 Found 488 hits with Last Name = 'garbaccio' and Initial = 'rm'
Found 488 hits with Last Name = 'garbaccio' and Initial = 'rm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50169690
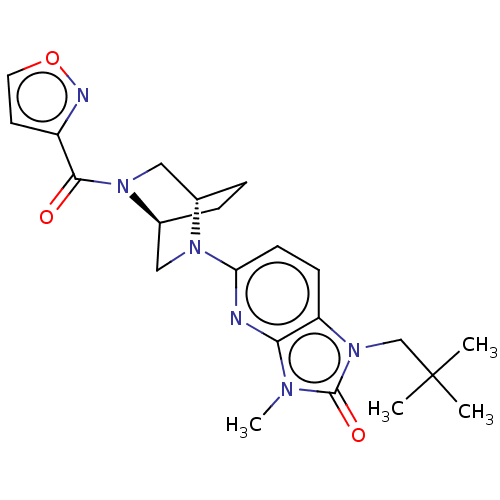
(CHEMBL3806137)Show SMILES [H][C@@]12CC[C@@]([H])(CN1C(=O)c1ccon1)N(C2)c1ccc2n(CC(C)(C)C)c(=O)n(C)c2n1 |r| Show InChI InChI=1S/C22H28N6O3/c1-22(2,3)13-28-17-7-8-18(23-19(17)25(4)21(28)30)26-11-15-6-5-14(26)12-27(15)20(29)16-9-10-31-24-16/h7-10,14-15H,5-6,11-13H2,1-4H3/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
ACS Med Chem Lett 7: 312-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00459
BindingDB Entry DOI: 10.7270/Q2W95C3K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50145198
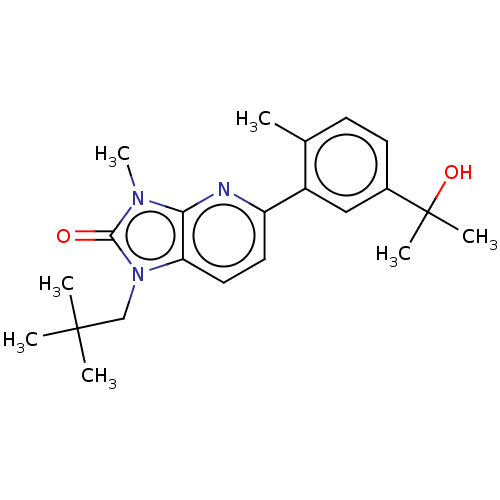
(CHEMBL3765778)Show SMILES Cc1ccc(cc1-c1ccc2n(CC(C)(C)C)c(=O)n(C)c2n1)C(C)(C)O Show InChI InChI=1S/C22H29N3O2/c1-14-8-9-15(22(5,6)27)12-16(14)17-10-11-18-19(23-17)24(7)20(26)25(18)13-21(2,3)4/h8-12,27H,13H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of 5-HT2B receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1260-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.021
BindingDB Entry DOI: 10.7270/Q2NV9M4S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50145198

(CHEMBL3765778)Show SMILES Cc1ccc(cc1-c1ccc2n(CC(C)(C)C)c(=O)n(C)c2n1)C(C)(C)O Show InChI InChI=1S/C22H29N3O2/c1-14-8-9-15(22(5,6)27)12-16(14)17-10-11-18-19(23-17)24(7)20(26)25(18)13-21(2,3)4/h8-12,27H,13H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of platelet-activating factor receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1260-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.021
BindingDB Entry DOI: 10.7270/Q2NV9M4S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM476583
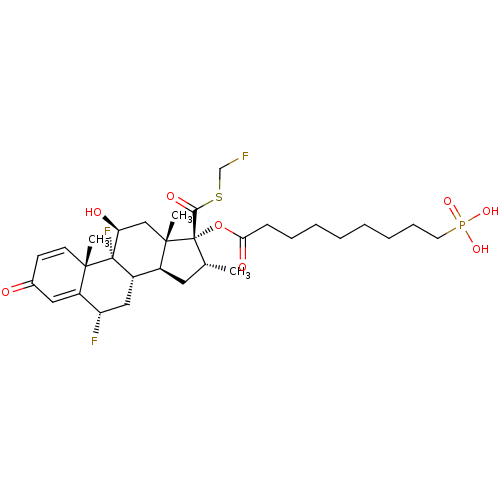
(US10869929, Compound 18 | US11554172, Compound 18)Show SMILES C[C@@H]1C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)CCCCCCCCP(O)(O)=O)C(=O)SCF |r,c:12,t:8| Show InChI InChI=1S/C31H44F3O8PS/c1-19-14-21-22-16-24(33)23-15-20(35)11-12-28(23,2)30(22,34)25(36)17-29(21,3)31(19,27(38)44-18-32)42-26(37)10-8-6-4-5-7-9-13-43(39,40)41/h11-12,15,19,21-22,24-25,36H,4-10,13-14,16-18H2,1-3H3,(H2,39,40,41)/t19-,21+,22+,24+,25+,28+,29+,30+,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
TSC22 domain family protein 3
(Human) | BDBM476583

(US10869929, Compound 18 | US11554172, Compound 18)Show SMILES C[C@@H]1C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)CCCCCCCCP(O)(O)=O)C(=O)SCF |r,c:12,t:8| Show InChI InChI=1S/C31H44F3O8PS/c1-19-14-21-22-16-24(33)23-15-20(35)11-12-28(23,2)30(22,34)25(36)17-29(21,3)31(19,27(38)44-18-32)42-26(37)10-8-6-4-5-7-9-13-43(39,40)41/h11-12,15,19,21-22,24-25,36H,4-10,13-14,16-18H2,1-3H3,(H2,39,40,41)/t19-,21+,22+,24+,25+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM589245

(US11554172, Compound 19)Show SMILES C[C@@H]1C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)CCCCCP(O)(O)=O)C(=O)SCF |r,c:12,t:8| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
TSC22 domain family protein 3
(Human) | BDBM476584

(US10869929, Compound 19)Show SMILES C[C@@H]1CC2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)CCCCCP(O)(O)=O)C(=O)SCF |r,c:12,t:8| Show InChI InChI=1S/C28H38F3O8PS/c1-16-11-18-19-13-21(30)20-12-17(32)8-9-25(20,2)27(19,31)22(33)14-26(18,3)28(16,24(35)41-15-29)39-23(34)7-5-4-6-10-40(36,37)38/h8-9,12,16,18-19,21-22,33H,4-7,10-11,13-15H2,1-3H3,(H2,36,37,38)/t16-,18?,19+,21+,22+,25+,26+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM589230

