

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354647 (CHEMBL1834244) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354644 (CHEMBL1834218) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266025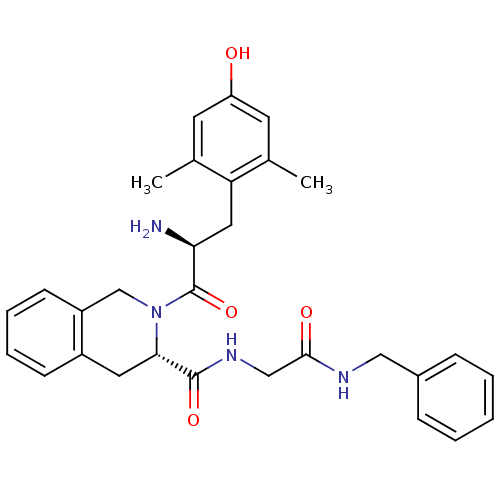 ((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354650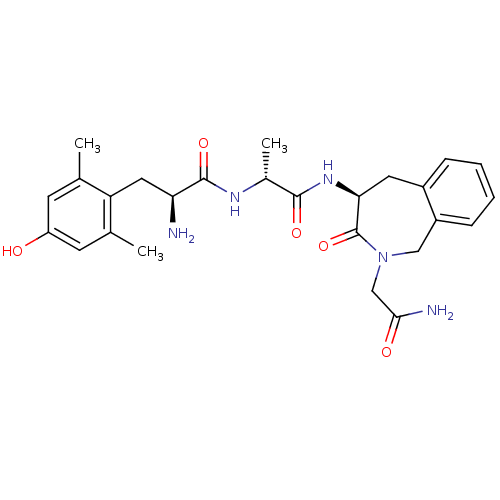 (CHEMBL1834247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266025 ((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50079527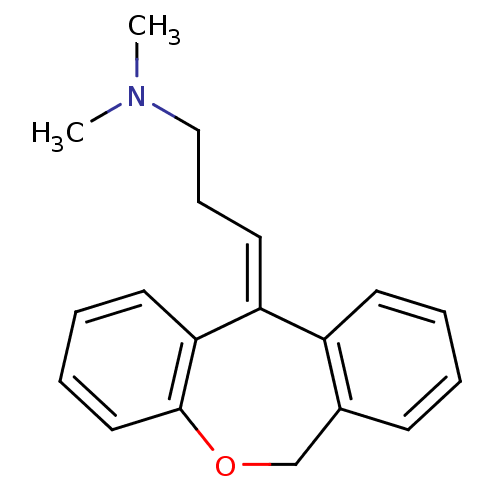 ((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8195-206 (2011) Article DOI: 10.1021/jm2011589 BindingDB Entry DOI: 10.7270/Q2QF8T85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM104692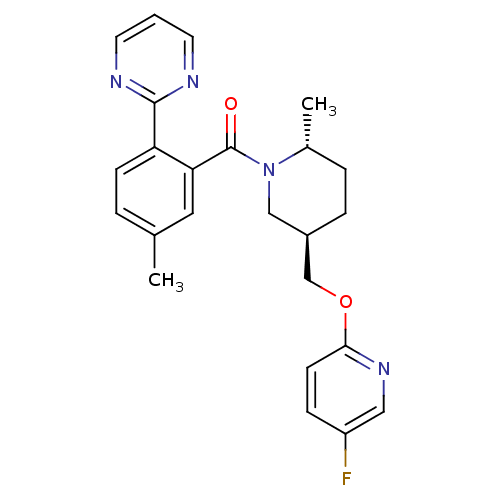 (US8569311, E-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50079527 ((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human wild type N-terminal hemagglutinin-tagged histamine H1 receptor expressed in HEK293T cells after 4 hrs by m... | J Med Chem 59: 9047-9061 (2016) Article DOI: 10.1021/acs.jmedchem.6b00981 BindingDB Entry DOI: 