 Found 243 hits with Last Name = 'giridhar' and Initial = 'r'
Found 243 hits with Last Name = 'giridhar' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50004154

(2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCC1=NC(Cl)=C(C(C)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(O)=O |c:7,t:4| Show InChI InChI=1S/C24H25ClN6O2/c1-3-4-9-20-26-22(25)21(24(32)33)15(2)31(20)14-16-10-12-17(13-11-16)18-7-5-6-8-19(18)23-27-29-30-28-23/h5-8,10-13,15H,3-4,9,14H2,1-2H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030727
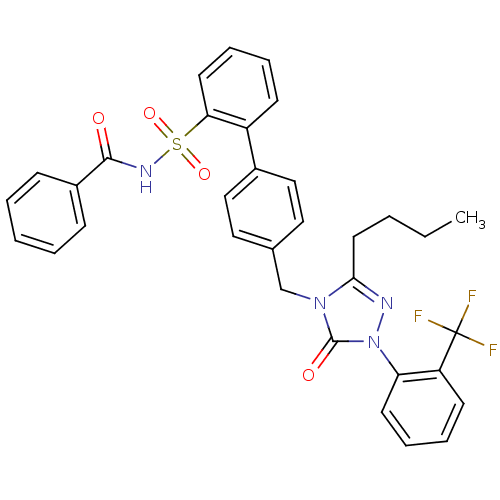
(4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...)Show SMILES CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C33H29F3N4O4S/c1-2-3-17-30-37-40(28-15-9-8-14-27(28)33(34,35)36)32(42)39(30)22-23-18-20-24(21-19-23)26-13-7-10-16-29(26)45(43,44)38-31(41)25-11-5-4-6-12-25/h4-16,18-21H,2-3,17,22H2,1H3,(H,38,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11548

(CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(CN2C(CCS2(=O)=O)C(=O)NO)cc1 Show InChI InChI=1S/C18H20N2O5S/c1-25-16-8-6-15(7-9-16)14-4-2-13(3-5-14)12-20-17(18(21)19-22)10-11-26(20,23)24/h2-9,17,22H,10-12H2,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50044739
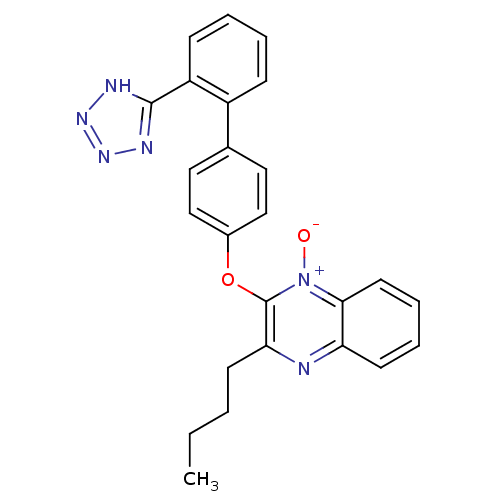
(3-Butyl-2-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yloxy]...)Show SMILES CCCCc1nc2ccccc2[n+]([O-])c1Oc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22N6O2/c1-2-3-10-22-25(31(32)23-12-7-6-11-21(23)26-22)33-18-15-13-17(14-16-18)19-8-4-5-9-20(19)24-27-29-30-28-24/h4-9,11-16H,2-3,10H2,1H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT2 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50247674
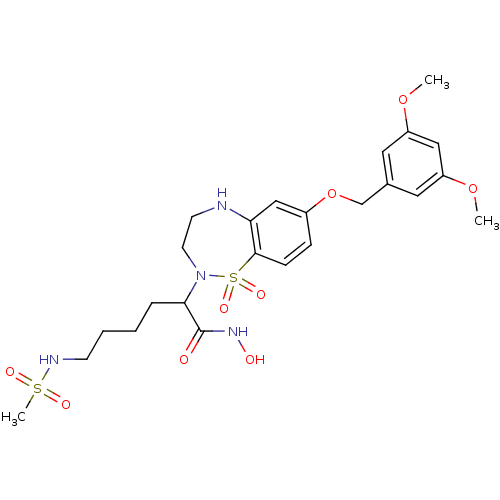
(2-[7-(3,5-Dimethoxy-benzyloxy)-1,1-dioxo-1,3,4,5-t...)Show SMILES COc1cc(COc2ccc3c(NCCN(C(CCCCNS(C)(=O)=O)C(=O)NO)S3(=O)=O)c2)cc(OC)c1 Show InChI InChI=1S/C24H34N4O9S2/c1-35-19-12-17(13-20(14-19)36-2)16-37-18-7-8-23-21(15-18)25-10-11-28(39(23,33)34)22(24(29)27-30)6-4-5-9-26-38(3,31)32/h7-8,12-15,22,25-26,30H,4-6,9-11,16H2,1-3H3,(H,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of pig TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50335930
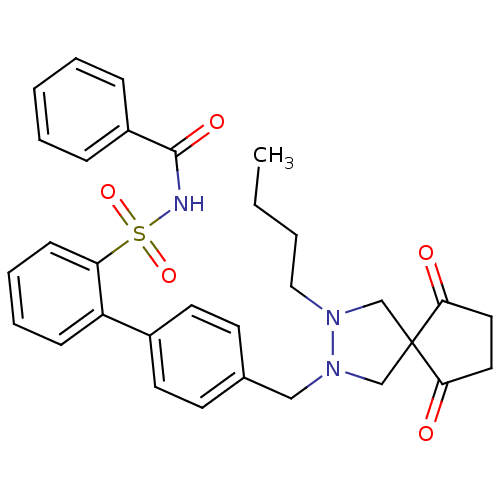
(CHEMBL1668000 | N-(4'-((3-butyl-6,9-dioxo-2,3-diaz...)Show SMILES CCCCN1CC2(CN1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1)C(=O)CCC2=O Show InChI InChI=1S/C31H33N3O5S/c1-2-3-19-33-21-31(28(35)17-18-29(31)36)22-34(33)20-23-13-15-24(16-14-23)26-11-7-8-12-27(26)40(38,39)32-30(37)25-9-5-4-6-10-25/h4-16H,2-3,17-22H2,1H3,(H,32,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Displacement of [3H]-angiotensin 2 from angiotensin AT1 receptor in human PLC-PRF5 cells |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50039342
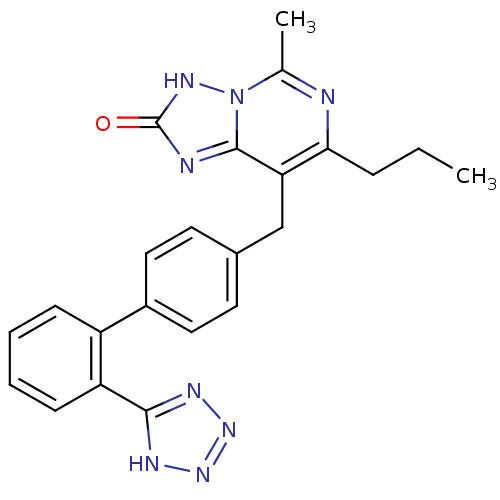
(5-Methyl-7-propyl-8-[2'-(1H-tetrazol-5-yl)-bipheny...)Show SMILES CCCc1nc(C)n2[nH]c(=O)nc2c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H22N8O/c1-3-6-20-19(22-25-23(32)28-31(22)14(2)24-20)13-15-9-11-16(12-10-15)17-7-4-5-8-18(17)21-26-29-30-27-21/h4-5,7-12H,3,6,13H2,1-2H3,(H,28,32)(H,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50335929
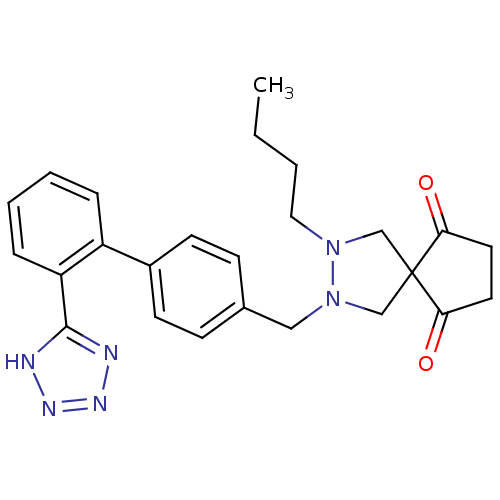
(2-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-3-b...)Show SMILES CCCCN1CC2(CN1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)CCC2=O Show InChI InChI=1S/C25H28N6O2/c1-2-3-14-30-16-25(22(32)12-13-23(25)33)17-31(30)15-18-8-10-19(11-9-18)20-6-4-5-7-21(20)24-26-28-29-27-24/h4-11H,2-3,12-17H2,1H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Displacement of [3H]-angiotensin 2 from angiotensin AT1 receptor in human PLC-PRF5 cells |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50335923
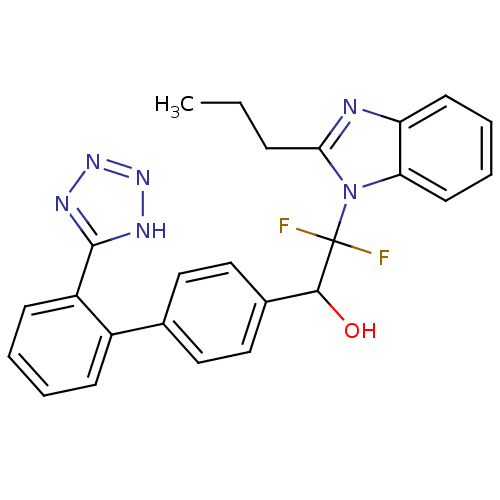
(1-(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)-2,2-difluor...)Show SMILES CCCc1nc2ccccc2n1C(F)(F)C(O)c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22F2N6O/c1-2-7-22-28-20-10-5-6-11-21(20)33(22)25(26,27)23(34)17-14-12-16(13-15-17)18-8-3-4-9-19(18)24-29-31-32-30-24/h3-6,8-15,23,34H,2,7H2,1H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11548

(CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(CN2C(CCS2(=O)=O)C(=O)NO)cc1 Show InChI InChI=1S/C18H20N2O5S/c1-25-16-8-6-15(7-9-16)14-4-2-13(3-5-14)12-20-17(18(21)19-22)10-11-26(20,23)24/h2-9,17,22H,10-12H2,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11548

(CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(CN2C(CCS2(=O)=O)C(=O)NO)cc1 Show InChI InChI=1S/C18H20N2O5S/c1-25-16-8-6-15(7-9-16)14-4-2-13(3-5-14)12-20-17(18(21)19-22)10-11-26(20,23)24/h2-9,17,22H,10-12H2,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50335923

(1-(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)-2,2-difluor...)Show SMILES CCCc1nc2ccccc2n1C(F)(F)C(O)c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22F2N6O/c1-2-7-22-28-20-10-5-6-11-21(20)33(22)25(26,27)23(34)17-14-12-16(13-15-17)18-8-3-4-9-19(18)24-29-31-32-30-24/h3-6,8-15,23,34H,2,7H2,1H3,(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT2 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86095

(2-Cyanopyrrolidine Derivative, 24)Show SMILES CC(C)CC(NC(=O)C(=C\c1ccccc1)\c1ccccc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C26H29N3O2/c1-19(2)16-24(26(31)29-15-9-14-22(29)18-27)28-25(30)23(21-12-7-4-8-13-21)17-20-10-5-3-6-11-20/h3-8,10-13,17,19,22,24H,9,14-16H2,1-2H3,(H,28,30)/b23-17+/t22-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86096
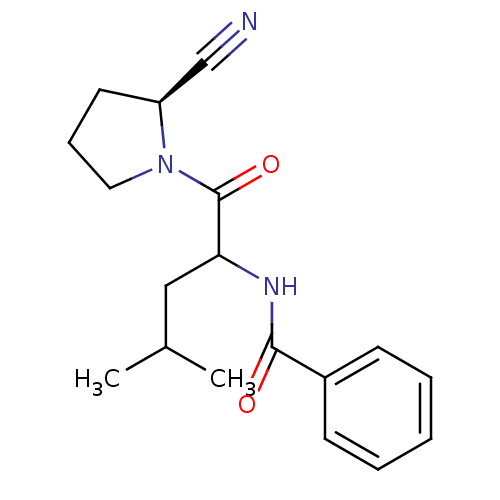
(2-Cyanopyrrolidine Derivative, 25)Show SMILES CC(C)CC(NC(=O)c1ccccc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H23N3O2/c1-13(2)11-16(18(23)21-10-6-9-15(21)12-19)20-17(22)14-7-4-3-5-8-14/h3-5,7-8,13,15-16H,6,9-11H2,1-2H3,(H,20,22)/t15-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60E+3 | -28.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86094

(2-Cyanopyrrolidine Derivative, 23)Show SMILES COc1ccc(\C=C\C(=O)NC(CC(C)C)C(=O)N2CCC[C@H]2C#N)cc1 |r| Show InChI InChI=1S/C21H27N3O3/c1-15(2)13-19(21(26)24-12-4-5-17(24)14-22)23-20(25)11-8-16-6-9-18(27-3)10-7-16/h6-11,15,17,19H,4-5,12-13H2,1-3H3,(H,23,25)/b11-8+/t17-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70E+4 | -23.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86099

(2-Cyanopyrrolidine Derivative, 28)Show SMILES O=C(NC(Cc1ccccc1)C(=O)N1CCC[C@H]1C#N)C(=C\c1ccccc1)\c1ccccc1 |r| Show InChI InChI=1S/C29H27N3O2/c30-21-25-17-10-18-32(25)29(34)27(20-23-13-6-2-7-14-23)31-28(33)26(24-15-8-3-9-16-24)19-22-11-4-1-5-12-22/h1-9,11-16,19,25,27H,10,17-18,20H2,(H,31,33)/b26-19+/t25-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+4 | -23.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86093
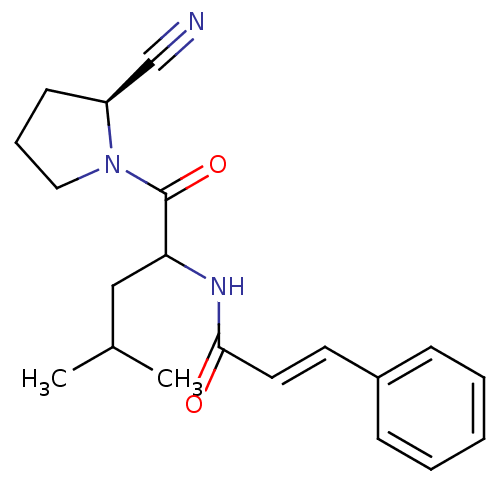
(2-Cyanopyrrolidine Derivative, 22)Show SMILES CC(C)CC(NC(=O)\C=C\c1ccccc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H25N3O2/c1-15(2)13-18(20(25)23-12-6-9-17(23)14-21)22-19(24)11-10-16-7-4-3-5-8-16/h3-5,7-8,10-11,15,17-18H,6,9,12-13H2,1-2H3,(H,22,24)/b11-10+/t17-,18?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.50E+4 | -23.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86094