(US11554172, Compound Fluticasone-Propionate)Show SMILES C[C@@H]1C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)SCF |c:12,t:8| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
TSC22 domain family protein 3
(Human) | BDBM50354849
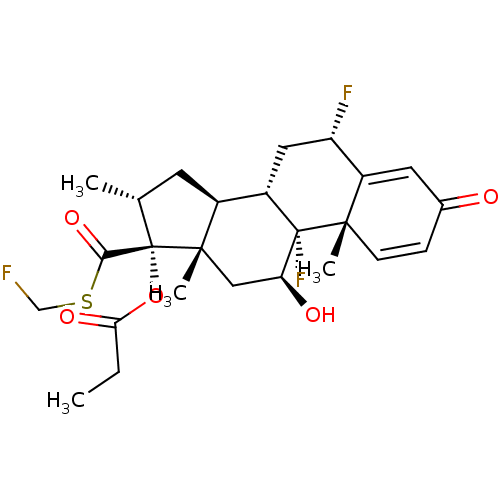
(CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCF |r,c:18,t:14| Show InChI InChI=1S/C25H31F3O5S/c1-5-20(31)33-25(21(32)34-12-26)13(2)8-15-16-10-18(27)17-9-14(29)6-7-22(17,3)24(16,28)19(30)11-23(15,25)4/h6-7,9,13,15-16,18-19,30H,5,8,10-12H2,1-4H3/t13-,15+,16+,18+,19+,22+,23+,24+,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM476575

(US10869929, Compound 8 | US11554172, Compound 8)Show SMILES C[C@@H]1C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)SCCCCCCCCCCP(O)(O)=O |r,c:12,t:8| Show InChI InChI=1S/C31H47F2O7PS/c1-20-16-22-23-18-25(32)24-17-21(34)12-13-28(24,2)30(23,33)26(35)19-29(22,3)31(20,37)27(36)42-15-11-9-7-5-4-6-8-10-14-41(38,39)40/h12-13,17,20,22-23,25-26,35,37H,4-11,14-16,18-19H2,1-3H3,(H2,38,39,40)/t20-,22+,23+,25+,26+,28+,29+,30+,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
TSC22 domain family protein 3
(Human) | BDBM476575

(US10869929, Compound 8 | US11554172, Compound 8)Show SMILES C[C@@H]1C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)SCCCCCCCCCCP(O)(O)=O |r,c:12,t:8| Show InChI InChI=1S/C31H47F2O7PS/c1-20-16-22-23-18-25(32)24-17-21(34)12-13-28(24,2)30(23,33)26(35)19-29(22,3)31(20,37)27(36)42-15-11-9-7-5-4-6-8-10-14-41(38,39)40/h12-13,17,20,22-23,25-26,35,37H,4-11,14-16,18-19H2,1-3H3,(H2,38,39,40)/t20-,22+,23+,25+,26+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223460
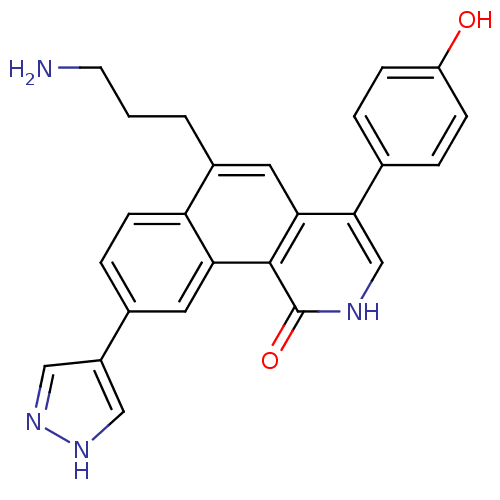
(6-(3-aminopropyl)-4-(4-hydroxyphenyl)-9-(1H-pyrazo...)Show SMILES NCCCc1cc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)-c1ccc(O)cc1 Show InChI InChI=1S/C25H22N4O2/c26-9-1-2-17-11-22-23(15-3-6-19(30)7-4-15)14-27-25(31)24(22)21-10-16(5-8-20(17)21)18-12-28-29-13-18/h3-8,10-14,30H,1-2,9,26H2,(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
TSC22 domain family protein 3
(Human) | BDBM476572
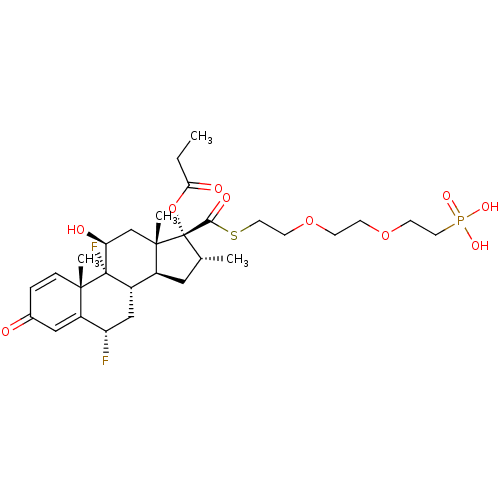
(US10869929, Compound 6 | US11554172, Compound 6)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCCOCCOCCP(O)(O)=O |r,c:18,t:14| Show InChI InChI=1S/C30H43F2O10PS/c1-5-25(35)42-30(26(36)44-13-11-41-9-8-40-10-12-43(37,38)39)18(2)14-20-21-16-23(31)22-15-19(33)6-7-27(22,3)29(21,32)24(34)17-28(20,30)4/h6-7,15,18,20-21,23-24,34H,5,8-14,16-17H2,1-4H3,(H2,37,38,39)/t18-,20+,21+,23+,24+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM476572

