 Found 251 hits with Last Name = 'grbic' and Initial = 'h'
Found 251 hits with Last Name = 'grbic' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518185

(CHEMBL4571977)Show SMILES CC(C)(O)c1ccc2OCc3ncccc3\C(=C\CCN3CC[C@](O)(c4ccc(Cl)cc4)C(C)(C)C3)c2c1 |r| Show InChI InChI=1S/C32H37ClN2O3/c1-30(2)21-35(18-15-32(30,37)22-9-12-24(33)13-10-22)17-6-8-25-26-7-5-16-34-28(26)20-38-29-14-11-23(19-27(25)29)31(3,4)36/h5,7-14,16,19,36-37H,6,15,17-18,20-21H2,1-4H3/b25-8-/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50208999
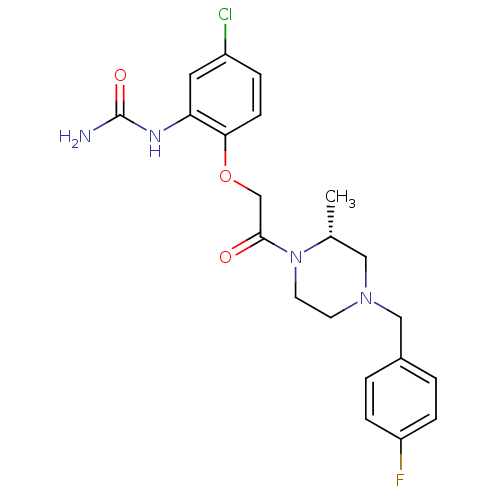
((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O |r| Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 in human THP1 cells assessed as inhibition of chemotaxis after 30 mins by Celltiter-glo reagent based luminescence assay |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50208999

((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O |r| Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056504

(CHEMBL3334824)Show SMILES CC(C)[C@@H](NC(=O)NCC(C)(C)O)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H36ClN3O4/c1-15(2)18(26-20(29)25-13-22(5,6)30)19(28)27-12-11-23(31,21(3,4)14-27)16-7-9-17(24)10-8-16/h7-10,15,18,30-31H,11-14H2,1-6H3,(H2,25,26,29)/t18-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 in human THP1 cells assessed as inhibition of chemotaxis after 30 mins by Celltiter-glo reagent based luminescence assay |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518185

(CHEMBL4571977)Show SMILES CC(C)(O)c1ccc2OCc3ncccc3\C(=C\CCN3CC[C@](O)(c4ccc(Cl)cc4)C(C)(C)C3)c2c1 |r| Show InChI InChI=1S/C32H37ClN2O3/c1-30(2)21-35(18-15-32(30,37)22-9-12-24(33)13-10-22)17-6-8-25-26-7-5-16-34-28(26)20-38-29-14-11-23(19-27(25)29)31(3,4)36/h5,7-14,16,19,36-37H,6,15,17-18,20-21H2,1-4H3/b25-8-/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 in human THP1 cells assessed as inhibition of chemotaxis after 30 mins by Celltiter-glo reagent based luminescence assay |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518184
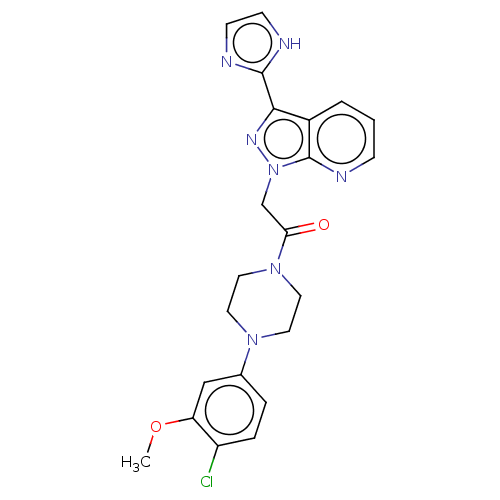
(CHEMBL4444976)Show SMILES COc1cc(ccc1Cl)N1CCN(CC1)C(=O)Cn1nc(-c2ncc[nH]2)c2cccnc12 Show InChI InChI=1S/C22H22ClN7O2/c1-32-18-13-15(4-5-17(18)23)28-9-11-29(12-10-28)19(31)14-30-22-16(3-2-6-26-22)20(27-30)21-24-7-8-25-21/h2-8,13H,9-12,14H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196941
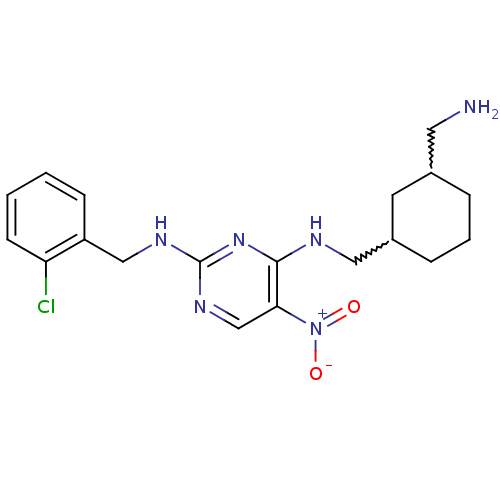
(CHEMBL246365 | N2-(2-chlorobenzyl)-N4-((3-(aminome...)Show SMILES NCC1CCCC(CNc2nc(NCc3ccccc3Cl)ncc2[N+]([O-])=O)C1 |w:6.6,2.1| Show InChI InChI=1S/C19H25ClN6O2/c20-16-7-2-1-6-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-5-3-4-13(8-14)9-21/h1-2,6-7,12-14H,3-5,8-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056504