(2-Cyanopyrrolidine Derivative, 23)Show SMILES COc1ccc(\C=C\C(=O)NC(CC(C)C)C(=O)N2CCC[C@H]2C#N)cc1 |r| Show InChI InChI=1S/C21H27N3O3/c1-15(2)13-19(21(26)24-12-4-5-17(24)14-22)23-20(25)11-8-16-6-9-18(27-3)10-7-16/h6-11,15,17,19H,4-5,12-13H2,1-3H3,(H,23,25)/b11-8+/t17-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20E+4 | -22.9 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86096

(2-Cyanopyrrolidine Derivative, 25)Show SMILES CC(C)CC(NC(=O)c1ccccc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H23N3O2/c1-13(2)11-16(18(23)21-10-6-9-15(21)12-19)20-17(22)14-7-4-3-5-8-14/h3-5,7-8,13,15-16H,6,9-11H2,1-2H3,(H,20,22)/t15-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86095

(2-Cyanopyrrolidine Derivative, 24)Show SMILES CC(C)CC(NC(=O)C(=C\c1ccccc1)\c1ccccc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C26H29N3O2/c1-19(2)16-24(26(31)29-15-9-14-22(29)18-27)28-25(30)23(21-12-7-4-8-13-21)17-20-10-5-3-6-11-20/h3-8,10-13,17,19,22,24H,9,14-16H2,1-2H3,(H,28,30)/b23-17+/t22-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86100

(2-Cyanopyrrolidine Derivative, 29)Show SMILES O=C(NC(Cc1ccccc1)C(=O)N1CCC[C@H]1C#N)c1ccccc1 |r| Show InChI InChI=1S/C21H21N3O2/c22-15-18-12-7-13-24(18)21(26)19(14-16-8-3-1-4-9-16)23-20(25)17-10-5-2-6-11-17/h1-6,8-11,18-19H,7,12-14H2,(H,23,25)/t18-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86100

(2-Cyanopyrrolidine Derivative, 29)Show SMILES O=C(NC(Cc1ccccc1)C(=O)N1CCC[C@H]1C#N)c1ccccc1 |r| Show InChI InChI=1S/C21H21N3O2/c22-15-18-12-7-13-24(18)21(26)19(14-16-8-3-1-4-9-16)23-20(25)17-10-5-2-6-11-17/h1-6,8-11,18-19H,7,12-14H2,(H,23,25)/t18-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86098

(2-Cyanopyrrolidine Derivative, 27)Show SMILES COc1ccc(\C=C\C(=O)NC(Cc2ccccc2)C(=O)N2CCC[C@H]2C#N)cc1 |r| Show InChI InChI=1S/C24H25N3O3/c1-30-21-12-9-18(10-13-21)11-14-23(28)26-22(16-19-6-3-2-4-7-19)24(29)27-15-5-8-20(27)17-25/h2-4,6-7,9-14,20,22H,5,8,15-16H2,1H3,(H,26,28)/b14-11+/t20-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86097

(2-Cyanopyrrolidine Derivative, 26)Show SMILES O=C(NC(Cc1ccccc1)C(=O)N1CCC[C@H]1C#N)\C=C\c1ccccc1 |r| Show InChI InChI=1S/C23H23N3O2/c24-17-20-12-7-15-26(20)23(28)21(16-19-10-5-2-6-11-19)25-22(27)14-13-18-8-3-1-4-9-18/h1-6,8-11,13-14,20-21H,7,12,15-16H2,(H,25,27)/b14-13+/t20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86097

(2-Cyanopyrrolidine Derivative, 26)Show SMILES O=C(NC(Cc1ccccc1)C(=O)N1CCC[C@H]1C#N)\C=C\c1ccccc1 |r| Show InChI InChI=1S/C23H23N3O2/c24-17-20-12-7-15-26(20)23(28)21(16-19-10-5-2-6-11-19)25-22(27)14-13-18-8-3-1-4-9-18/h1-6,8-11,13-14,20-21H,7,12,15-16H2,(H,25,27)/b14-13+/t20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86098

(2-Cyanopyrrolidine Derivative, 27)Show SMILES COc1ccc(\C=C\C(=O)NC(Cc2ccccc2)C(=O)N2CCC[C@H]2C#N)cc1 |r| Show InChI InChI=1S/C24H25N3O3/c1-30-21-12-9-18(10-13-21)11-14-23(28)26-22(16-19-6-3-2-4-7-19)24(29)27-15-5-8-20(27)17-25/h2-4,6-7,9-14,20,22H,5,8,15-16H2,1H3,(H,26,28)/b14-11+/t20-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86099