(US10869929, Compound 6 | US11554172, Compound 6)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCCOCCOCCP(O)(O)=O |r,c:18,t:14| Show InChI InChI=1S/C30H43F2O10PS/c1-5-25(35)42-30(26(36)44-13-11-41-9-8-40-10-12-43(37,38)39)18(2)14-20-21-16-23(31)22-15-19(33)6-7-27(22,3)29(21,32)24(34)17-28(20,30)4/h6-7,15,18,20-21,23-24,34H,5,8-14,16-17H2,1-4H3,(H2,37,38,39)/t18-,20+,21+,23+,24+,27+,28+,29+,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195211
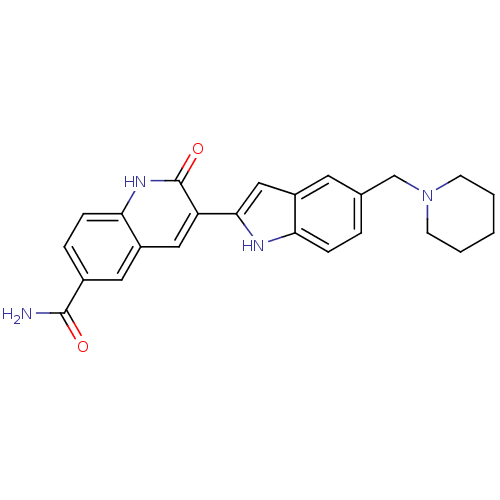
(2-oxo-3-(5-(piperidin-1-ylmethyl)-1H-indol-2-yl)-1...)Show SMILES NC(=O)c1ccc2[nH]c(=O)c(cc2c1)-c1cc2cc(CN3CCCCC3)ccc2[nH]1 Show InChI InChI=1S/C24H24N4O2/c25-23(29)16-5-7-21-18(11-16)12-19(24(30)27-21)22-13-17-10-15(4-6-20(17)26-22)14-28-8-2-1-3-9-28/h4-7,10-13,26H,1-3,8-9,14H2,(H2,25,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220966

(1-[(3R,3aR)-8-fluoro-3-(3-morpholin-4-yl-propyl)-3...)Show SMILES CC(=O)C1=NN2[C@@H](COc3ccc(F)cc23)[C@@]1(CCCN1CCOCC1)c1ccccc1 |t:3| Show InChI InChI=1S/C25H28FN3O3/c1-18(30)24-25(19-6-3-2-4-7-19,10-5-11-28-12-14-31-15-13-28)23-17-32-22-9-8-20(26)16-21(22)29(23)27-24/h2-4,6-9,16,23H,5,10-15,17H2,1H3/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of kinesin spindle protein |
Bioorg Med Chem Lett 17: 5671-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.067
BindingDB Entry DOI: 10.7270/Q261101N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
TSC22 domain family protein 3
(Human) | BDBM476567
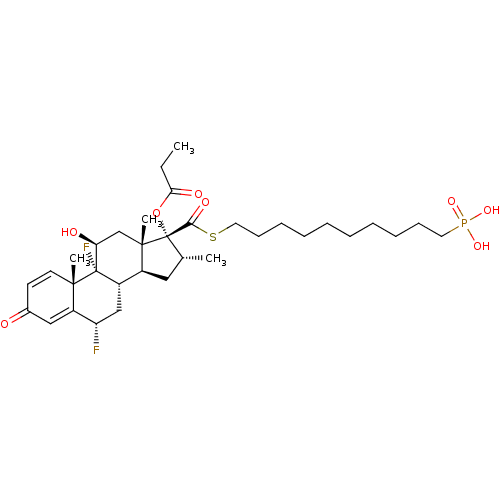
(US10869929, Compound 3 | US11554172, Compound 3)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCCCCCCCCCCP(O)(O)=O |r,c:18,t:14| Show InChI InChI=1S/C34H51F2O8PS/c1-5-29(39)44-34(30(40)46-17-13-11-9-7-6-8-10-12-16-45(41,42)43)22(2)18-24-25-20-27(35)26-19-23(37)14-15-31(26,3)33(25,36)28(38)21-32(24,34)4/h14-15,19,22,24-25,27-28,38H,5-13,16-18,20-21H2,1-4H3,(H2,41,42,43)/t22-,24+,25+,27+,28+,31+,32+,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM476567

(US10869929, Compound 3 | US11554172, Compound 3)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCCCCCCCCCCP(O)(O)=O |r,c:18,t:14| Show InChI InChI=1S/C34H51F2O8PS/c1-5-29(39)44-34(30(40)46-17-13-11-9-7-6-8-10-12-16-45(41,42)43)22(2)18-24-25-20-27(35)26-19-23(37)14-15-31(26,3)33(25,36)28(38)21-32(24,34)4/h14-15,19,22,24-25,27-28,38H,5-13,16-18,20-21H2,1-4H3,(H2,41,42,43)/t22-,24+,25+,27+,28+,31+,32+,33+,34+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
TSC22 domain family protein 3
(Human) | BDBM476573
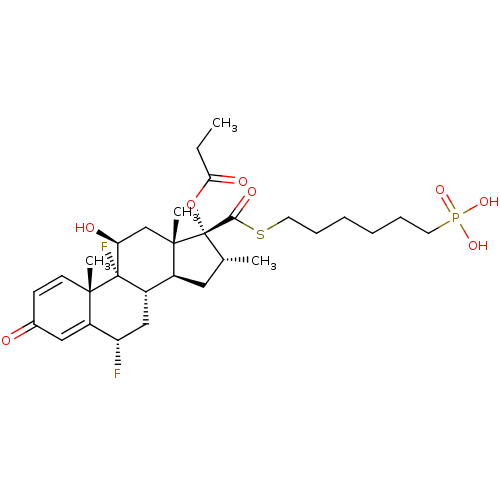
(US10869929, Compound 7 | US11554172, Compound 7)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCCCCCCP(O)(O)=O |r,c:18,t:14| Show InChI InChI=1S/C30H43F2O8PS/c1-5-25(35)40-30(26(36)42-13-9-7-6-8-12-41(37,38)39)18(2)14-20-21-16-23(31)22-15-19(33)10-11-27(22,3)29(21,32)24(34)17-28(20,30)4/h10-11,15,18,20-21,23-24,34H,5-9,12-14,16-17H2,1-4H3,(H2,37,38,39)/t18-,20+,21+,23+,24+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM476573