(CHEMBL3334824)Show SMILES CC(C)[C@@H](NC(=O)NCC(C)(C)O)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H36ClN3O4/c1-15(2)18(26-20(29)25-13-22(5,6)30)19(28)27-12-11-23(31,21(3,4)14-27)16-7-9-17(24)10-8-16/h7-10,15,18,30-31H,11-14H2,1-6H3,(H2,25,26,29)/t18-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518184

(CHEMBL4444976)Show SMILES COc1cc(ccc1Cl)N1CCN(CC1)C(=O)Cn1nc(-c2ncc[nH]2)c2cccnc12 Show InChI InChI=1S/C22H22ClN7O2/c1-32-18-13-15(4-5-17(18)23)28-9-11-29(12-10-28)19(31)14-30-22-16(3-2-6-26-22)20(27-30)21-24-7-8-25-21/h2-8,13H,9-12,14H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 in human THP1 cells assessed as inhibition of chemotaxis after 30 mins by Celltiter-glo reagent based luminescence assay |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196957
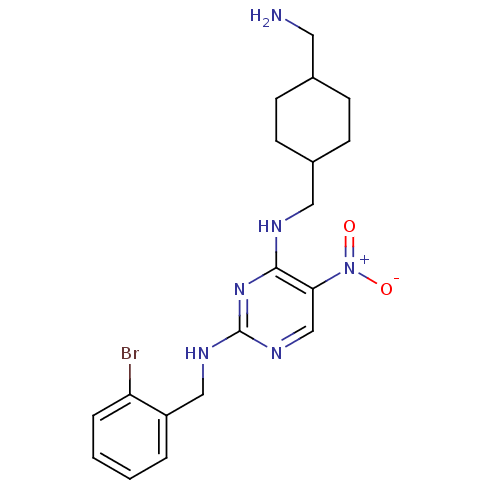
(CHEMBL246175 | N2-(2-bromobenzyl)-N4-((4-(aminomet...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3Br)ncc2[N+]([O-])=O)CC1 |(5.93,-24.27,;5.93,-22.73,;4.6,-21.95,;3.26,-22.72,;1.93,-21.95,;1.94,-20.42,;.61,-19.65,;.6,-18.11,;-.73,-17.34,;-2.07,-18.11,;-3.4,-17.34,;-4.74,-18.11,;-6.07,-17.34,;-7.41,-18.11,;-8.74,-17.33,;-10.07,-18.1,;-10.07,-19.64,;-8.73,-20.41,;-7.4,-19.64,;-6.07,-20.41,;-3.4,-15.8,;-2.07,-15.03,;-.74,-15.79,;.6,-15.01,;1.93,-15.78,;.59,-13.47,;3.26,-19.63,;4.6,-20.41,)| Show InChI InChI=1S/C19H25BrN6O2/c20-16-4-2-1-3-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-7-5-13(9-21)6-8-14/h1-4,12-14H,5-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196932

(CHEMBL392642 | N2-(2-(methylthio)benzyl)-N4-((4-(a...)Show SMILES CSc1ccccc1CNc1ncc(c(NCC2CCC(CN)CC2)n1)[N+]([O-])=O |(25.65,-13.62,;25.64,-12.08,;24.31,-11.32,;22.98,-12.09,;21.64,-11.32,;21.64,-9.78,;22.97,-9.01,;24.3,-9.79,;25.64,-9.02,;26.97,-9.79,;28.31,-9.02,;28.31,-7.47,;29.64,-6.7,;30.97,-7.47,;30.98,-9.02,;32.31,-9.79,;32.32,-11.33,;33.65,-12.09,;33.64,-13.63,;34.97,-14.4,;36.31,-13.63,;37.64,-14.4,;37.64,-15.94,;36.31,-12.09,;34.97,-11.31,;29.64,-9.79,;32.31,-6.69,;33.64,-7.45,;32.3,-5.15,)| Show InChI InChI=1S/C20H28N6O2S/c1-29-18-5-3-2-4-16(18)12-23-20-24-13-17(26(27)28)19(25-20)22-11-15-8-6-14(10-21)7-9-15/h2-5,13-15H,6-12,21H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196961
(CHEMBL246174 | N2-(2-(trifluoromethylthio)benzyl)-...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3SC(F)(F)F)ncc2[N+]([O-])=O)CC1 |(21.67,-14.36,;21.67,-12.82,;20.34,-12.05,;19,-12.82,;17.68,-12.05,;17.68,-10.51,;16.35,-9.75,;16.35,-8.21,;15.01,-7.44,;13.67,-8.21,;12.34,-7.44,;11,-8.21,;9.67,-7.44,;8.34,-8.21,;7.01,-7.43,;5.67,-8.2,;5.67,-9.74,;7.01,-10.51,;8.34,-9.74,;9.68,-10.51,;9.68,-12.05,;9.67,-13.58,;11.22,-12.05,;8.14,-12.05,;12.34,-5.9,;13.67,-5.12,;15,-5.89,;16.34,-5.11,;17.68,-5.88,;16.33,-3.57,;19.01,-9.73,;20.34,-10.51,)| Show InChI InChI=1S/C20H25F3N6O2S/c21-20(22,23)32-17-4-2-1-3-15(17)11-26-19-27-12-16(29(30)31)18(28-19)25-10-14-7-5-13(9-24)6-8-14/h1-4,12-14H,5-11,24H2,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196936
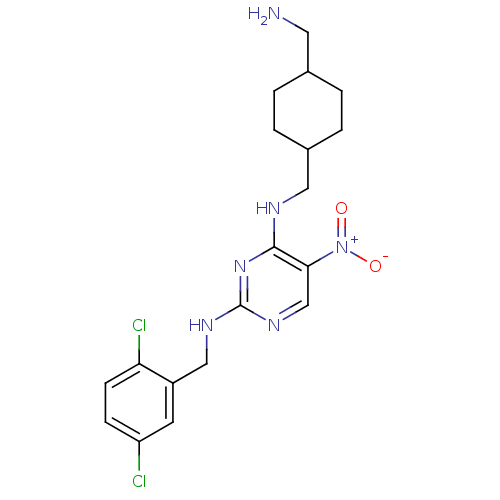
(CHEMBL246386 | N2-(2,5-dichlorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3cc(Cl)ccc3Cl)ncc2[N+]([O-])=O)CC1 |(6.39,-4.5,;6.4,-2.96,;5.07,-2.19,;3.73,-2.95,;2.4,-2.19,;2.41,-.65,;1.07,.12,;1.07,1.66,;-.27,2.42,;-1.6,1.65,;-2.94,2.42,;-4.27,1.65,;-5.6,2.42,;-6.94,1.66,;-8.27,2.43,;-9.6,1.66,;-10.94,2.43,;-9.61,.12,;-8.26,-.65,;-6.94,.12,;-5.6,-.64,;-2.93,3.97,;-1.61,4.74,;-.27,3.97,;1.06,4.75,;2.4,3.99,;1.06,6.29,;3.73,.13,;5.07,-.65,)| Show InChI InChI=1S/C19H24Cl2N6O2/c20-15-5-6-16(21)14(7-15)10-24-19-25-11-17(27(28)29)18(26-19)23-9-13-3-1-12(8-22)2-4-13/h5-7,11-13H,1-4,8-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196978

