

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity at alpha4-beta2 nACh receptors in rat brain (minus cerebellum) homogenates. | Bioorg Med Chem Lett 13: 733-5 (2003) BindingDB Entry DOI: 10.7270/Q2FT8NKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50143279 ((S)-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards nicotinic acetylcholine receptor alpha4-beta2 | Bioorg Med Chem Lett 14: 1841-4 (2004) Article DOI: 10.1016/j.bmcl.2003.07.035 BindingDB Entry DOI: 10.7270/Q2MC917Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50144508 (4-Methyl-1-(5-phenyl-pentyl)-piperidine | CHEMBL30...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand | Bioorg Med Chem Lett 14: 2217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.018 BindingDB Entry DOI: 10.7270/Q2R210T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50144508 (4-Methyl-1-(5-phenyl-pentyl)-piperidine | CHEMBL30...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by scintillation spectrometric method | J Med Chem 60: 2605-2628 (2017) Article DOI: 10.1021/acs.jmedchem.7b00085 BindingDB Entry DOI: 10.7270/Q2DJ5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50236721 (CHEMBL4073530) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description The compound was tested for inhibition of [3H]-5-hydroxy tryptamine uptake in HEK cells by expressing cDNA for human tryptamine transporter | J Med Chem 60: 2605-2628 (2017) Article DOI: 10.1021/acs.jmedchem.7b00085 BindingDB Entry DOI: 10.7270/Q2DJ5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50144518 (1-(5,5-Diphenyl-pentyl)-4-methyl-piperidine | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand | Bioorg Med Chem Lett 14: 2217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.018 BindingDB Entry DOI: 10.7270/Q2R210T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581191 (CHEMBL5070876) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581204 (CHEMBL5076637) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50005257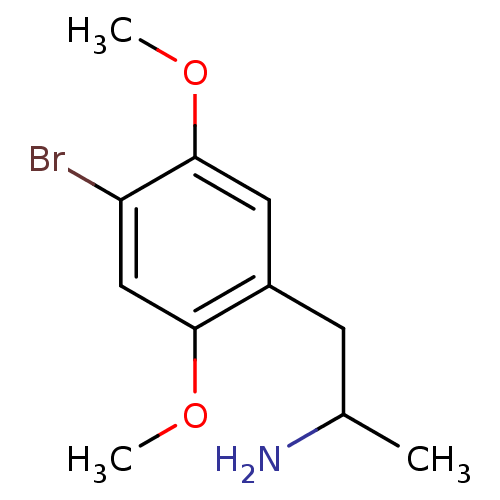 ((+)-2-(4-Bromo-2,5-dimethoxy-phenyl)-1-methyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for 5-HT2A serotonin receptor | J Med Chem 47: 6034-41 (2004) Article DOI: 10.1021/jm040082s BindingDB Entry DOI: 