10.7270/Q2G44S8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354649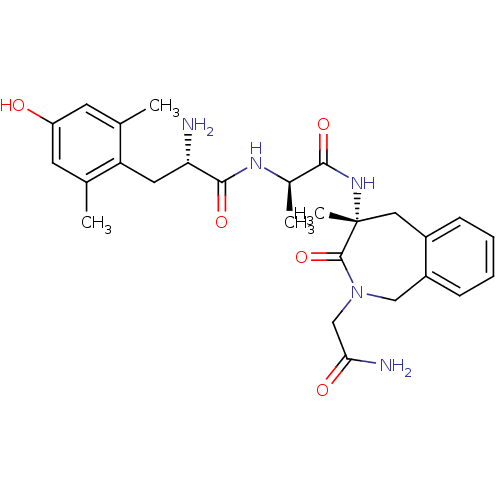 (CHEMBL1834246) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354651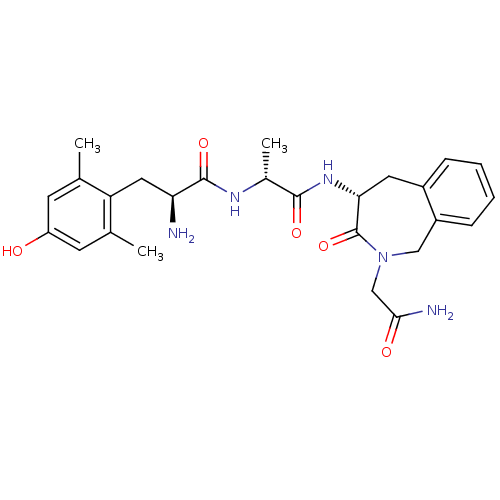 (CHEMBL1834248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50031228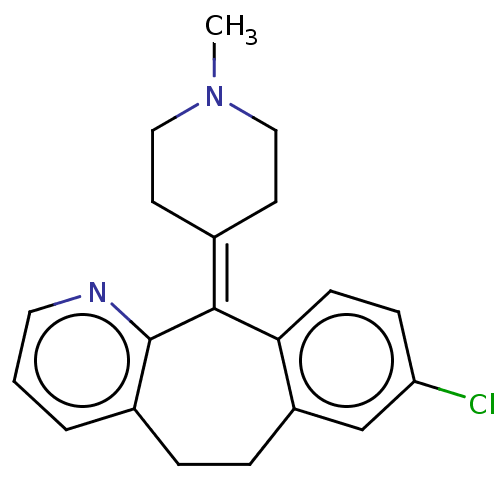 (CHEMBL420316) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354645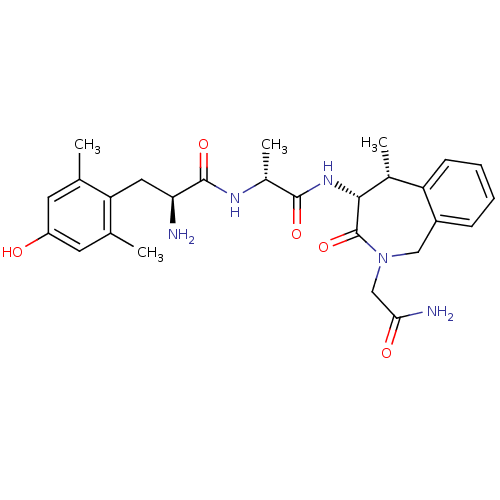 (CHEMBL1834219) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516710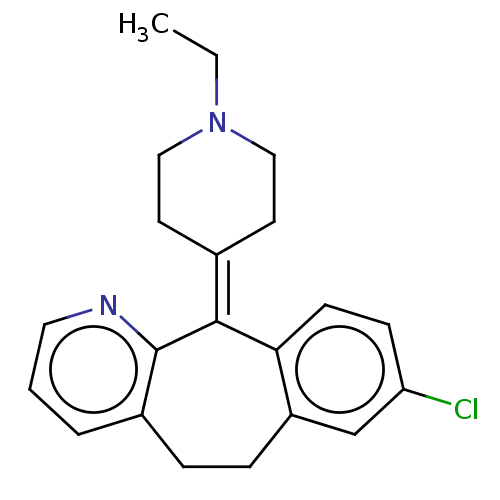 (CHEMBL4469024) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50189920 ((S)-2-amino-N-((S)-2-(2-(benzylamino)-2-oxoethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093793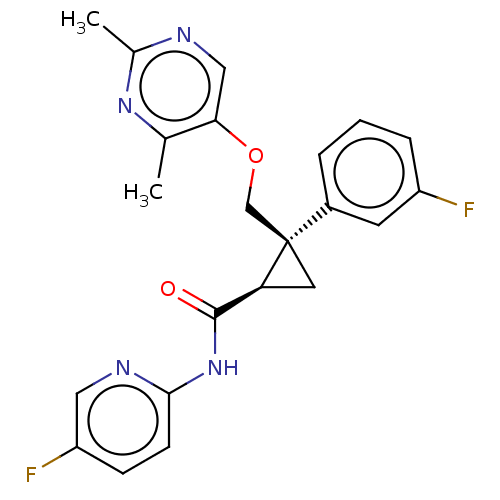 (E-2006 | Lemborexant) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50335557 (CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM104692 (US8569311, E-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM154947 (US9000029, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516713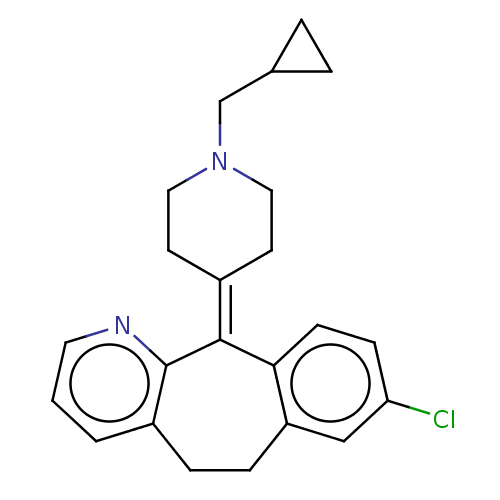 (CHEMBL4448535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50427452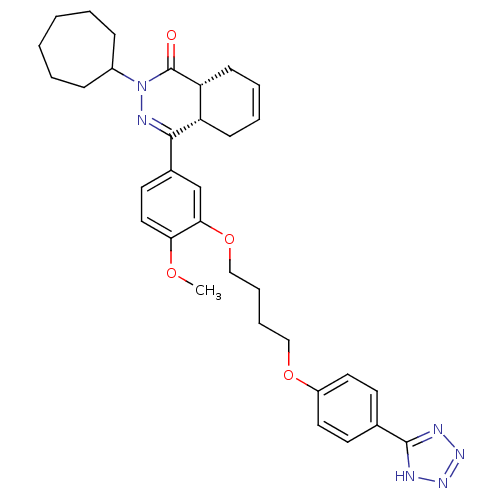 (CHEMBL2326941) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22916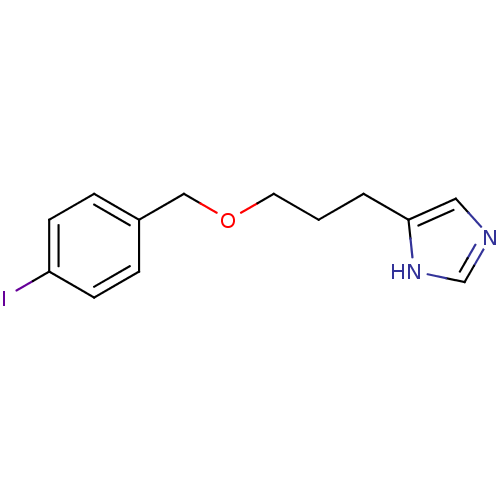 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516707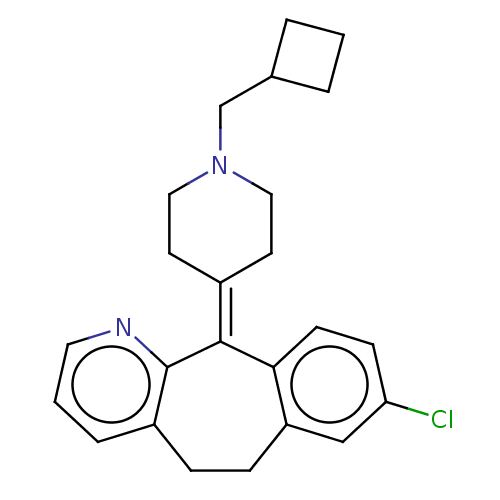 (CHEMBL4588983) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516717 (CHEMBL4582006) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419448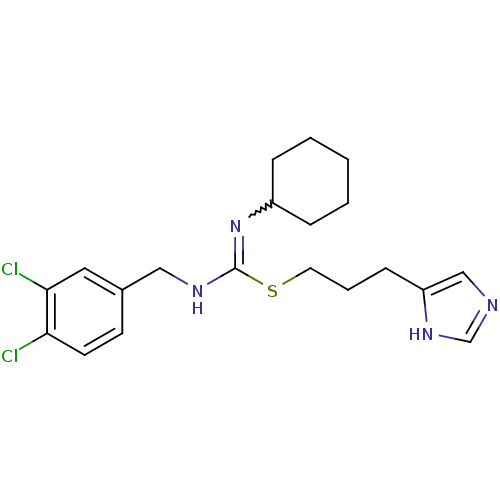 (CHEMBL1923026) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419444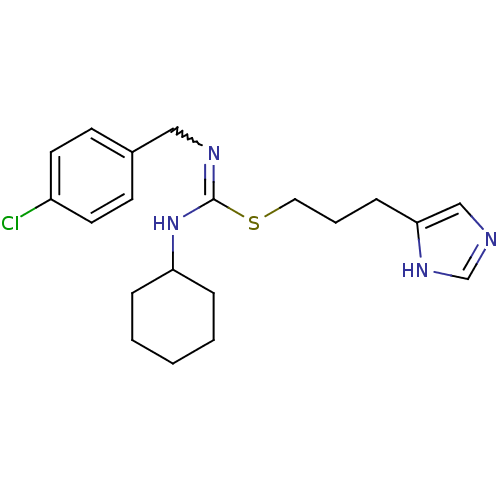 (CHEMBL43934 | VUF-5228) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419456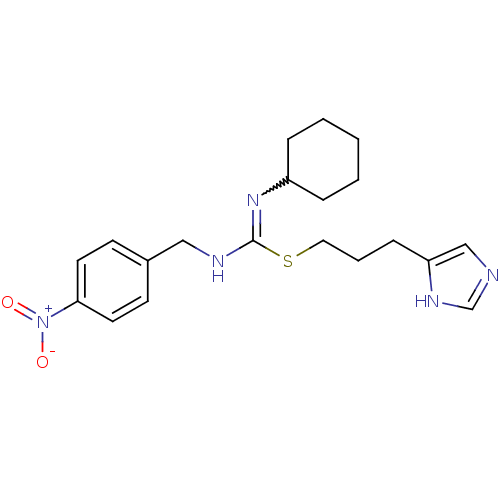 (CHEMBL1923034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human wild type N-terminal hemagglutinin-tagged histamine H1 receptor expressed in HEK293T cells after 4 hrs by m... | J Med Chem 59: 9047-9061 (2016) Article DOI: 10.1021/acs.jmedchem.6b00981 BindingDB Entry DOI: 10.7270/Q2G44S8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516716 (CHEMBL4474226) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM334973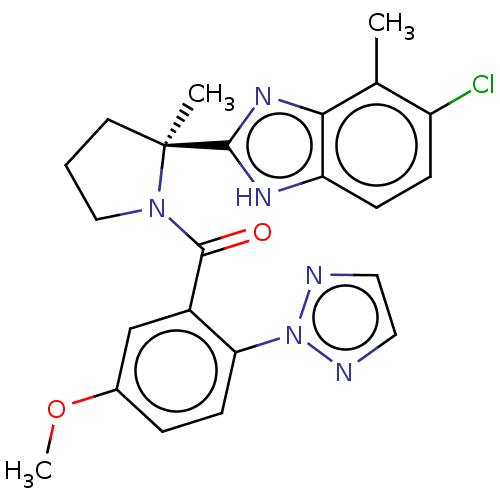 (US11040966, Example 5.36 | US9732075, Example 5.36...