(CHEMBL246183 | N2-(2,3-dichlorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3cccc(Cl)c3Cl)ncc2[N+]([O-])=O)CC1 |(21.13,-47,;21.14,-45.46,;19.8,-44.69,;18.47,-45.45,;17.14,-44.68,;17.15,-43.15,;15.81,-42.38,;15.81,-40.84,;14.47,-40.07,;13.14,-40.85,;11.8,-40.08,;10.47,-40.84,;9.13,-40.07,;7.8,-40.84,;6.47,-40.07,;5.14,-40.83,;5.13,-42.38,;6.48,-43.15,;6.48,-44.69,;7.8,-42.38,;9.14,-43.14,;11.8,-38.53,;13.13,-37.76,;14.47,-38.52,;15.8,-37.75,;17.14,-38.51,;15.8,-36.21,;18.47,-42.37,;19.81,-43.14,)| Show InChI InChI=1S/C19H24Cl2N6O2/c20-15-3-1-2-14(17(15)21)10-24-19-25-11-16(27(28)29)18(26-19)23-9-13-6-4-12(8-22)5-7-13/h1-3,11-13H,4-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
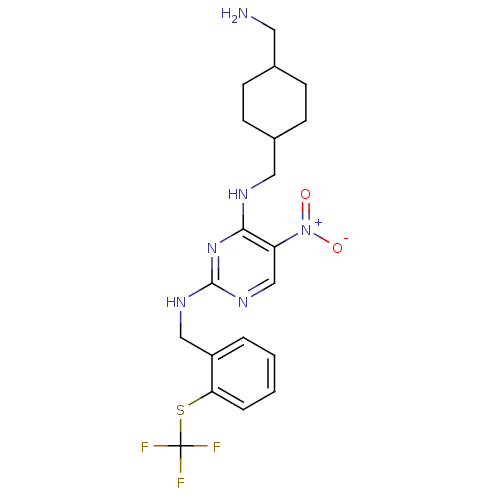
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518200
(CHEMBL4436061)Show SMILES CCC[C@H](NC(=O)c1cnn(c1C)-c1ccc(Cl)cc1)c1cccc(c1F)C(F)(F)F |r| Show InChI InChI=1S/C22H20ClF4N3O/c1-3-5-19(16-6-4-7-18(20(16)24)22(25,26)27)29-21(31)17-12-28-30(13(17)2)15-10-8-14(23)9-11-15/h4,6-12,19H,3,5H2,1-2H3,(H,29,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196959

(CHEMBL396929 | N2-(2-nitrobenzyl)-N4-((4-(aminomet...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3[N+]([O-])=O)ncc2[N+]([O-])=O)CC1 |(36.8,-26.77,;36.8,-25.23,;35.47,-24.46,;34.13,-25.22,;32.8,-24.45,;32.81,-22.92,;31.48,-22.15,;31.47,-20.61,;30.14,-19.84,;28.8,-20.62,;27.47,-19.85,;26.13,-20.61,;24.8,-19.84,;23.46,-20.61,;22.13,-19.84,;20.8,-20.6,;20.8,-22.15,;22.14,-22.92,;23.47,-22.15,;24.8,-22.91,;24.81,-24.46,;26.14,-22.14,;27.47,-18.3,;28.8,-17.53,;30.13,-18.29,;31.47,-17.52,;32.8,-18.28,;31.46,-15.98,;34.13,-22.14,;35.47,-22.91,)| Show InChI InChI=1S/C19H25N7O4/c20-9-13-5-7-14(8-6-13)10-21-18-17(26(29)30)12-23-19(24-18)22-11-15-3-1-2-4-16(15)25(27)28/h1-4,12-14H,5-11,20H2,(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196951
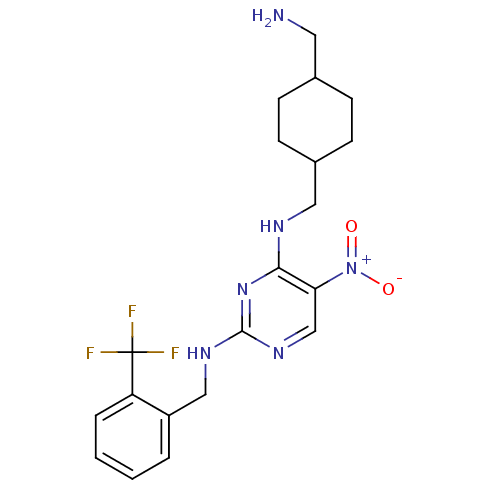
(CHEMBL247979 | N2-(2-(trifluoromethyl)benzyl)-N4-(...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3C(F)(F)F)ncc2[N+]([O-])=O)CC1 |(20.15,-4.22,;20.15,-2.68,;18.82,-1.91,;17.48,-2.67,;16.15,-1.91,;16.16,-.37,;14.82,.4,;14.82,1.94,;13.49,2.7,;12.15,1.93,;10.81,2.7,;9.48,1.93,;8.15,2.7,;6.81,1.94,;5.48,2.71,;4.15,1.94,;4.15,.4,;5.49,-.37,;6.82,.4,;8.15,-.36,;9.48,-1.13,;8.92,.97,;7.39,-1.7,;10.82,4.25,;12.15,5.02,;13.48,4.25,;14.82,5.03,;16.15,4.27,;14.81,6.57,;17.48,.41,;18.82,-.37,)| Show InChI InChI=1S/C20H25F3N6O2/c21-20(22,23)16-4-2-1-3-15(16)11-26-19-27-12-17(29(30)31)18(28-19)25-10-14-7-5-13(9-24)6-8-14/h1-4,12-14H,5-11,24H2,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518188