10.7270/Q2R78DQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50014997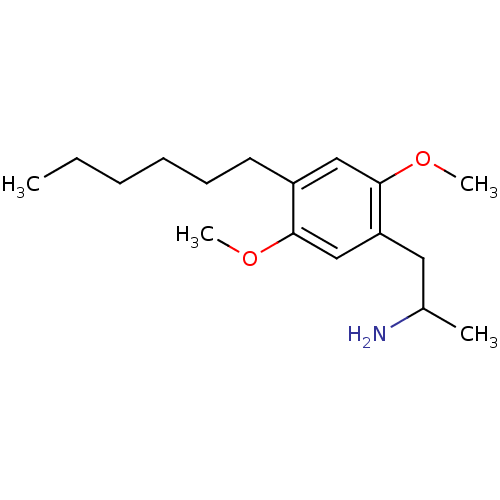 (1-(4-hexyl-2,5-dimethoxyphenyl)propan-2-amine | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 359: 1-6 (1999) Article DOI: 10.1007/pl00005315 BindingDB Entry DOI: 10.7270/Q2862F09 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038677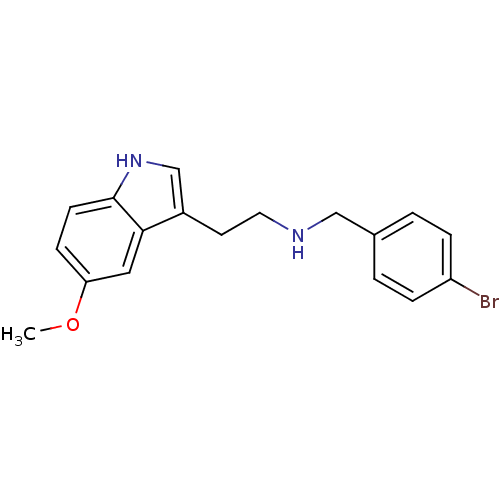 ((4-Bromo-benzyl)-[2-(5-methoxy-1H-indol-3-yl)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581204 (CHEMBL5076637) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581192 (CHEMBL5091461) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581209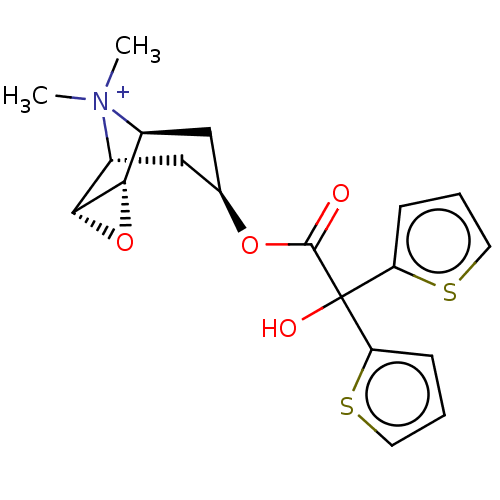 (CHEMBL4650755) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581203 (CHEMBL5074599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581203 (CHEMBL5074599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50144512 (1-(5,5-Diphenyl-pent-4-enyl)-4-methyl-piperidine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand | Bioorg Med Chem Lett 14: 2217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.018 BindingDB Entry DOI: 10.7270/Q2R210T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50236715 (CHEMBL4067210) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 | J Med Chem 60: 2605-2628 (2017) Article DOI: 10.1021/acs.jmedchem.7b00085 BindingDB Entry DOI: 10.7270/Q2DJ5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50236730 (CHEMBL4072342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against human progesterone receptor (hPR) in a competitive binding assay | J Med Chem 60: 2605-2628 (2017) Article DOI: 10.1021/acs.jmedchem.7b00085 BindingDB Entry DOI: 10.7270/Q2DJ5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581185 (CHEMBL5076558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581187 (CHEMBL5077161) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50581209 (CHEMBL4650755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581189 (CHEMBL5075132) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50041259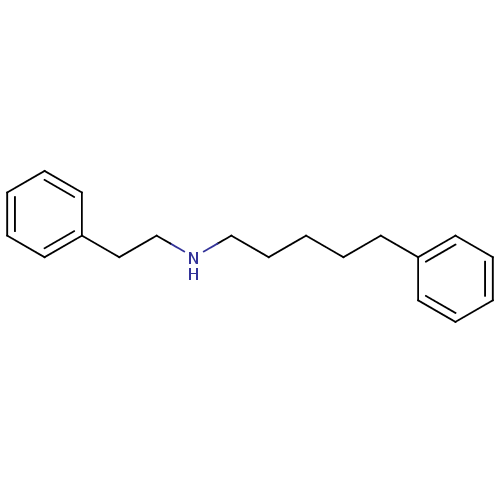 (CHEMBL19355 | Phenethyl-(5-phenyl-pentyl)-amine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand | Bioorg Med Chem Lett 14: 2217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.018 