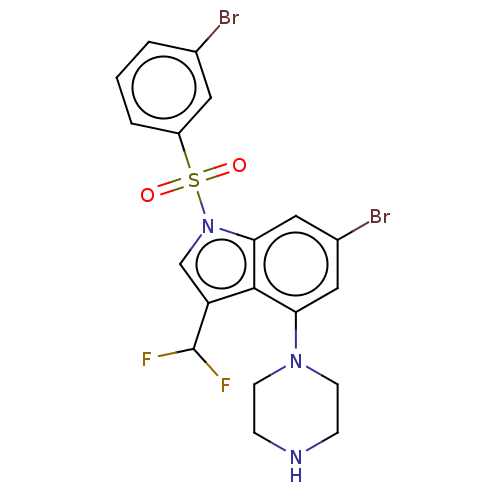
(US9974785, Example 37)Show SMILES FC(F)c1cn(c2cc(Br)cc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Br)c1 Show InChI InChI=1S/C19H17Br2F2N3O2S/c20-12-2-1-3-14(8-12)29(27,28)26-11-15(19(22)23)18-16(9-13(21)10-17(18)26)25-6-4-24-5-7-25/h1-3,8-11,19,24H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc.
| Assay Description
32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... |
J Med Chem 52: 3047-62 (2009)
BindingDB Entry DOI: 10.7270/Q27W6FH4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50048340
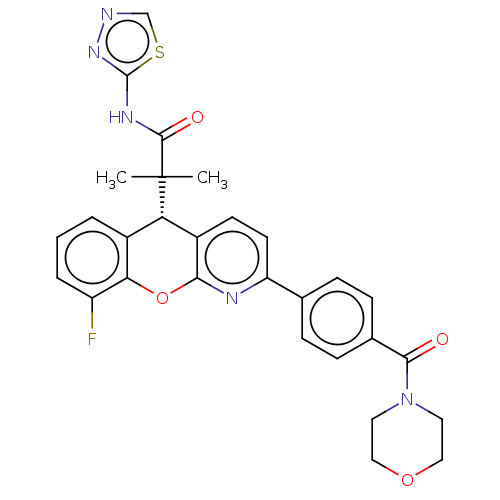
(CHEMBL3315062)Show SMILES CC(C)([C@@H]1c2ccc(nc2Oc2c(F)cccc12)-c1ccc(cc1)C(=O)N1CCOCC1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C29H26FN5O4S/c1-29(2,27(37)33-28-34-31-16-40-28)23-19-4-3-5-21(30)24(19)39-25-20(23)10-11-22(32-25)17-6-8-18(9-7-17)26(36)35-12-14-38-15-13-35/h3-11,16,23H,12-15H2,1-2H3,(H,33,34,37)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 3268-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.010
BindingDB Entry DOI: 10.7270/Q21N82SN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50048327
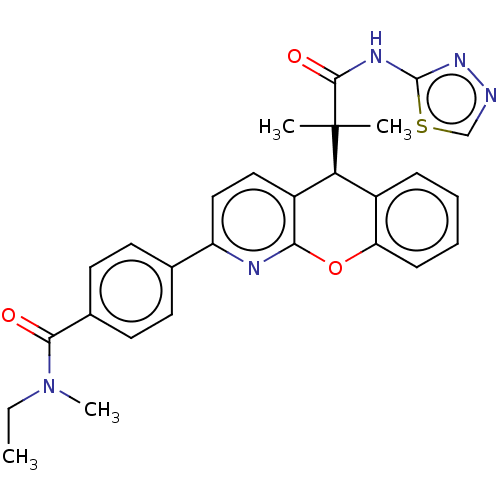
(CHEMBL3315049)Show SMILES CCN(C)C(=O)c1ccc(cc1)-c1ccc2[C@H](c3ccccc3Oc2n1)C(C)(C)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C28H27N5O3S/c1-5-33(4)25(34)18-12-10-17(11-13-18)21-15-14-20-23(19-8-6-7-9-22(19)36-24(20)30-21)28(2,3)26(35)31-27-32-29-16-37-27/h6-16,23H,5H2,1-4H3,(H,31,32,35)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 3268-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.010
BindingDB Entry DOI: 10.7270/Q21N82SN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data