(CHEMBL4577357)Show SMILES CC[C@H](NC(=O)c1cnn(c1C)-c1ccc(Cl)cc1)c1cccc(c1F)C(F)(F)F |r| Show InChI InChI=1S/C21H18ClF4N3O/c1-3-18(15-5-4-6-17(19(15)23)21(24,25)26)28-20(30)16-11-27-29(12(16)2)14-9-7-13(22)8-10-14/h4-11,18H,3H2,1-2H3,(H,28,30)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196987
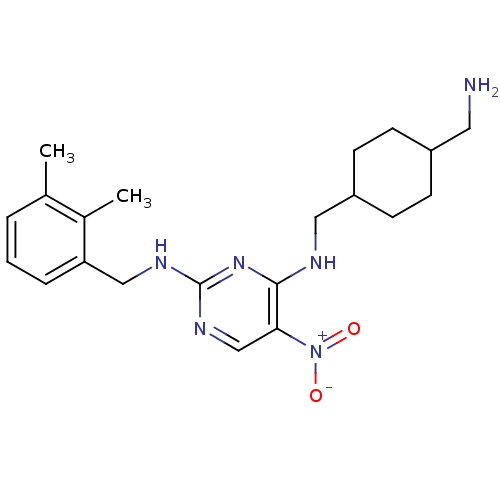
(CHEMBL245912 | N2-(2,3-dimethylbenzyl)-N4-((4-(ami...)Show SMILES Cc1cccc(CNc2ncc(c(NCC3CCC(CN)CC3)n2)[N+]([O-])=O)c1C |(20.87,-.91,;20.86,.63,;19.52,1.4,;19.52,2.95,;20.86,3.71,;22.19,2.94,;23.52,3.71,;24.86,2.94,;26.19,3.71,;26.19,5.25,;27.52,6.02,;28.86,5.26,;28.86,3.71,;30.2,2.94,;30.2,1.4,;31.53,.63,;31.53,-.9,;32.86,-1.67,;34.19,-.91,;35.53,-1.68,;35.52,-3.22,;34.2,.64,;32.86,1.41,;27.53,2.93,;30.19,6.03,;31.53,5.27,;30.19,7.58,;22.19,1.41,;23.53,.64,)| Show InChI InChI=1S/C21H30N6O2/c1-14-4-3-5-18(15(14)2)12-24-21-25-13-19(27(28)29)20(26-21)23-11-17-8-6-16(10-22)7-9-17/h3-5,13,16-17H,6-12,22H2,1-2H3,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196991
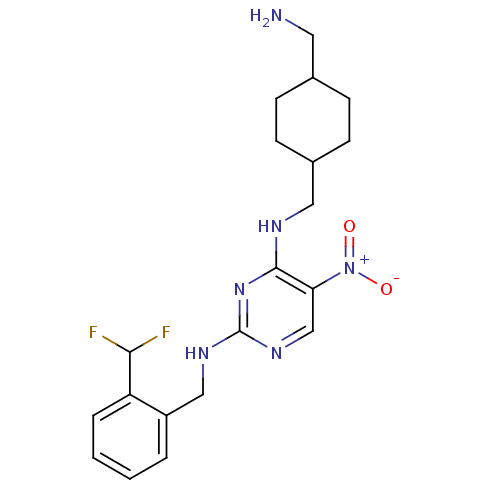
(CHEMBL245977 | N2-(2-(difluoromethyl)benzyl)-N4-((...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3C(F)F)ncc2[N+]([O-])=O)CC1 |(5.34,-13.55,;5.35,-12.01,;4.02,-11.23,;2.68,-12,;1.35,-11.23,;1.36,-9.7,;.02,-8.93,;.02,-7.39,;-1.32,-6.62,;-2.65,-7.39,;-3.99,-6.62,;-5.32,-7.39,;-6.65,-6.62,;-7.99,-7.39,;-9.32,-6.61,;-10.65,-7.38,;-10.66,-8.92,;-9.31,-9.69,;-7.99,-8.92,;-6.65,-9.69,;-6.65,-11.23,;-5.32,-8.92,;-3.98,-5.08,;-2.66,-4.31,;-1.32,-5.07,;.01,-4.29,;1.35,-5.06,;.01,-2.75,;2.68,-8.92,;4.02,-9.69,)| Show InChI InChI=1S/C20H26F2N6O2/c21-18(22)16-4-2-1-3-15(16)11-25-20-26-12-17(28(29)30)19(27-20)24-10-14-7-5-13(9-23)6-8-14/h1-4,12-14,18H,5-11,23H2,(H2,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518186
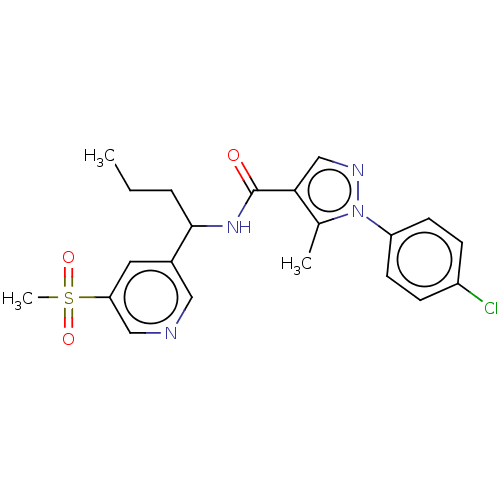