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50414391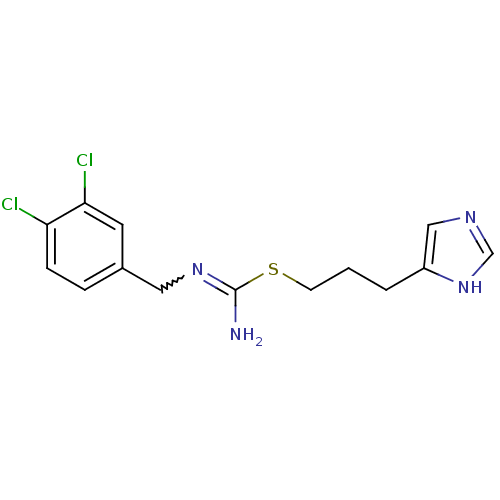 (CHEMBL1202332 | CHEMBL553423) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Agonistic activity at histamine H4 receptor | J Med Chem 54: 1693-703 (2011) Article DOI: 10.1021/jm1013488 BindingDB Entry DOI: 10.7270/Q20G3MDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50419048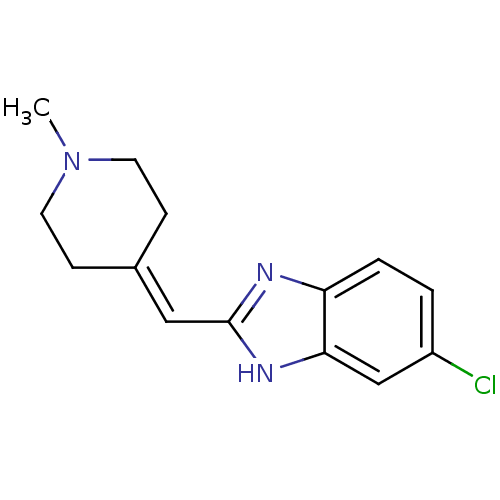 (CHEMBL1824048) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5-HT3AR expressed in HEK293 cells after 24 hrs by liquid scintillation counting | Bioorg Med Chem Lett 21: 5460-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.123 BindingDB Entry DOI: 10.7270/Q2ZC844K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM334973 (US11040966, Example 5.36 | US9732075, Example 5.36...