

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479680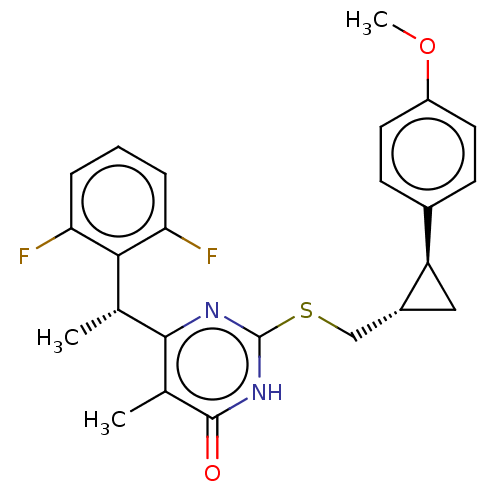 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant free reverse transcriptase K103N mutant expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant free reverse transcriptase K103N mutant expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant free reverse transcriptase K103N mutant expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant free reverse transcriptase K103N mutant expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50151798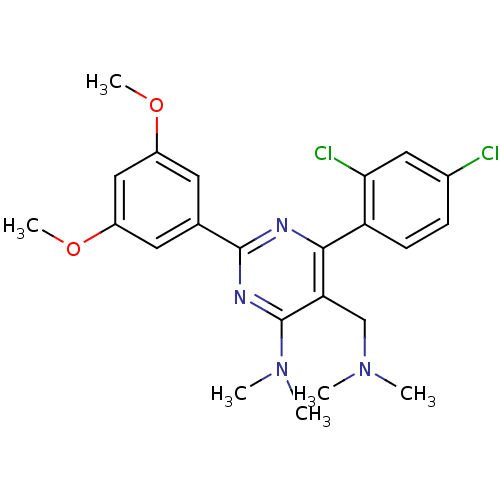 (CHEMBL186877 | [6-(2,4-Dichloro-phenyl)-2-(3,5-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibitory concentration against Dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4759-62 (2004) Article DOI: 10.1016/j.bmcl.2004.06.099 BindingDB Entry DOI: 10.7270/Q2XK8F1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50485779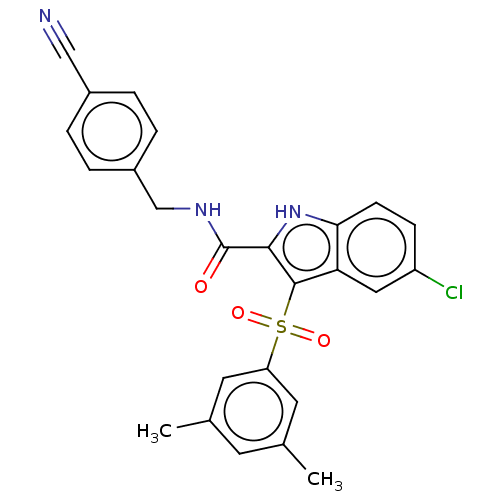 (CHEMBL2163850) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50485778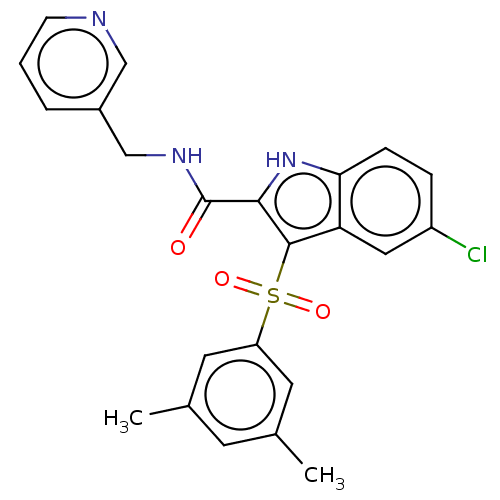 (CHEMBL2163488) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50485779 (CHEMBL2163850) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485779 (CHEMBL2163850) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50485781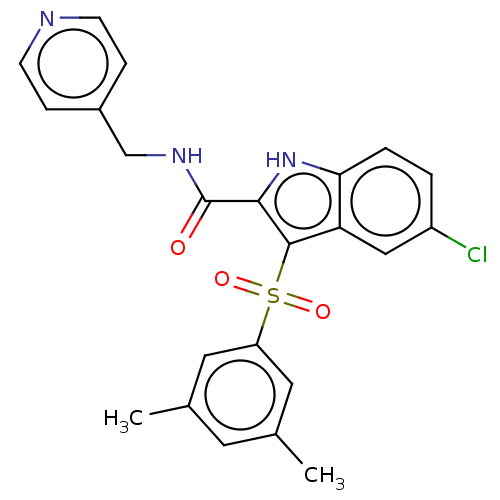 (CHEMBL2163842) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM161942 (US9682991, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
FUNDACIÓN CENTRO NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III US Patent | Assay Description The biochemical assay to measure PIM-1 activity relies on the ADP Hunter assay kit (DiscoveRx Corp., Cat. #90-0077), that determines the amount of AD... | US Patent US9682991 (2017) BindingDB Entry DOI: 10.7270/Q2513WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485778 (CHEMBL2163488) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485781 (CHEMBL2163842) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50223140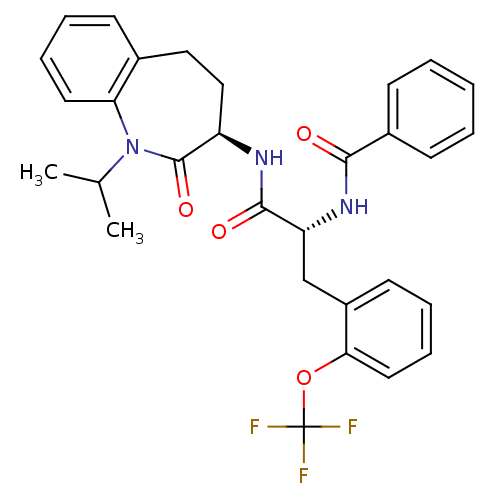 (CHEMBL429828 | N-((R)-1-((R)-1-isopropyl-2-oxo-2,3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human sodium channel Nav1.7 by FRET assay | Bioorg Med Chem Lett 17: 6172-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.032 BindingDB Entry DOI: 10.7270/Q2X92B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50485782 (CHEMBL2163845) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM161946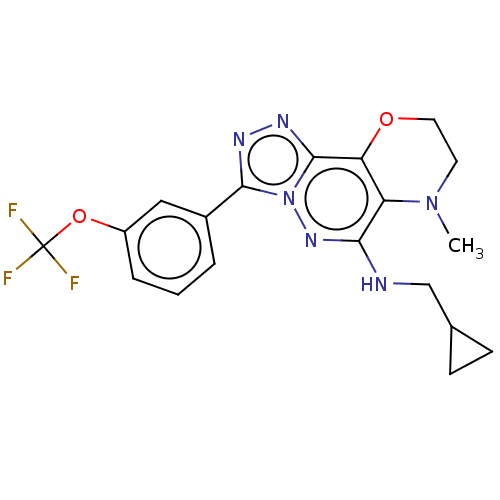 (US9682991, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
FUNDACIÓN CENTRO NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III US Patent | Assay Description The biochemical assay to measure PIM-1 activity relies on the ADP Hunter assay kit (DiscoveRx Corp., Cat. #90-0077), that determines the amount of AD... | US Patent US9682991 (2017) BindingDB Entry DOI: 10.7270/Q2513WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50223136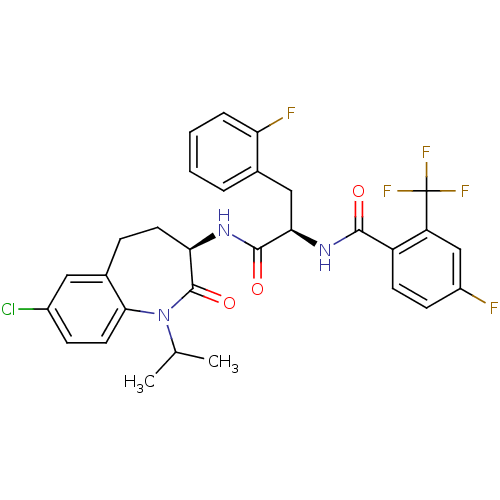 (CHEMBL249360 | N-((R)-1-((R)-7-chloro-1-isopropyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human sodium channel Nav1.7 by FRET assay | Bioorg Med Chem Lett 17: 6172-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.032 BindingDB Entry DOI: 10.7270/Q2X92B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50485778 (CHEMBL2163488) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50216671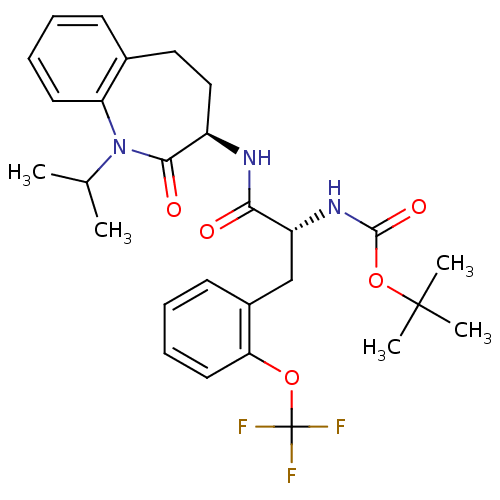 (CHEMBL247828 | tert-butyl (R)-1-((R)-1-isopropyl-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human sodium channel Nav1.7 by FRET assay | Bioorg Med Chem Lett 17: 6172-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.032 BindingDB Entry DOI: 10.7270/Q2X92B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50485780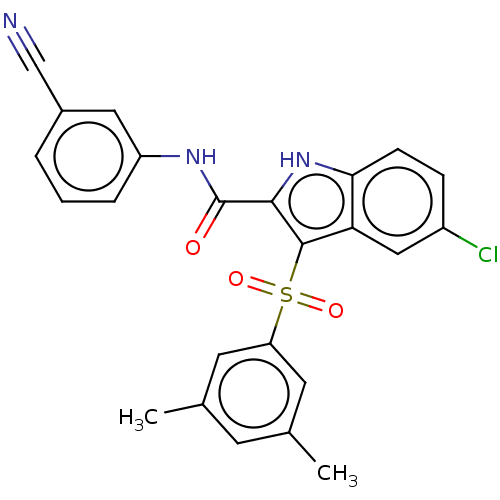 (CHEMBL2163848) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485782 (CHEMBL2163845) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50223139 (CHEMBL249358 | tert-butyl (R)-1-((R)-5-isopropyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human sodium channel Nav1.7 by FRET assay | Bioorg Med Chem Lett 17: 6172-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.032 BindingDB Entry DOI: 10.7270/Q2X92B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50485781 (CHEMBL2163842) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM161950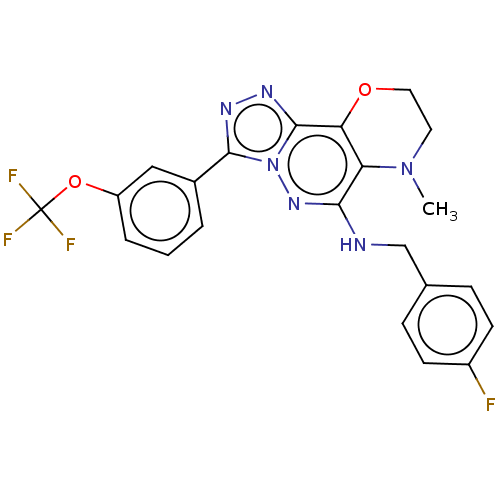 (US9682991, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
FUNDACIÓN CENTRO NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III US Patent | Assay Description The biochemical assay to measure PIM-1 activity relies on the ADP Hunter assay kit (DiscoveRx Corp., Cat. #90-0077), that determines the amount of AD... | US Patent US9682991 (2017) BindingDB Entry DOI: 10.7270/Q2513WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50223150 (CHEMBL250756 | tert-butyl (R)-1-(9-isopropyl-8-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human sodium channel Nav1.7 by FRET assay | Bioorg Med Chem Lett 17: 6172-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.032 BindingDB Entry DOI: 10.7270/Q2X92B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrate | J Med Chem 55: 6634-8 (2012) Article DOI: 10.1021/jm300477h BindingDB Entry DOI: 10.7270/Q24T6N7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50223153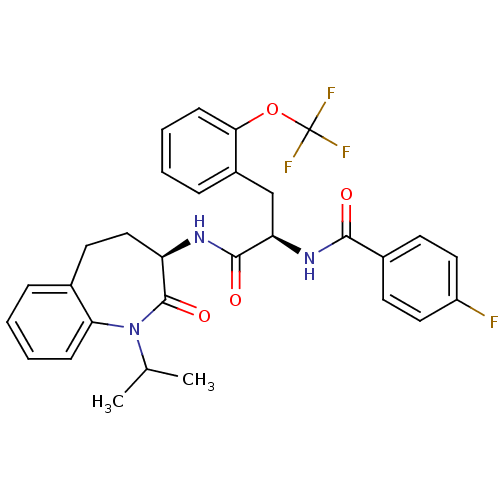 (4-fluoro-N-((R)-1-((R)-1-isopropyl-2-oxo-2,3,4,5-t...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human sodium channel Nav1.7 by FRET assay | Bioorg Med Chem Lett 17: 6172-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.032 BindingDB Entry DOI: 10.7270/Q2X92B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 489 total ) | Next | Last >> |