(CHEMBL4568048)Show SMILES CCCC(NC(=O)c1cnn(c1C)-c1ccc(Cl)cc1)c1cncc(c1)S(C)(=O)=O Show InChI InChI=1S/C21H23ClN4O3S/c1-4-5-20(15-10-18(12-23-11-15)30(3,28)29)25-21(27)19-13-24-26(14(19)2)17-8-6-16(22)7-9-17/h6-13,20H,4-5H2,1-3H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196952
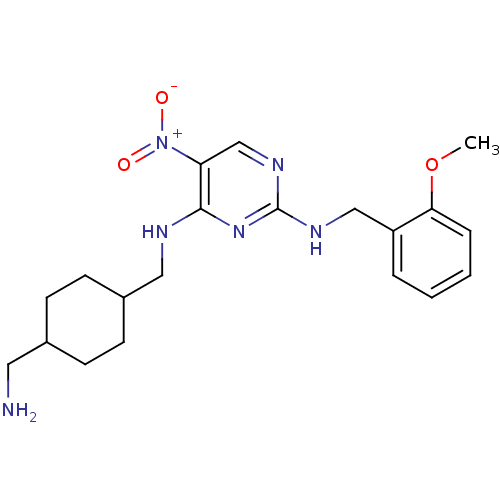
(CHEMBL395690 | N2-(2-methoxybenzyl)-N4-((4-(aminom...)Show SMILES COc1ccccc1CNc1ncc(c(NCC2CCC(CN)CC2)n1)[N+]([O-])=O |(25.24,-35.25,;25.23,-33.71,;23.9,-32.95,;22.57,-33.72,;21.23,-32.95,;21.23,-31.41,;22.56,-30.64,;23.89,-31.42,;25.23,-30.65,;26.56,-31.42,;27.89,-30.65,;27.9,-29.1,;29.23,-28.33,;30.56,-29.1,;30.57,-30.65,;31.9,-31.42,;31.9,-32.96,;33.24,-33.72,;33.23,-35.26,;34.56,-36.03,;35.9,-35.26,;37.23,-36.03,;37.23,-37.57,;35.9,-33.72,;34.56,-32.94,;29.23,-31.42,;31.9,-28.32,;33.23,-29.08,;31.89,-26.78,)| Show InChI InChI=1S/C20H28N6O3/c1-29-18-5-3-2-4-16(18)12-23-20-24-13-17(26(27)28)19(25-20)22-11-15-8-6-14(10-21)7-9-15/h2-5,13-15H,6-12,21H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196958
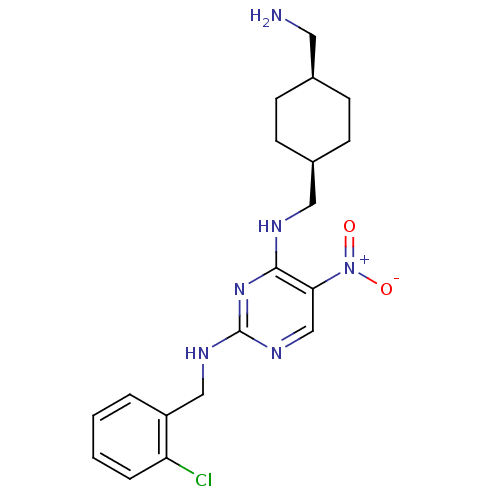
(CHEMBL246159 | N2-(2-chlorobenzyl)-N4-(((1s,4s)-4-...)Show SMILES NC[C@H]1CC[C@@H](CNc2nc(NCc3ccccc3Cl)ncc2[N+]([O-])=O)CC1 |wU:5.5,2.1,(4.42,-27.37,;4.42,-25.83,;3.09,-25.06,;1.75,-25.82,;.43,-25.05,;.43,-23.52,;-.9,-22.75,;-.91,-21.21,;-2.24,-20.44,;-3.58,-21.22,;-4.91,-20.44,;-6.25,-21.21,;-7.58,-20.44,;-8.91,-21.21,;-10.25,-20.44,;-11.58,-21.2,;-11.6,-22.75,;-10.24,-23.52,;-8.91,-22.74,;-7.58,-23.51,;-4.91,-18.9,;-3.58,-18.13,;-2.25,-18.89,;-.91,-18.12,;.43,-18.88,;-.92,-16.58,;1.76,-22.74,;3.09,-23.51,)| Show InChI InChI=1S/C19H25ClN6O2/c20-16-4-2-1-3-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-7-5-13(9-21)6-8-14/h1-4,12-14H,5-11,21H2,(H2,22,23,24,25)/t13-,14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196972