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50073179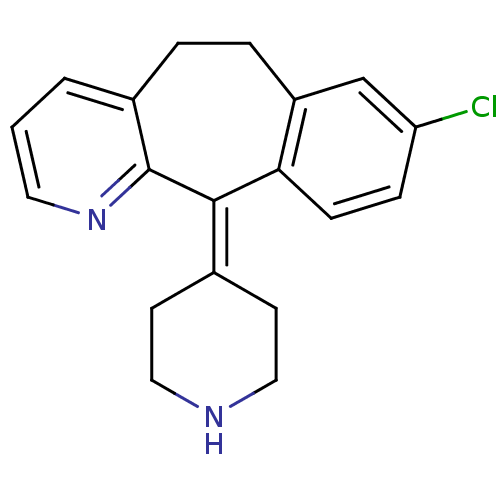 (8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419455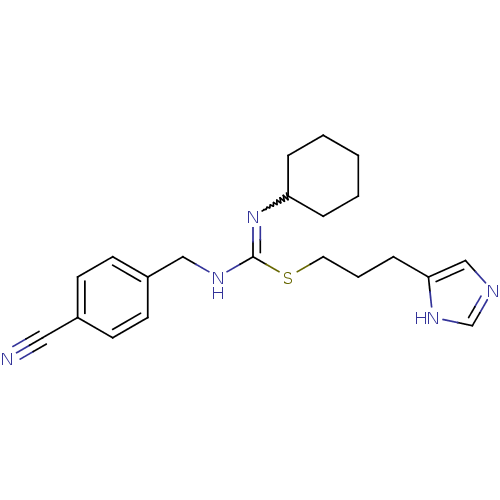 (CHEMBL1923033) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354644 (CHEMBL1834218) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]Ile-deltorphin-2 from rat brain delta-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516706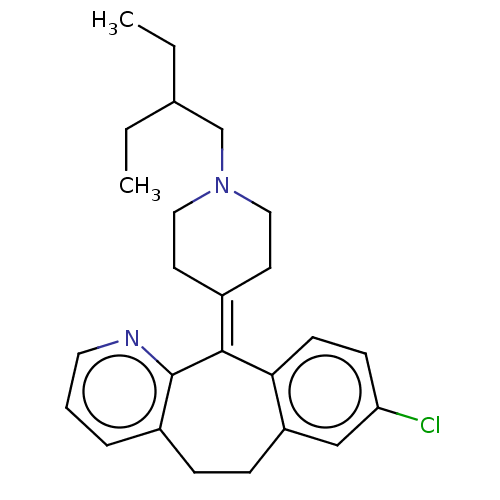 (CHEMBL4514700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50202722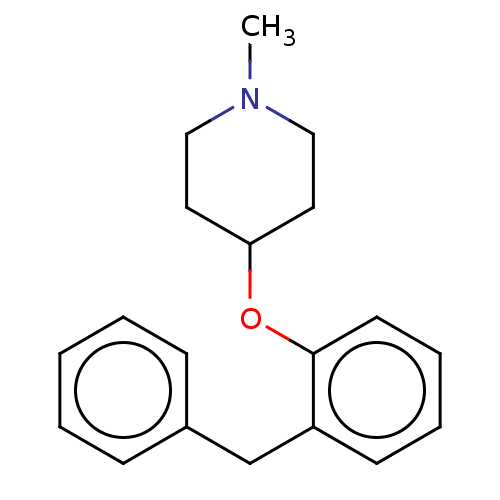 (CHEMBL3906929) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human wild type N-terminal hemagglutinin-tagged histamine H1 receptor expressed in HEK293T cells after 4 hrs by m... | J Med Chem 59: 9047-9061 (2016) Article DOI: 10.1021/acs.jmedchem.6b00981 BindingDB Entry DOI: 10.7270/Q2G44S8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50520181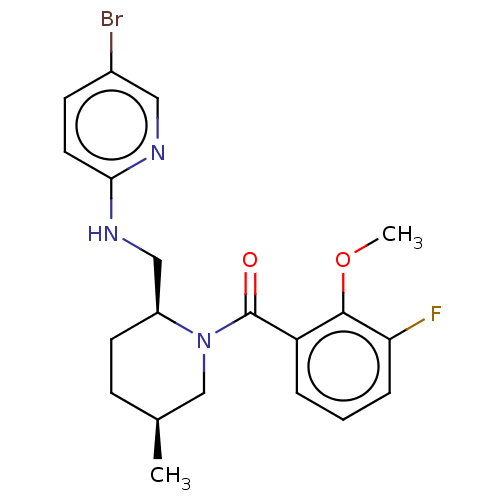 (CHEMBL2413522) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8195-206 (2011) Article DOI: 10.1021/jm2011589 BindingDB Entry DOI: 10.7270/Q2QF8T85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516708 (CHEMBL4581343) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354650 (CHEMBL1834247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]Ile-deltorphin-2 from rat brain delta-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50202830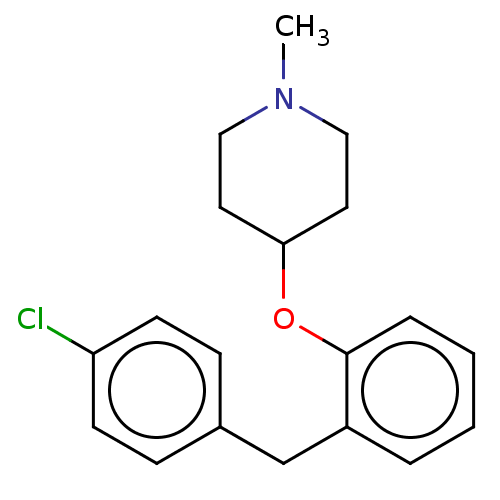 (CHEMBL3905009) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human wild type N-terminal hemagglutinin-tagged histamine H1 receptor expressed in HEK293T cells after 4 hrs by m... | J Med Chem 59: 9047-9061 (2016) Article DOI: 10.1021/acs.jmedchem.6b00981 BindingDB Entry DOI: 10.7270/Q2G44S8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50093793 (E-2006 | Lemborexant) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... | J Med Chem 63: 1528-1543 (2020) Article DOI: 10.1021/acs.jmedchem.9b01787 BindingDB Entry DOI: 10.7270/Q2474F8R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50516719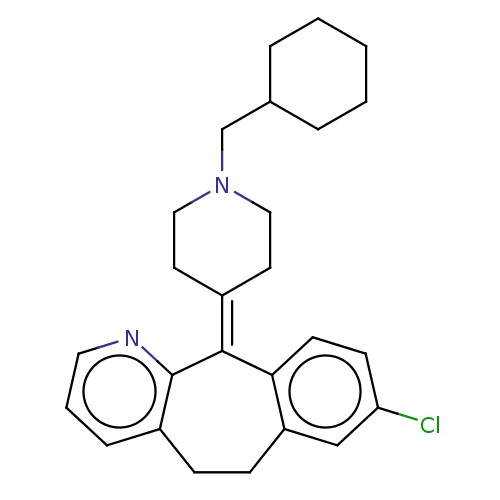 (CHEMBL4554533) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... | J Med Chem 62: 6630-6644 (2019) Article DOI: 10.1021/acs.jmedchem.9b00447 BindingDB Entry DOI: 10.7270/Q2HH6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 946 total ) | Next | Last >> |