(US10869929, Compound 7 | US11554172, Compound 7)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCCCCCCP(O)(O)=O |r,c:18,t:14| Show InChI InChI=1S/C30H43F2O8PS/c1-5-25(35)40-30(26(36)42-13-9-7-6-8-12-41(37,38)39)18(2)14-20-21-16-23(31)22-15-19(33)10-11-27(22,3)29(21,32)24(34)17-28(20,30)4/h10-11,15,18,20-21,23-24,34H,5-9,12-14,16-17H2,1-4H3,(H2,37,38,39)/t18-,20+,21+,23+,24+,27+,28+,29+,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181139
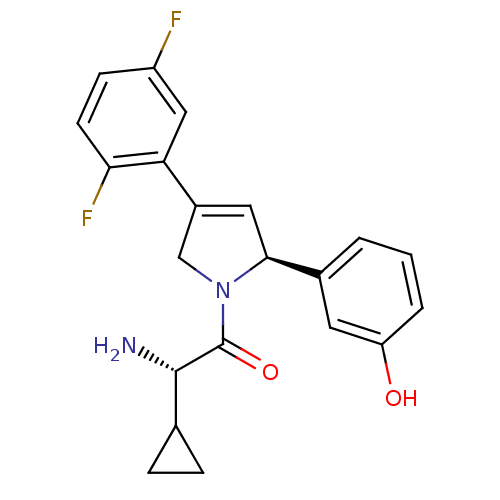
((S)-2-amino-2-cyclopropyl-1-((S)-4-(2,5-difluoroph...)Show SMILES N[C@@H](C1CC1)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:10| Show InChI InChI=1S/C21H20F2N2O2/c22-15-6-7-18(23)17(10-15)14-9-19(13-2-1-3-16(26)8-13)25(11-14)21(27)20(24)12-4-5-12/h1-3,6-10,12,19-20,26H,4-5,11,24H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223478
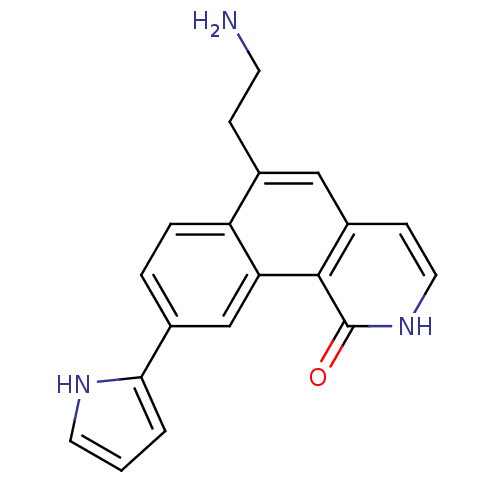
(6-(2-aminoethyl)-9-(1H-pyrrol-2-yl)benzo[h]isoquin...)Show SMILES NCCc1cc2cc[nH]c(=O)c2c2cc(ccc12)-c1ccc[nH]1 Show InChI InChI=1S/C19H17N3O/c20-7-5-12-10-14-6-9-22-19(23)18(14)16-11-13(3-4-15(12)16)17-2-1-8-21-17/h1-4,6,8-11,21H,5,7,20H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220958
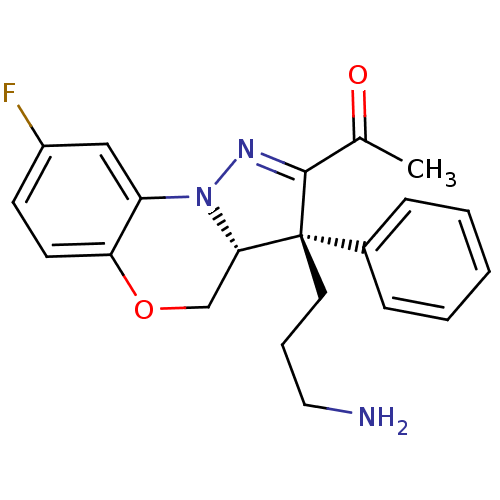
(1-[(3R,3aR)-3-(3-amino-propyl)-8-fluoro-3-phenyl-3...)Show SMILES CC(=O)C1=NN2[C@@H](COc3ccc(F)cc23)[C@@]1(CCCN)c1ccccc1 |t:3| Show InChI InChI=1S/C21H22FN3O2/c1-14(26)20-21(10-5-11-23,15-6-3-2-4-7-15)19-13-27-18-9-8-16(22)12-17(18)25(19)24-20/h2-4,6-9,12,19H,5,10-11,13,23H2,1H3/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of kinesin spindle protein |
Bioorg Med Chem Lett 17: 5671-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.067
BindingDB Entry DOI: 10.7270/Q261101N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223480
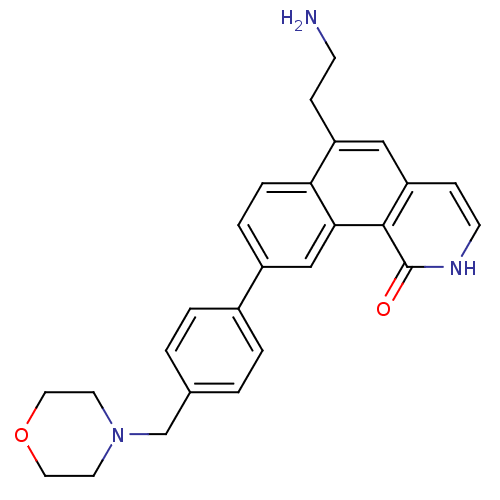
(6-(2-aminoethyl)-9-(4-(morpholinomethyl)phenyl)ben...)Show SMILES NCCc1cc2cc[nH]c(=O)c2c2cc(ccc12)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C26H27N3O2/c27-9-7-21-15-22-8-10-28-26(30)25(22)24-16-20(5-6-23(21)24)19-3-1-18(2-4-19)17-29-11-13-31-14-12-29/h1-6,8,10,15-16H,7,9,11-14,17,27H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195197