(CHEMBL248183 | N2-(2-(trifluoromethoxy)benzyl)-N4-...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3OC(F)(F)F)ncc2[N+]([O-])=O)CC1 |(34.71,-3.87,;34.71,-2.33,;33.38,-1.56,;32.04,-2.32,;30.71,-1.56,;30.72,-.02,;29.38,.75,;29.38,2.29,;28.05,3.05,;26.71,2.28,;25.37,3.05,;24.04,2.28,;22.71,3.05,;21.37,2.29,;20.04,3.06,;18.71,2.29,;18.71,.75,;20.05,-.02,;21.38,.75,;22.71,-.01,;22.72,-1.55,;22.7,-3.09,;24.26,-1.56,;21.18,-1.56,;25.38,4.6,;26.7,5.37,;28.04,4.6,;29.38,5.38,;30.71,4.62,;29.37,6.92,;32.04,.76,;33.38,-.02,)| Show InChI InChI=1S/C20H25F3N6O3/c21-20(22,23)32-17-4-2-1-3-15(17)11-26-19-27-12-16(29(30)31)18(28-19)25-10-14-7-5-13(9-24)6-8-14/h1-4,12-14H,5-11,24H2,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196975

(CHEMBL245783 | N2-(2,3-difluorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3cccc(F)c3F)ncc2[N+]([O-])=O)CC1 |(5.69,-35.63,;5.7,-34.09,;4.37,-33.32,;3.03,-34.08,;1.7,-33.31,;1.71,-31.78,;.37,-31.01,;.37,-29.47,;-.97,-28.7,;-2.3,-29.48,;-3.64,-28.7,;-4.97,-29.47,;-6.3,-28.7,;-7.64,-29.47,;-8.97,-28.7,;-10.3,-29.46,;-10.31,-31.01,;-8.96,-31.78,;-8.96,-33.32,;-7.64,-31,;-6.3,-31.77,;-3.63,-27.16,;-2.31,-26.39,;-.97,-27.15,;.36,-26.38,;1.7,-27.14,;.36,-24.84,;3.03,-31,;4.37,-31.77,)| Show InChI InChI=1S/C19H24F2N6O2/c20-15-3-1-2-14(17(15)21)10-24-19-25-11-16(27(28)29)18(26-19)23-9-13-6-4-12(8-22)5-7-13/h1-3,11-13H,4-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196990
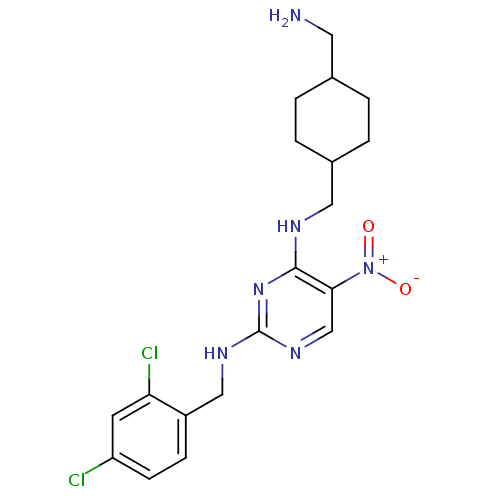
(CHEMBL246184 | N2-(2,4-dichlorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3ccc(Cl)cc3Cl)ncc2[N+]([O-])=O)CC1 |(38.9,-46.88,;38.9,-45.34,;37.57,-44.56,;36.23,-45.33,;34.9,-44.56,;34.91,-43.03,;33.58,-42.26,;33.57,-40.72,;32.24,-39.95,;30.9,-40.72,;29.57,-39.95,;28.23,-40.72,;26.9,-39.95,;25.56,-40.72,;24.23,-39.94,;22.9,-40.71,;22.9,-42.25,;21.56,-43.02,;24.24,-43.02,;25.57,-42.25,;26.9,-43.02,;29.57,-38.41,;30.9,-37.64,;32.23,-38.4,;33.57,-37.62,;34.9,-38.39,;33.56,-36.08,;36.23,-42.24,;37.57,-43.02,)| Show InChI InChI=1S/C19H24Cl2N6O2/c20-15-6-5-14(16(21)7-15)10-24-19-25-11-17(27(28)29)18(26-19)23-9-13-3-1-12(8-22)2-4-13/h5-7,11-13H,1-4,8-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518164

(CHEMBL4513370)Show SMILES C[C@H](NC(=O)c1cnn(c1C)-c1ccc(Cl)cc1)c1cccc(c1F)C(F)(F)F |r| Show InChI InChI=1S/C20H16ClF4N3O/c1-11(15-4-3-5-17(18(15)22)20(23,24)25)27-19(29)16-10-26-28(12(16)2)14-8-6-13(21)7-9-14/h3-11H,1-2H3,(H,27,29)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196982