(2-Cyanopyrrolidine Derivative, 28)Show SMILES O=C(NC(Cc1ccccc1)C(=O)N1CCC[C@H]1C#N)C(=C\c1ccccc1)\c1ccccc1 |r| Show InChI InChI=1S/C29H27N3O2/c30-21-25-17-10-18-32(25)29(34)27(20-23-13-6-2-7-14-23)31-28(33)26(24-15-8-3-9-16-24)19-22-11-4-1-5-12-22/h1-9,11-16,19,25,27H,10,17-18,20H2,(H,31,33)/b26-19+/t25-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM86093

(2-Cyanopyrrolidine Derivative, 22)Show SMILES CC(C)CC(NC(=O)\C=C\c1ccccc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H25N3O2/c1-15(2)13-18(20(25)23-12-6-9-17(23)14-21)22-19(24)11-10-16-7-4-3-5-8-16/h3-5,7-8,10-11,15,17-18H,6,9,12-13H2,1-2H3,(H,22,24)/b11-10+/t17-,18?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda
| Assay Description
The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... |
J Enzyme Inhib Med Chem 23: 190-7 (2008)
Article DOI: 10.1080/14756360701504842
BindingDB Entry DOI: 10.7270/Q2DV1HFC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50195927

(CHEMBL391851 | N-(5-methyl-2,4,6-trioxo-hexahydrop...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C23H20N4O5/c1-13-11-15(17-5-3-4-6-18(17)24-13)12-32-16-9-7-14(8-10-16)19(28)27-23(2)20(29)25-22(31)26-21(23)30/h3-11H,12H2,1-2H3,(H,27,28)(H2,25,26,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50044402
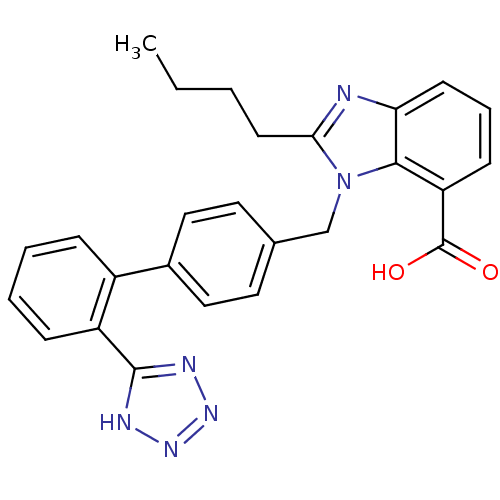
(2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H24N6O2/c1-2-3-11-23-27-22-10-6-9-21(26(33)34)24(22)32(23)16-17-12-14-18(15-13-17)19-7-4-5-8-20(19)25-28-30-31-29-25/h4-10,12-15H,2-3,11,16H2,1H3,(H,33,34)(H,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aortic strip assessed as inhibition of angiotensin 2-induced contractile response |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50247788