BindingDB Entry DOI: 10.7270/Q2R210T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50041259 (CHEMBL19355 | Phenethyl-(5-phenyl-pentyl)-amine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand | J Med Chem 37: 1214-9 (1994) BindingDB Entry DOI: 10.7270/Q29P30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50041268 (Benzyl-methyl-(5-phenyl-pentyl)-amine | CHEMBL1931...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand | J Med Chem 37: 1214-9 (1994) BindingDB Entry DOI: 10.7270/Q29P30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581193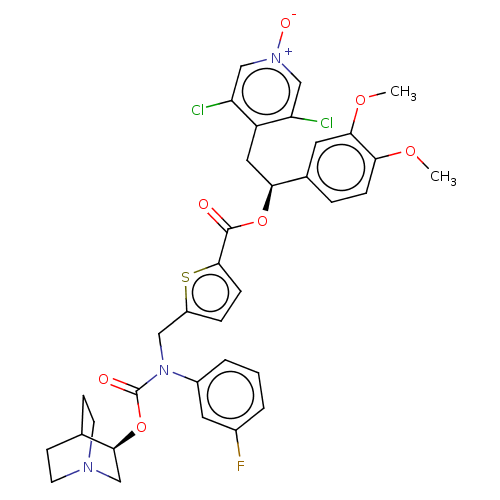 (CHEMBL5084383) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50581203 (CHEMBL5074599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581190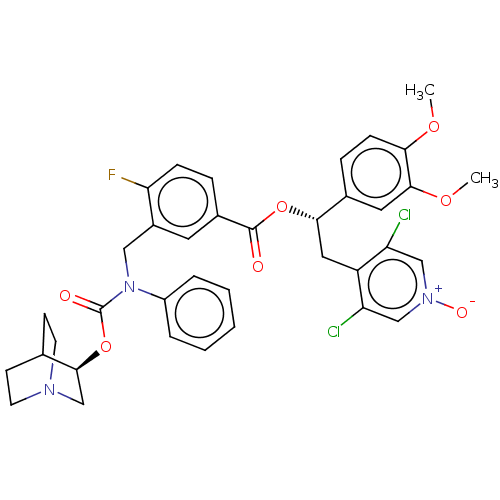 (CHEMBL5076266) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581202 (CHEMBL5090464) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for the binding affinity to hippocampus striatal membranes at 5-hydroxytryptamine 1A receptor binding site by using [3H]-8-OH- DPAT as a ra... | J Med Chem 30: 1-12 (1987) BindingDB Entry DOI: 10.7270/Q29K4BS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50064708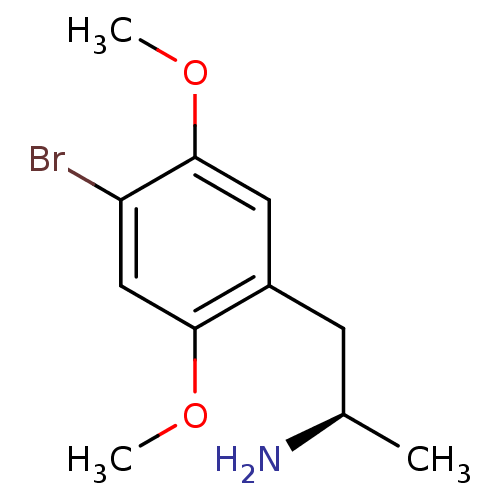 ((R)-1-(4-bromo-2,5-dimethoxyphenyl)propan-2-amine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for 5-HT2A serotonin receptor | J Med Chem 47: 6034-41 (2004) Article DOI: 10.1021/jm040082s BindingDB Entry DOI: 10.7270/Q2R78DQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Tested for binding affinity against dopamine receptor D2 | J Med Chem 36: 2519-25 (1993) BindingDB Entry DOI: 10.7270/Q2QF8RZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50064708 ((R)-1-(4-bromo-2,5-dimethoxyphenyl)propan-2-amine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity against Prothrombin | J Med Chem 60: 2605-2628 (2017) Article DOI: 10.1021/acs.jmedchem.7b00085 BindingDB Entry DOI: 10.7270/Q2DJ5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50041246 (CHEMBL19544 | Methyl-phenethyl-(5-phenyl-pentyl)-a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by scintillation spectrometric method | J Med Chem 60: 2605-2628 (2017) Article DOI: 10.1021/acs.jmedchem.7b00085 