(CHEMBL395691 | N2-(2-chlorobenzyl)-N4-(((1r,4r)-4-...)Show SMILES NC[C@H]1CC[C@H](CNc2nc(NCc3ccccc3Cl)ncc2[N+]([O-])=O)CC1 |wU:5.5,wD:2.1,(19.68,-26.91,;19.68,-25.37,;18.35,-24.6,;18.35,-23.05,;17.02,-22.28,;15.69,-23.06,;14.36,-22.29,;14.35,-20.75,;13.02,-19.98,;11.68,-20.76,;10.35,-19.99,;9.01,-20.75,;7.68,-19.98,;6.35,-20.75,;5.01,-19.98,;3.68,-20.74,;3.68,-22.29,;5.02,-23.06,;6.35,-22.29,;7.68,-23.05,;10.35,-18.44,;11.68,-17.67,;13.01,-18.43,;14.35,-17.66,;15.69,-18.42,;14.34,-16.12,;15.69,-24.59,;17.01,-25.36,)| Show InChI InChI=1S/C19H25ClN6O2/c20-16-4-2-1-3-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-7-5-13(9-21)6-8-14/h1-4,12-14H,5-11,21H2,(H2,22,23,24,25)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196980
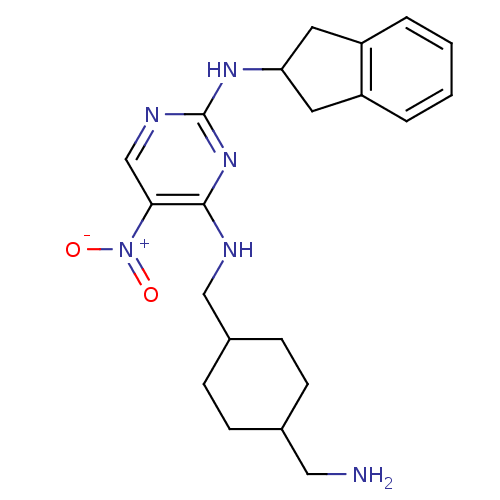
(CHEMBL247572 | N4-((4-(aminomethyl)cyclohexyl)meth...)Show SMILES NCC1CCC(CNc2nc(NC3Cc4ccccc4C3)ncc2[N+]([O-])=O)CC1 |(5.74,-49.23,;5.75,-47.69,;4.41,-46.92,;3.08,-47.68,;1.75,-46.92,;1.75,-45.38,;.41,-44.61,;.41,-43.07,;-.93,-42.31,;-2.26,-43.08,;-3.6,-42.31,;-4.93,-43.08,;-6.27,-42.31,;-6.43,-40.78,;-7.94,-40.46,;-8.71,-39.14,;-10.24,-39.15,;-11,-40.49,;-10.22,-41.8,;-8.7,-41.79,;-7.67,-42.93,;-3.6,-40.76,;-2.27,-39.99,;-.93,-40.76,;.4,-39.98,;1.74,-40.74,;.4,-38.44,;3.08,-44.6,;4.41,-45.38,)| Show InChI InChI=1S/C21H28N6O2/c22-11-14-5-7-15(8-6-14)12-23-20-19(27(28)29)13-24-21(26-20)25-18-9-16-3-1-2-4-17(16)10-18/h1-4,13-15,18H,5-12,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196948
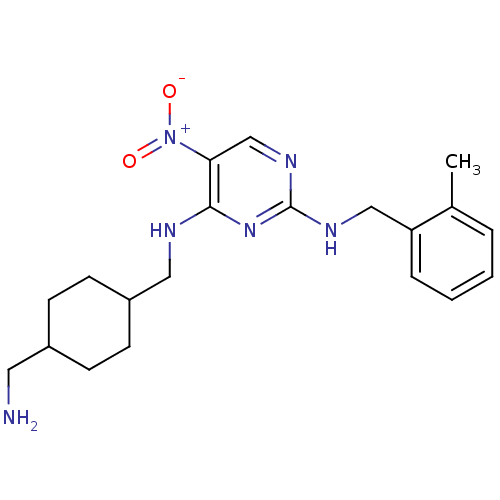
(CHEMBL428751 | N*4*-(4-aminomethyl-cyclohexylmethy...)Show SMILES Cc1ccccc1CNc1ncc(c(NCC2CCC(CN)CC2)n1)[N+]([O-])=O |(26.51,-43.74,;25.18,-42.97,;23.85,-43.75,;22.51,-42.98,;22.51,-41.43,;23.84,-40.67,;25.18,-41.44,;26.51,-40.67,;27.84,-41.44,;29.18,-40.67,;29.18,-39.13,;30.51,-38.36,;31.84,-39.12,;31.85,-40.67,;33.18,-41.44,;33.19,-42.98,;34.52,-43.75,;34.52,-45.28,;35.84,-46.05,;37.18,-45.29,;38.51,-46.06,;38.51,-47.6,;37.18,-43.74,;35.85,-42.97,;30.51,-41.45,;33.18,-38.35,;34.52,-39.11,;33.17,-36.81,)| Show InChI InChI=1S/C20H28N6O2/c1-14-4-2-3-5-17(14)12-23-20-24-13-18(26(27)28)19(25-20)22-11-16-8-6-15(10-21)7-9-16/h2-5,13,15-16H,6-12,21H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196956
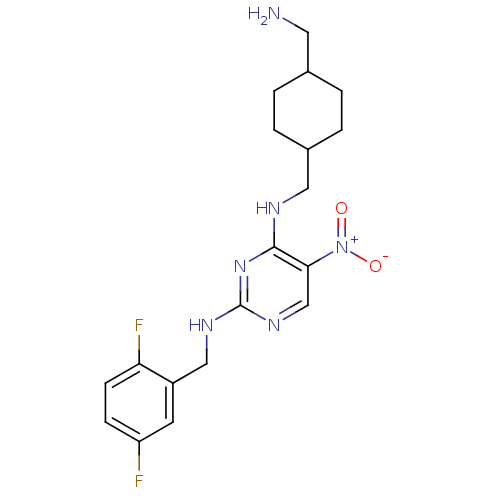
(CHEMBL396370 | N2-(2,5-difluorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3cc(F)ccc3F)ncc2[N+]([O-])=O)CC1 |(39.3,-37.78,;39.31,-36.24,;37.97,-35.47,;36.64,-36.24,;35.31,-35.47,;35.31,-33.93,;33.98,-33.17,;33.98,-31.63,;32.64,-30.86,;31.31,-31.63,;29.97,-30.86,;28.64,-31.63,;27.3,-30.86,;25.97,-31.63,;24.64,-30.85,;23.3,-31.62,;21.97,-30.85,;23.3,-33.16,;24.64,-33.93,;25.97,-33.16,;27.31,-33.92,;29.97,-29.31,;31.3,-28.54,;32.64,-29.31,;33.97,-28.53,;35.31,-29.29,;33.97,-26.99,;36.64,-33.15,;37.98,-33.93,)| Show InChI InChI=1S/C19H24F2N6O2/c20-15-5-6-16(21)14(7-15)10-24-19-25-11-17(27(28)29)18(26-19)23-9-13-3-1-12(8-22)2-4-13/h5-7,11-13H,1-4,8-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196973