(6-(isothiazol-4-yl)-3-(5-(piperidin-1-ylmethyl)-1H...)Show SMILES O=c1[nH]c2ccc(cc2cc1-c1cc2cc(CN3CCCCC3)ccc2[nH]1)-c1cnsc1 Show InChI InChI=1S/C26H24N4OS/c31-26-22(12-20-11-18(5-7-24(20)29-26)21-14-27-32-16-21)25-13-19-10-17(4-6-23(19)28-25)15-30-8-2-1-3-9-30/h4-7,10-14,16,28H,1-3,8-9,15H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195213
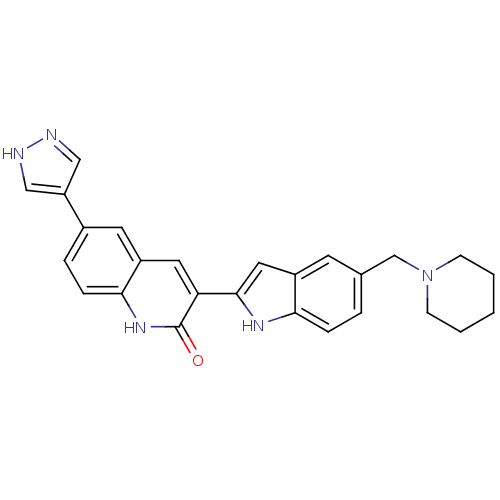
(3-(5-(piperidin-1-ylmethyl)-1H-indol-2-yl)-6-(1H-p...)Show SMILES O=c1[nH]c2ccc(cc2cc1-c1cc2cc(CN3CCCCC3)ccc2[nH]1)-c1cn[nH]c1 Show InChI InChI=1S/C26H25N5O/c32-26-22(12-20-11-18(5-7-24(20)30-26)21-14-27-28-15-21)25-13-19-10-17(4-6-23(19)29-25)16-31-8-2-1-3-9-31/h4-7,10-15,29H,1-3,8-9,16H2,(H,27,28)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195198
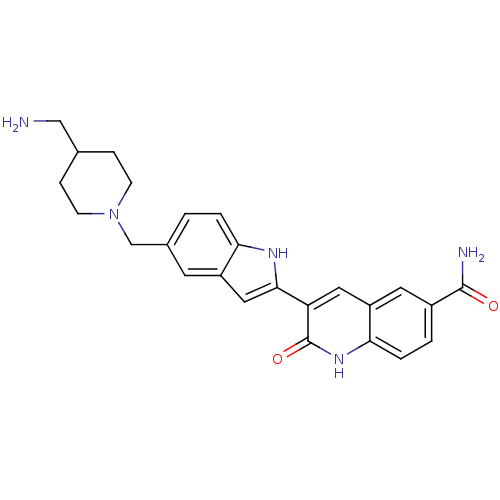
(3-(5-((4-(aminomethyl)piperidin-1-yl)methyl)-1H-in...)Show SMILES NCC1CCN(Cc2ccc3[nH]c(cc3c2)-c2cc3cc(ccc3[nH]c2=O)C(N)=O)CC1 Show InChI InChI=1S/C25H27N5O2/c26-13-15-5-7-30(8-6-15)14-16-1-3-21-18(9-16)12-23(28-21)20-11-19-10-17(24(27)31)2-4-22(19)29-25(20)32/h1-4,9-12,15,28H,5-8,13-14,26H2,(H2,27,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
TSC22 domain family protein 3
(Human) | BDBM476568
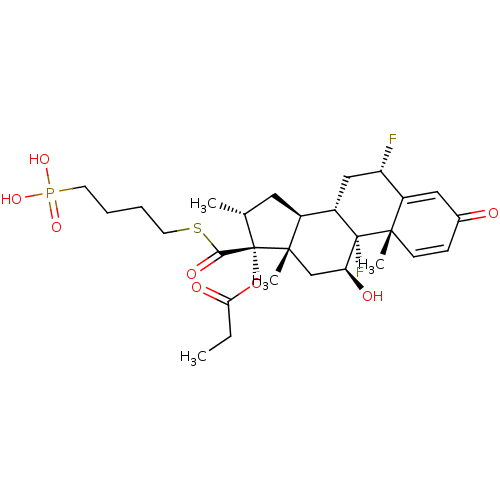
(US10869929, Compound 4 | US11554172, Compound 4)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCCCCP(O)(O)=O |r,c:18,t:14| Show InChI InChI=1S/C28H39F2O8PS/c1-5-23(33)38-28(24(34)40-11-7-6-10-39(35,36)37)16(2)12-18-19-14-21(29)20-13-17(31)8-9-25(20,3)27(19,30)22(32)15-26(18,28)4/h8-9,13,16,18-19,21-22,32H,5-7,10-12,14-15H2,1-4H3,(H2,35,36,37)/t16-,18+,19+,21+,22+,25+,26+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM476568

(US10869929, Compound 4 | US11554172, Compound 4)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCCCCP(O)(O)=O |r,c:18,t:14| Show InChI InChI=1S/C28H39F2O8PS/c1-5-23(33)38-28(24(34)40-11-7-6-10-39(35,36)37)16(2)12-18-19-14-21(29)20-13-17(31)8-9-25(20,3)27(19,30)22(32)15-26(18,28)4/h8-9,13,16,18-19,21-22,32H,5-7,10-12,14-15H2,1-4H3,(H2,35,36,37)/t16-,18+,19+,21+,22+,25+,26+,27+,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195200
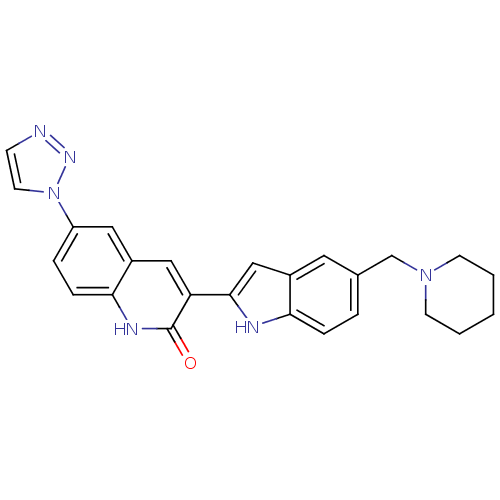
(3-(5-(piperidin-1-ylmethyl)-1H-indol-2-yl)-6-(1H-1...)Show SMILES O=c1[nH]c2ccc(cc2cc1-c1cc2cc(CN3CCCCC3)ccc2[nH]1)-n1ccnn1 Show InChI InChI=1S/C25H24N6O/c32-25-21(14-19-13-20(5-7-23(19)28-25)31-11-8-26-29-31)24-15-18-12-17(4-6-22(18)27-24)16-30-9-2-1-3-10-30/h4-8,11-15,27H,1-3,9-10,16H2,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223454