BindingDB Entry DOI: 10.7270/Q2DJ5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50041246 (CHEMBL19544 | Methyl-phenethyl-(5-phenyl-pentyl)-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand | Bioorg Med Chem Lett 14: 2217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.018 BindingDB Entry DOI: 10.7270/Q2R210T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50041246 (CHEMBL19544 | Methyl-phenethyl-(5-phenyl-pentyl)-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand | J Med Chem 37: 1214-9 (1994) BindingDB Entry DOI: 10.7270/Q29P30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581199 (CHEMBL5090179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50041277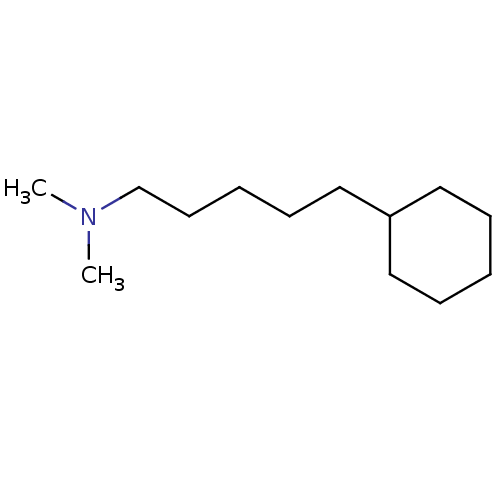 ((5-Cyclohexyl-pentyl)-dimethyl-amine | CHEMBL20036) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand | J Med Chem 37: 1214-9 (1994) BindingDB Entry DOI: 10.7270/Q29P30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50041257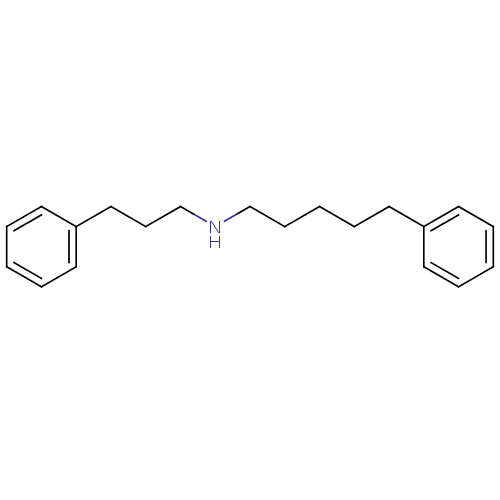 ((5-Phenyl-pentyl)-(3-phenyl-propyl)-amine | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand | J Med Chem 37: 1214-9 (1994) BindingDB Entry DOI: 10.7270/Q29P30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50064708 ((R)-1-(4-bromo-2,5-dimethoxyphenyl)propan-2-amine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor expressed in NIH3T3 cells | J Med Chem 51: 6808-28 (2008) Article DOI: 10.1021/jm800771x BindingDB Entry DOI: 10.7270/Q2KD1ZTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50041275 (CHEMBL20348 | Methyl-(5-phenyl-pentyl)-propyl-amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from guinea pig brain membrane sigma1 receptor by scintillation spectrometric method | J Med Chem 60: 2605-2628 (2017) Article DOI: 10.1021/acs.jmedchem.7b00085 BindingDB Entry DOI: 10.7270/Q2DJ5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50041275 (CHEMBL20348 | Methyl-(5-phenyl-pentyl)-propyl-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand | J Med Chem 37: 1214-9 (1994) BindingDB Entry DOI: 10.7270/Q29P30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50169599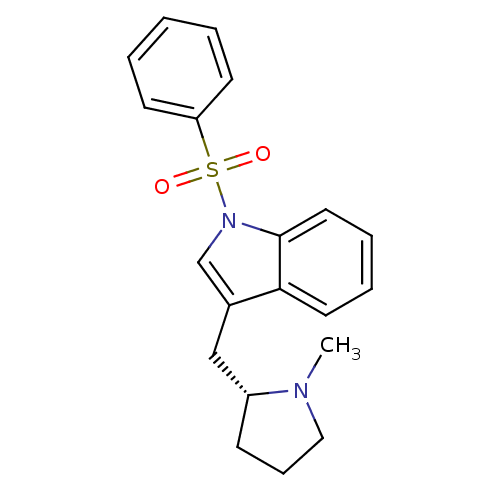 (1-Benzenesulfonyl-3-((R)-1-methyl-pyrrolidin-2-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 