((2R,3S)-N1-((S)-3-(4-guanidinophenyl)-1-(methylami...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#6@H](-[#6]-[#6]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#8] |r| Show InChI InChI=1S/C28H40N6O4/c1-18(2)16-23(22(26(36)34-38)11-7-10-19-8-5-4-6-9-19)25(35)33-24(27(37)31-3)17-20-12-14-21(15-13-20)32-28(29)30/h4-6,8-9,12-15,18,22-24,38H,7,10-11,16-17H2,1-3H3,(H,31,37)(H,33,35)(H,34,36)(H4,29,30,32)/t22-,23+,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50229322
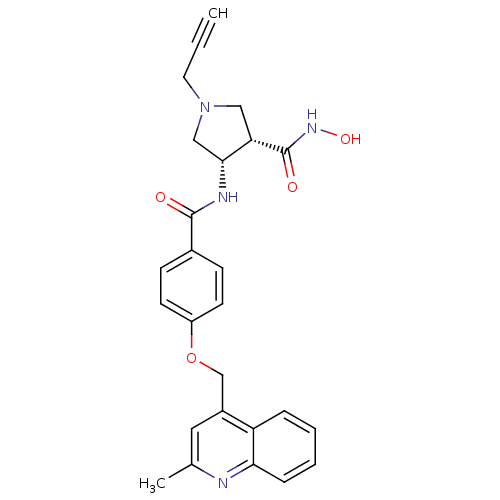
((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC#C)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C26H26N4O4/c1-3-12-30-14-22(26(32)29-33)24(15-30)28-25(31)18-8-10-20(11-9-18)34-16-19-13-17(2)27-23-7-5-4-6-21(19)23/h1,4-11,13,22,24,33H,12,14-16H2,2H3,(H,28,31)(H,29,32)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50247606
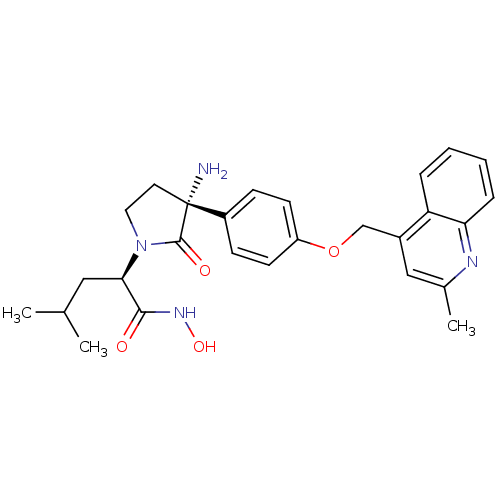
((R)-2-((R)-3-amino-3-(4-((2-methylquinolin-4-yl)me...)Show SMILES CC(C)C[C@@H](N1CC[C@](N)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H32N4O4/c1-17(2)14-24(25(32)30-34)31-13-12-27(28,26(31)33)20-8-10-21(11-9-20)35-16-19-15-18(3)29-23-7-5-4-6-22(19)23/h4-11,15,17,24,34H,12-14,16,28H2,1-3H3,(H,30,32)/t24-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50041677
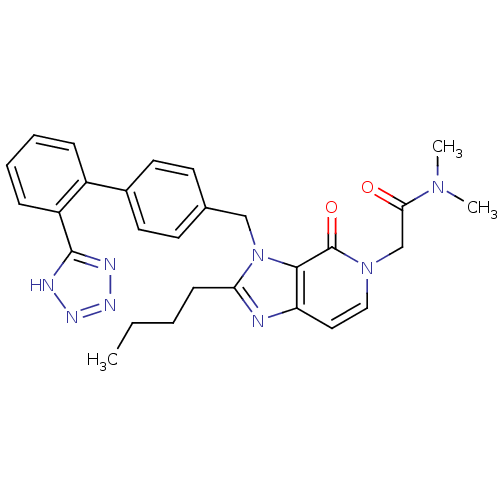
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(CC(=O)N(C)C)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H30N8O2/c1-4-5-10-24-29-23-15-16-35(18-25(37)34(2)3)28(38)26(23)36(24)17-19-11-13-20(14-12-19)21-8-6-7-9-22(21)27-30-32-33-31-27/h6-9,11-16H,4-5,10,17-18H2,1-3H3,(H,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009718
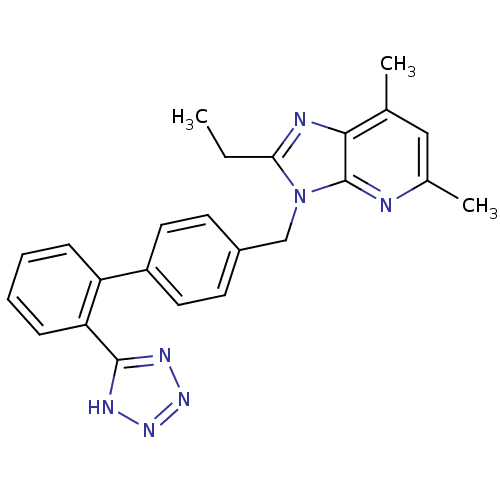
(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT2 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50038189

(4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H28N4O3S/c1-4-27-32-28-20(2)18-21(3)31-29(28)34(27)19-22-14-16-23(17-15-22)25-12-8-9-13-26(25)38(36,37)33-30(35)24-10-6-5-7-11-24/h5-18H,4,19H2,1-3H3,(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor in rabbit aortic rings |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50009718

(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor in rabbit aortic rings |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50287290
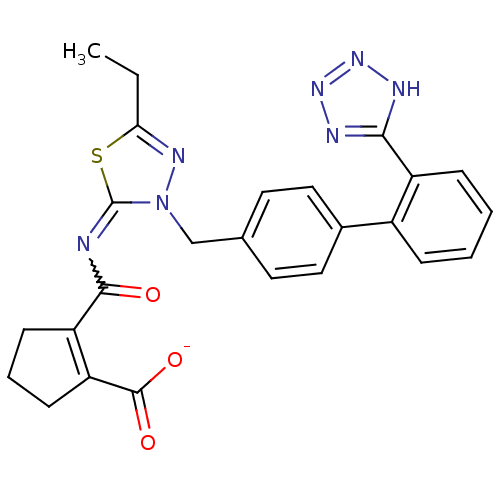
(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50042534
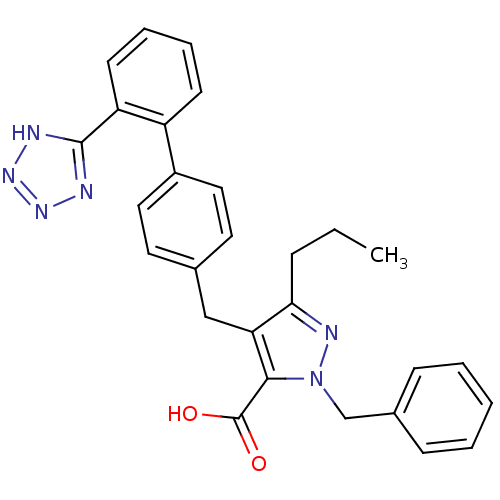
(2-Benzyl-5-propyl-4-[2'-(1H-tetrazol-5-yl)-bipheny...)Show SMILES CCCc1nn(Cc2ccccc2)c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H26N6O2/c1-2-8-25-24(26(28(35)36)34(31-25)18-20-9-4-3-5-10-20)17-19-13-15-21(16-14-19)22-11-6-7-12-23(22)27-29-32-33-30-27/h3-7,9-16H,2,8,17-18H2,1H3,(H,35,36)(H,29,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50042580