(6-(3-aminopropyl)-4-(2-chlorophenyl)-9-(1H-pyrazol...)Show SMILES NCCCc1cc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)-c1ccccc1Cl Show InChI InChI=1S/C25H21ClN4O/c26-23-6-2-1-5-19(23)22-14-28-25(31)24-20-10-15(17-12-29-30-13-17)7-8-18(20)16(4-3-9-27)11-21(22)24/h1-2,5-8,10-14H,3-4,9,27H2,(H,28,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220324
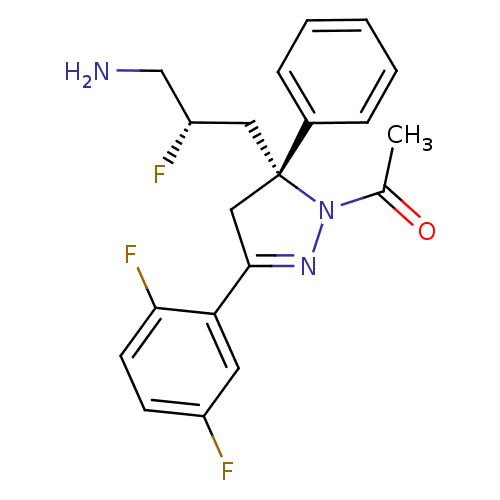
(1-((R)-5-((S)-3-amino-2-fluoropropyl)-3-(2,5-diflu...)Show SMILES CC(=O)N1N=C(C[C@@]1(C[C@H](F)CN)c1ccccc1)c1cc(F)ccc1F |c:4| Show InChI InChI=1S/C20H20F3N3O/c1-13(27)26-20(10-16(22)12-24,14-5-3-2-4-6-14)11-19(25-26)17-9-15(21)7-8-18(17)23/h2-9,16H,10-12,24H2,1H3/t16-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 17: 5390-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.046
BindingDB Entry DOI: 10.7270/Q2RX9BSM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195218
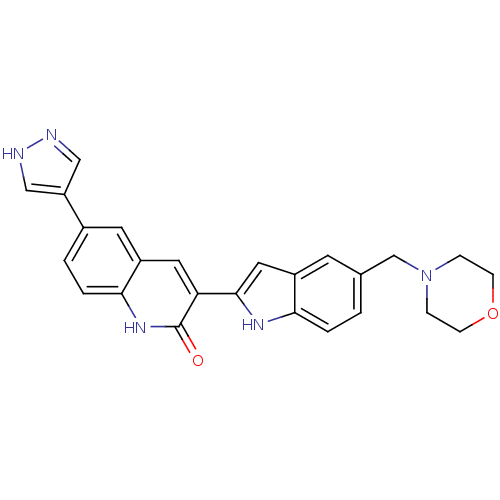
(3-(5-(morpholinomethyl)-1H-indol-2-yl)-6-(1H-pyraz...)Show SMILES O=c1[nH]c2ccc(cc2cc1-c1cc2cc(CN3CCOCC3)ccc2[nH]1)-c1cn[nH]c1 Show InChI InChI=1S/C25H23N5O2/c31-25-21(11-19-10-17(2-4-23(19)29-25)20-13-26-27-14-20)24-12-18-9-16(1-3-22(18)28-24)15-30-5-7-32-8-6-30/h1-4,9-14,28H,5-8,15H2,(H,26,27)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50354850

(BUDESONIDE | US10869929, Compound Budesonide | US1...)Show SMILES CCCC1O[C@@H]2C[C@H]3[C@@H]4CCC5=CC(=O)C=C[C@]5(C)[C@H]4[C@@H](O)C[C@]3(C)[C@@]2(O1)C(=O)CO |r,c:15,t:11| Show InChI InChI=1S/C25H34O6/c1-4-5-21-30-20-11-17-16-7-6-14-10-15(27)8-9-23(14,2)22(16)18(28)12-24(17,3)25(20,31-21)19(29)13-26/h8-10,16-18,20-22,26,28H,4-7,11-13H2,1-3H3/t16-,17-,18-,20+,21?,22+,23-,24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639TPT |
More data for this
Ligand-Target Pair | |
TSC22 domain family protein 3
(Human) | BDBM50354850

(BUDESONIDE | US10869929, Compound Budesonide | US1...)Show SMILES CCCC1O[C@@H]2C[C@H]3[C@@H]4CCC5=CC(=O)C=C[C@]5(C)[C@H]4[C@@H](O)C[C@]3(C)[C@@]2(O1)C(=O)CO |r,c:15,t:11| Show InChI InChI=1S/C25H34O6/c1-4-5-21-30-20-11-17-16-7-6-14-10-15(27)8-9-23(14,2)22(16)18(28)12-24(17,3)25(20,31-21)19(29)13-26/h8-10,16-18,20-22,26,28H,4-7,11-13H2,1-3H3/t16-,17-,18-,20+,21?,22+,23-,24-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... |
US Patent US10869929 (2020)
BindingDB Entry DOI: 10.7270/Q2N58QG7 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220964
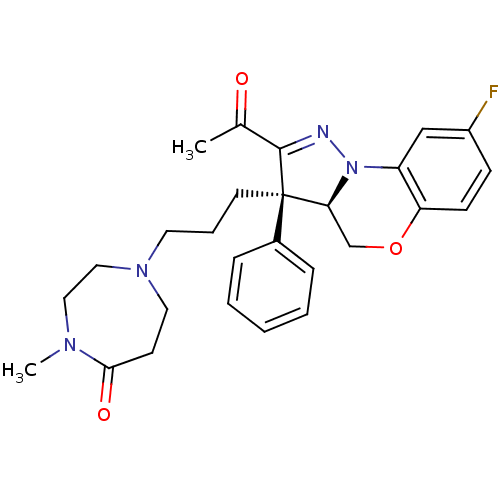
(1-[3-((3R,3aR)-2-acetyl-8-fluoro-3-phenyl-3a,4-dih...)Show SMILES CN1CCN(CCC[C@]2([C@@H]3COc4ccc(F)cc4N3N=C2C(C)=O)c2ccccc2)CCC1=O |c:22| Show InChI InChI=1S/C27H31FN4O3/c1-19(33)26-27(20-7-4-3-5-8-20,12-6-13-31-14-11-25(34)30(2)15-16-31)24-18-35-23-10-9-21(28)17-22(23)32(24)29-26/h3-5,7-10,17,24H,6,11-16,18H2,1-2H3/t24-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of kinesin spindle protein |
Bioorg Med Chem Lett 17: 5671-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.067
BindingDB Entry DOI: 10.7270/Q261101N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220326

((S)-1-(5-(3-aminopropyl)-3-(2,5-difluorophenyl)-5-...)Show SMILES CC(=O)N1N=C(C[C@@]1(CCCN)c1ccccc1)c1cc(F)ccc1F |c:4| Show InChI InChI=1S/C20H21F2N3O/c1-14(26)25-20(10-5-11-23,15-6-3-2-4-7-15)13-19(24-25)17-12-16(21)8-9-18(17)22/h2-4,6-9,12H,5,10-11,13,23H2,1H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 17: 5390-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.046
BindingDB Entry DOI: 10.7270/Q2RX9BSM |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220320