51: 603-11 (2008) Article DOI: 10.1021/jm070910s BindingDB Entry DOI: 10.7270/Q2CJ8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50143487 (CHEMBL353552 | [9-(2,5-Dimethoxy-benzenesulfonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ability to displace [3H]LSD from human 5-hydroxytryptamine 6 receptor transiently expressed in COS-7 cells | Bioorg Med Chem Lett 14: 1961-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.071 BindingDB Entry DOI: 10.7270/Q29887J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50130285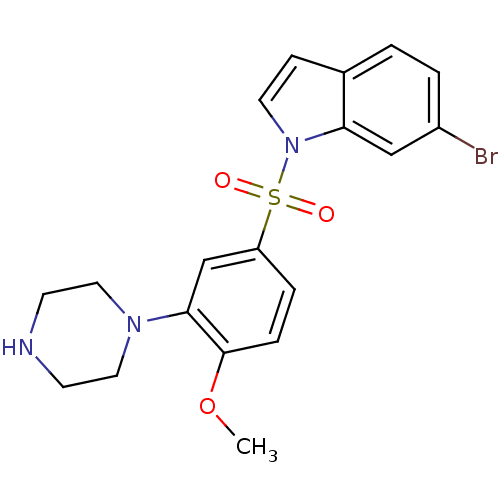 (6-Bromo-1-(4-methoxy-3-piperazin-1-yl-benzenesulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 6 receptor | J Med Chem 46: 2795-812 (2003) Article DOI: 10.1021/jm030030n BindingDB Entry DOI: 10.7270/Q2M0465F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50169599 (1-Benzenesulfonyl-3-((R)-1-methyl-pyrrolidin-2-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against h5-HT6 receptor transiently expressed in HEK293 cells | Bioorg Med Chem Lett 15: 3510-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.092 BindingDB Entry DOI: 10.7270/Q20Z7411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50143274 (3-(4-Methyl-isoxazol-5-ylmethyl)-1-aza-bicyclo[2.2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards nicotinic acetylcholine receptor alpha4-beta2 | Bioorg Med Chem Lett 14: 1841-4 (2004) Article DOI: 10.1016/j.bmcl.2003.07.035 BindingDB Entry DOI: 10.7270/Q2MC917Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50151705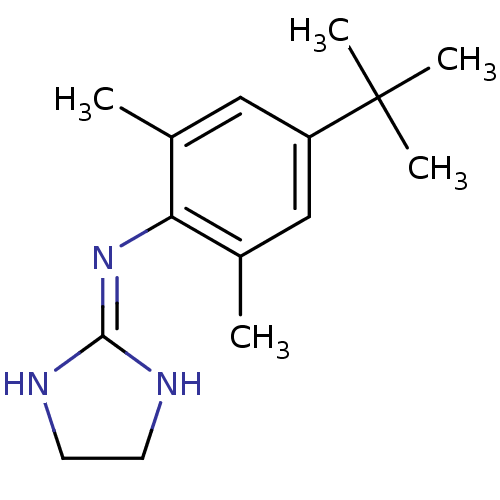 ((4-tert-Butyl-2,6-dimethyl-phenyl)-(4,5-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor | Bioorg Med Chem Lett 14: 4697-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.085 BindingDB Entry DOI: 10.7270/Q2FJ2G7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50156192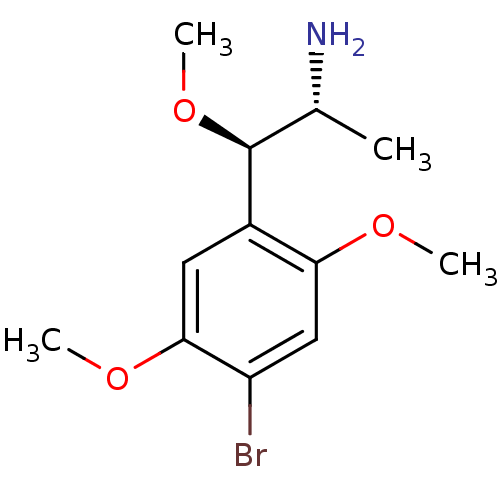 ((1R,2R)-2-(4-Bromo-2,5-dimethoxy-phenyl)-2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from rat 5HT2A after 1 hr by beta scintillation counting method | J Med Chem 60: 2605-2628 (2017) Article DOI: 10.1021/acs.jmedchem.7b00085 BindingDB Entry DOI: 10.7270/Q2DJ5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4648 total ) | Next | Last >> |