(2-Butyl-5-(2-chloro-phenyl)-3-[2'-(1H-tetrazol-5-y...)Show SMILES CCCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1ccccc1Cl Show InChI InChI=1S/C28H25ClN6O2/c1-2-3-12-24-30-25(22-10-6-7-11-23(22)29)26(28(36)37)35(24)17-18-13-15-19(16-14-18)20-8-4-5-9-21(20)27-31-33-34-32-27/h4-11,13-16H,2-3,12,17H2,1H3,(H,36,37)(H,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta assessed as reduction of angiotensin 2-induced contractile response |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50282383
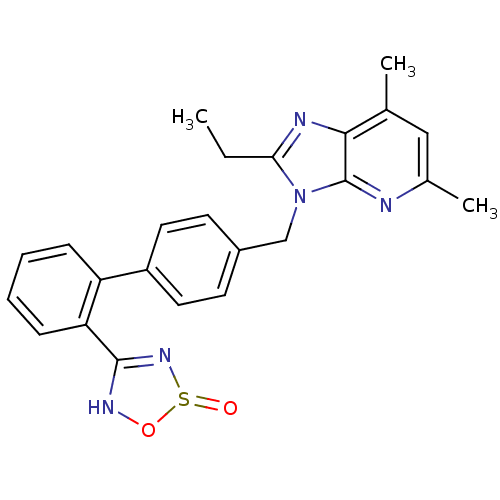
(2-Ethyl-5,7-dimethyl-3-[2'-(2-oxo-2,3-dihydro-2lam...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1C1=NS(=O)ON1 |t:30| Show InChI InChI=1S/C24H23N5O2S/c1-4-21-26-22-15(2)13-16(3)25-24(22)29(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-31-32(30)28-23/h5-13H,4,14H2,1-3H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor in rabbit aortic rings |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50335928
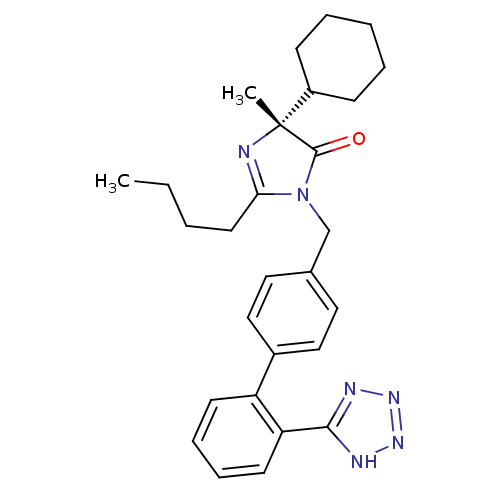
((R)-1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)...)Show SMILES CCCCC1=N[C@](C)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |r,t:4| Show InChI InChI=1S/C28H34N6O/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(35)34(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26-30-32-33-31-26/h8-9,12-13,15-18,22H,3-7,10-11,14,19H2,1-2H3,(H,30,31,32,33)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor in rabbit aortic rings |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50043450
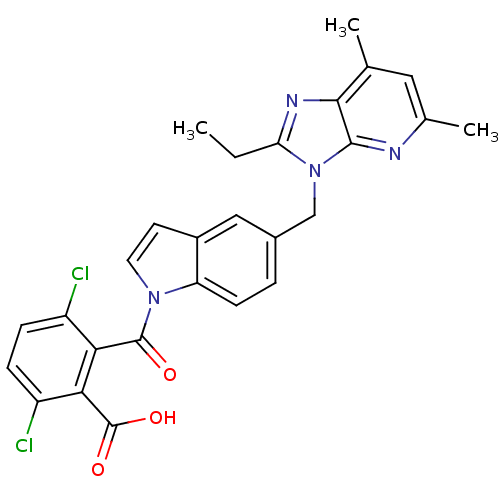
(3,6-Dichloro-2-[5-(2-ethyl-5,7-dimethyl-3H-imidazo...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc2n(ccc2c1)C(=O)c1c(Cl)ccc(Cl)c1C(O)=O Show InChI InChI=1S/C27H22Cl2N4O3/c1-4-21-31-24-14(2)11-15(3)30-25(24)33(21)13-16-5-8-20-17(12-16)9-10-32(20)26(34)22-18(28)6-7-19(29)23(22)27(35)36/h5-12H,4,13H2,1-3H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor in rabbit aortic rings |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50043434
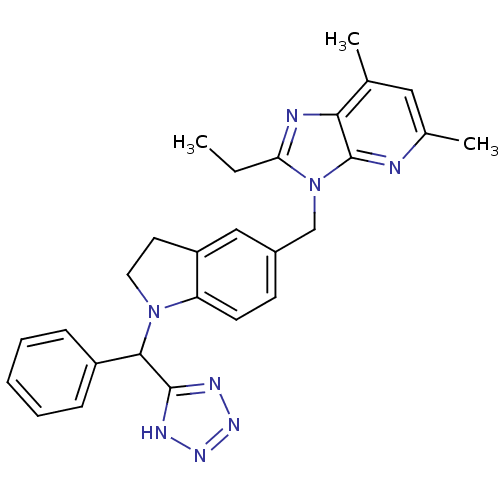
(2-Ethyl-5,7-dimethyl-4-{1-[phenyl-(1H-tetrazol-5-y...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc2N(CCc2c1)C(c1nnn[nH]1)c1ccccc1 Show InChI InChI=1S/C27H28N8/c1-4-23-29-24-17(2)14-18(3)28-27(24)35(23)16-19-10-11-22-21(15-19)12-13-34(22)25(26-30-32-33-31-26)20-8-6-5-7-9-20/h5-11,14-15,25H,4,12-13,16H2,1-3H3,(H,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor in rabbit aortic rings |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26526
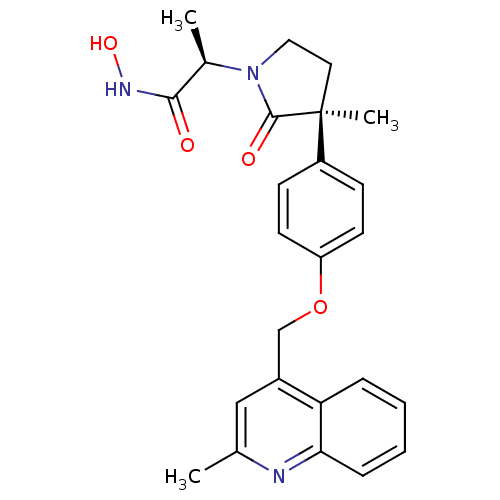
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50247605
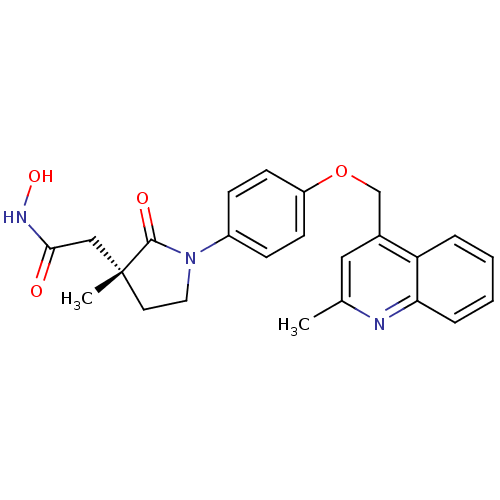
((R)-N-hydroxy-2-(3-methyl-1-(4-((2-methylquinolin-...)Show SMILES Cc1cc(COc2ccc(cc2)N2CC[C@](C)(CC(=O)NO)C2=O)c2ccccc2n1 |r| Show InChI InChI=1S/C24H25N3O4/c1-16-13-17(20-5-3-4-6-21(20)25-16)15-31-19-9-7-18(8-10-19)27-12-11-24(2,23(27)29)14-22(28)26-30/h3-10,13,30H,11-12,14-15H2,1-2H3,(H,26,28)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50152901