(1-((S)-3-(2,5-difluorophenyl)-5-(3-((S)-3-fluoropy...)Show SMILES CC(=O)N1N=C(C[C@@]1(CCCN1CC[C@H](F)C1)c1ccccc1)c1cc(F)ccc1F |c:4| Show InChI InChI=1S/C24H26F3N3O/c1-17(31)30-24(18-6-3-2-4-7-18,11-5-12-29-13-10-20(26)16-29)15-23(28-30)21-14-19(25)8-9-22(21)27/h2-4,6-9,14,20H,5,10-13,15-16H2,1H3/t20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 17: 5390-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.046
BindingDB Entry DOI: 10.7270/Q2RX9BSM |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220331
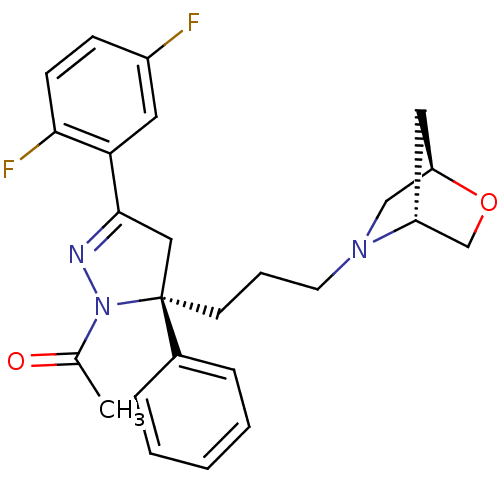
(1-((S)-5-(3-((1R,4R)-5-oxa-2-aza-bicyclo[2.2.1]hep...)Show SMILES CC(=O)N1N=C(C[C@@]1(CCCN1C[C@H]2C[C@@H]1CO2)c1ccccc1)c1cc(F)ccc1F |c:4| Show InChI InChI=1S/C25H27F2N3O2/c1-17(31)30-25(18-6-3-2-4-7-18,10-5-11-29-15-21-13-20(29)16-32-21)14-24(28-30)22-12-19(26)8-9-23(22)27/h2-4,6-9,12,20-21H,5,10-11,13-16H2,1H3/t20-,21-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 17: 5390-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.046
BindingDB Entry DOI: 10.7270/Q2RX9BSM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195216
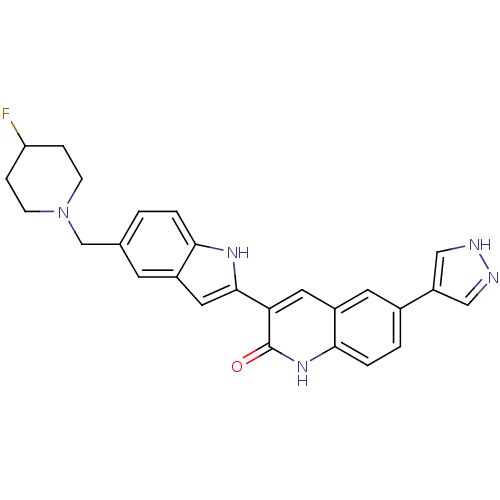
(3-(5-((4-fluoropiperidin-1-yl)methyl)-1H-indol-2-y...)Show SMILES FC1CCN(Cc2ccc3[nH]c(cc3c2)-c2cc3cc(ccc3[nH]c2=O)-c2cn[nH]c2)CC1 Show InChI InChI=1S/C26H24FN5O/c27-21-5-7-32(8-6-21)15-16-1-3-23-18(9-16)12-25(30-23)22-11-19-10-17(20-13-28-29-14-20)2-4-24(19)31-26(22)33/h1-4,9-14,21,30H,5-8,15H2,(H,28,29)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223484
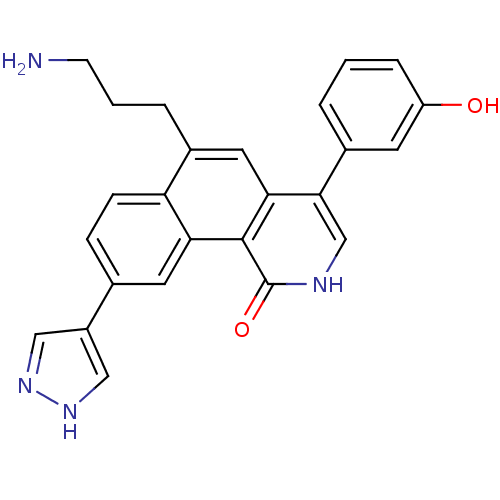
(6-(3-aminopropyl)-4-(3-hydroxyphenyl)-9-(1H-pyrazo...)Show SMILES NCCCc1cc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)-c1cccc(O)c1 Show InChI InChI=1S/C25H22N4O2/c26-8-2-4-17-11-22-23(16-3-1-5-19(30)9-16)14-27-25(31)24(22)21-10-15(6-7-20(17)21)18-12-28-29-13-18/h1,3,5-7,9-14,30H,2,4,8,26H2,(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220967

(1-[(3R,3aR)-8-chloro-3-(3-dimethylamino-propyl)-3-...)Show SMILES CN(C)CCC[C@]1([C@@H]2COc3ccc(Cl)cc3N2N=C1C(C)=O)c1ccccc1 |c:20| Show InChI InChI=1S/C23H26ClN3O2/c1-16(28)22-23(12-7-13-26(2)3,17-8-5-4-6-9-17)21-15-29-20-11-10-18(24)14-19(20)27(21)25-22/h4-6,8-11,14,21H,7,12-13,15H2,1-3H3/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of kinesin spindle protein |
Bioorg Med Chem Lett 17: 5671-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.067
BindingDB Entry DOI: 10.7270/Q261101N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220970

(1-[(3R,3aR)-8-methyl-3-(3-morpholin-4-yl-propyl)-3...)Show SMILES CC(=O)C1=NN2[C@@H](COc3ccc(C)cc23)[C@@]1(CCCN1CCOCC1)c1ccccc1 |t:3| Show InChI InChI=1S/C26H31N3O3/c1-19-9-10-23-22(17-19)29-24(18-32-23)26(25(27-29)20(2)30,21-7-4-3-5-8-21)11-6-12-28-13-15-31-16-14-28/h3-5,7-10,17,24H,6,11-16,18H2,1-2H3/t24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of kinesin spindle protein |
Bioorg Med Chem Lett 17: 5671-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.067
BindingDB Entry DOI: 10.7270/Q261101N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220969