(1-Methyl-2-[4-(2-methyl-quinolin-4-ylmethoxy)-benz...)Show SMILES CN1CCC(C1CS(=O)(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO Show InChI InChI=1S/C24H27N3O5S/c1-16-13-17(20-5-3-4-6-22(20)25-16)14-32-18-7-9-19(10-8-18)33(30,31)15-23-21(24(28)26-29)11-12-27(23)2/h3-10,13,21,23,29H,11-12,14-15H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50167485
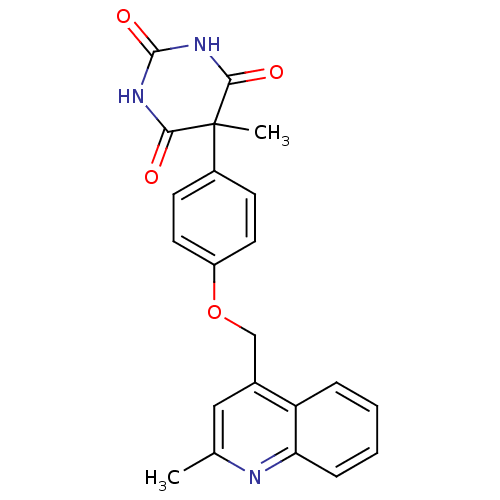
(5-Methyl-5-[4-(2-methyl-quinolin-4-ylmethoxy)-phen...)Show SMILES Cc1cc(COc2ccc(cc2)C2(C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C22H19N3O4/c1-13-11-14(17-5-3-4-6-18(17)23-13)12-29-16-9-7-15(8-10-16)22(2)19(26)24-21(28)25-20(22)27/h3-11H,12H2,1-2H3,(H2,24,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data