(1-{(3R,3aR)-3-[3-(4-acetyl-piperazin-1-yl)-propyl]...)Show SMILES CC(=O)N1CCN(CCC[C@]2([C@@H]3COc4ccc(Cl)cc4N3N=C2C(C)=O)c2ccccc2)CC1 |c:24| Show InChI InChI=1S/C27H31ClN4O3/c1-19(33)26-27(21-7-4-3-5-8-21,11-6-12-30-13-15-31(16-14-30)20(2)34)25-18-35-24-10-9-22(28)17-23(24)32(25)29-26/h3-5,7-10,17,25H,6,11-16,18H2,1-2H3/t25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of kinesin spindle protein |
Bioorg Med Chem Lett 17: 5671-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.067
BindingDB Entry DOI: 10.7270/Q261101N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220332
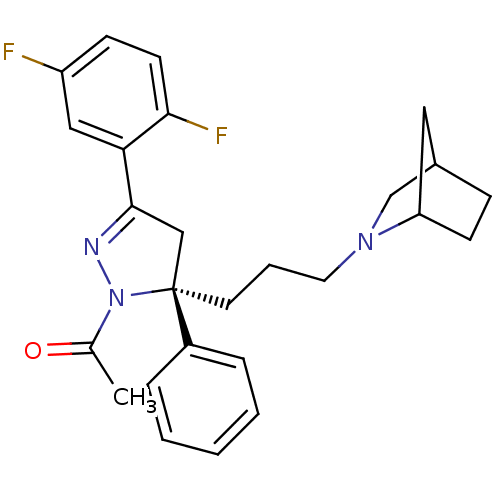
(1-((S)-5-(3-(2-aza-bicyclo[2.2.1]heptan-2-yl)propy...)Show SMILES CC(=O)N1N=C(C[C@@]1(CCCN1CC2CCC1C2)c1ccccc1)c1cc(F)ccc1F |c:4,TLB:10:11:17:14.15| Show InChI InChI=1S/C26H29F2N3O/c1-18(32)31-26(20-6-3-2-4-7-20,12-5-13-30-17-19-8-10-22(30)14-19)16-25(29-31)23-15-21(27)9-11-24(23)28/h2-4,6-7,9,11,15,19,22H,5,8,10,12-14,16-17H2,1H3/t19?,22?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 17: 5390-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.046
BindingDB Entry DOI: 10.7270/Q2RX9BSM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50220886
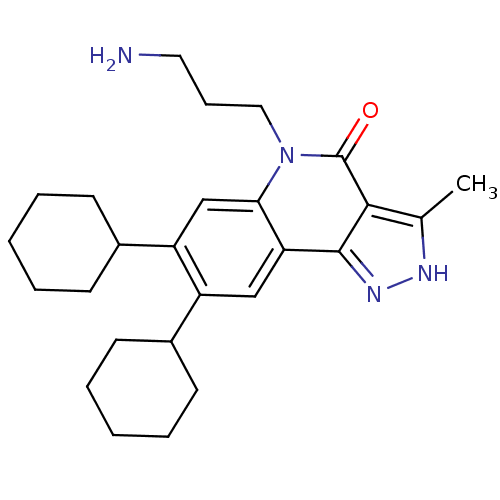
(5-(3-aminopropyl)-7,8-dicyclohexyl-3-methyl-2H-pyr...)Show SMILES Cc1[nH]nc2c1c(=O)n(CCCN)c1cc(C3CCCCC3)c(cc21)C1CCCCC1 Show InChI InChI=1S/C26H36N4O/c1-17-24-25(29-28-17)22-15-20(18-9-4-2-5-10-18)21(19-11-6-3-7-12-19)16-23(22)30(26(24)31)14-8-13-27/h15-16,18-19H,2-14,27H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 by fluorescence assay |
Bioorg Med Chem Lett 17: 5989-94 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.051
BindingDB Entry DOI: 10.7270/Q2K93775 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195209
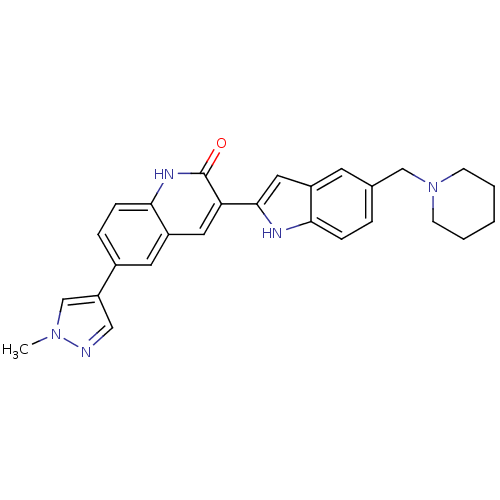
(6-(1-methyl-1H-pyrazol-4-yl)-3-(5-(piperidin-1-ylm...)Show SMILES Cn1cc(cn1)-c1ccc2[nH]c(=O)c(cc2c1)-c1cc2cc(CN3CCCCC3)ccc2[nH]1 Show InChI InChI=1S/C27H27N5O/c1-31-17-22(15-28-31)19-6-8-25-21(12-19)13-23(27(33)30-25)26-14-20-11-18(5-7-24(20)29-26)16-32-9-3-2-4-10-32/h5-8,11-15,17,29H,2-4,9-10,16H2,1H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM92404
(CHEMBL250843 | PDK1 inhibitor, 5)Show InChI InChI=1S/C14H15ClN4O/c1-8-12-13(18-17-8)10-7-9(15)3-4-11(10)19(14(12)20)6-2-5-16/h3-4,7H,2,5-6,16H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 by fluorescence assay |
Bioorg Med Chem Lett 17: 5989-94 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.051
BindingDB Entry DOI: 10.7270/Q2K93775 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223473
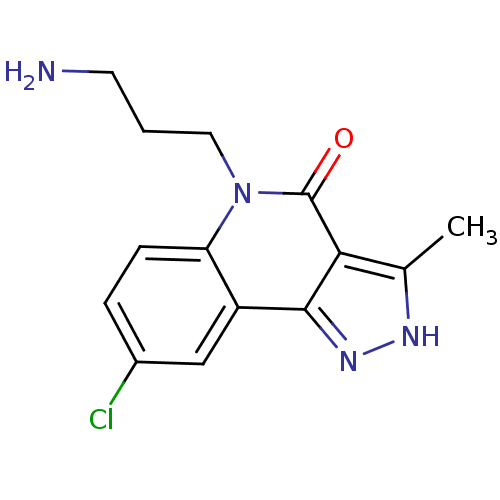
(6-(3-aminopropyl)-4-(4-chlorophenyl)-9-(1H-pyrazol...)Show SMILES NCCCc1cc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H21ClN4O/c26-19-6-3-15(4-7-19)23-14-28-25(31)24-21-10-16(18-12-29-30-13-18)5-8-20(21)17(2-1-9-27)11-22(23)24/h3-8,10-14H,1-2,9,27H2,(H,28,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223475
(6-(3-aminopropyl)-4-(2,4-dichlorophenyl)-9-(1H-pyr...)Show SMILES NCCCc1cc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H20Cl2N4O/c26-17-4-6-19(23(27)10-17)22-13-29-25(32)24-20-8-14(16-11-30-31-12-16)3-5-18(20)15(2-1-7-28)9-21(22)24/h3-6,8-13H,1-2,7,28H2,(H,29,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data