(CHEMBL438669 | N*4*-(4-aminomethyl-cyclohexylmethy...)Show SMILES Cc1cccc(CNc2ncc(c(NCC3CCC(CN)CC3)n2)[N+]([O-])=O)c1 |(-9.6,-1.63,;-9.6,-.09,;-10.94,.68,;-10.94,2.22,;-9.61,2.99,;-8.28,2.22,;-6.94,2.98,;-5.61,2.21,;-4.27,2.98,;-4.27,4.53,;-2.94,5.3,;-1.61,4.53,;-1.6,2.98,;-.27,2.22,;-.27,.68,;1.07,-.09,;1.06,-1.63,;2.39,-2.39,;3.73,-1.63,;5.06,-2.4,;5.06,-3.94,;3.73,-.09,;2.39,.69,;-2.94,2.21,;-.27,5.31,;1.06,4.55,;-.28,6.85,;-8.27,.68,)| Show InChI InChI=1S/C20H28N6O2/c1-14-3-2-4-17(9-14)12-23-20-24-13-18(26(27)28)19(25-20)22-11-16-7-5-15(10-21)6-8-16/h2-4,9,13,15-16H,5-8,10-12,21H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398338
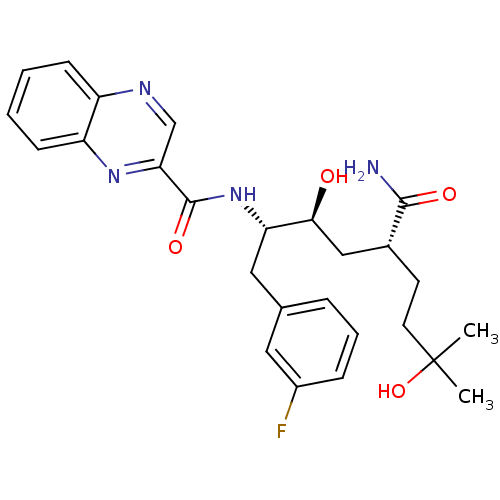
(CHEMBL1628706)Show SMILES CC(C)(O)CC[C@H](C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O |r| Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 in human THP1 cells assessed as inhibition of chemotaxis after 30 mins by Celltiter-glo reagent based luminescence assay |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196954
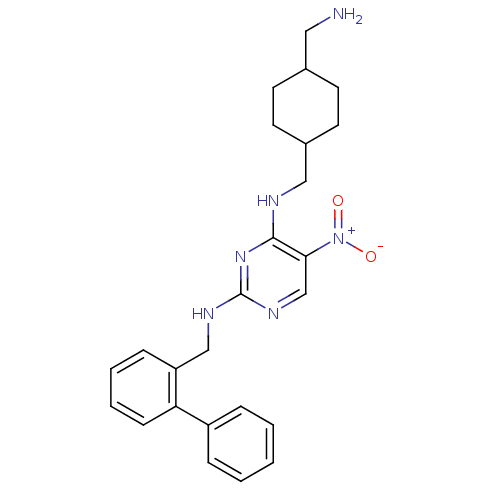
(CHEMBL246177 | N*4*-(4-aminomethyl-cyclohexylmethy...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3-c3ccccc3)ncc2[N+]([O-])=O)CC1 |(22.64,-27.42,;22.65,-25.88,;21.31,-25.1,;19.98,-25.87,;18.65,-25.1,;18.65,-23.57,;17.32,-22.8,;17.32,-21.26,;15.98,-20.49,;14.65,-21.26,;13.31,-20.49,;11.98,-21.26,;10.64,-20.49,;9.31,-21.26,;9.31,-22.79,;7.98,-23.56,;6.64,-22.79,;6.64,-21.25,;7.98,-20.48,;7.98,-18.94,;9.32,-18.18,;9.32,-16.64,;7.99,-15.87,;6.65,-16.64,;6.65,-18.18,;13.31,-18.95,;14.64,-18.18,;15.98,-18.94,;17.31,-18.16,;18.65,-18.93,;17.31,-16.62,;19.98,-22.78,;21.32,-23.56,)| Show InChI InChI=1S/C25H30N6O2/c26-14-18-10-12-19(13-11-18)15-27-24-23(31(32)33)17-29-25(30-24)28-16-21-8-4-5-9-22(21)20-6-2-1-3-7-20/h1-9,17-19H,10-16,26H2,(H2,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196935

(CHEMBL246182 | N2-(2,6-difluorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3c(F)cccc3F)ncc2[N+]([O-])=O)CC1 |(5.39,-47.23,;5.39,-45.69,;4.06,-44.92,;2.73,-45.69,;1.4,-44.92,;1.4,-43.38,;.07,-42.62,;.07,-41.08,;-1.27,-40.31,;-2.61,-41.08,;-3.94,-40.31,;-5.27,-41.08,;-6.61,-40.31,;-7.94,-41.08,;-7.94,-42.61,;-6.6,-43.37,;-9.27,-43.38,;-10.61,-42.61,;-10.61,-41.07,;-9.27,-40.3,;-9.27,-38.76,;-3.94,-38.76,;-2.61,-37.99,;-1.27,-38.76,;.06,-37.98,;1.4,-38.74,;.05,-36.44,;2.73,-42.6,;4.06,-43.38,)| Show InChI InChI=1S/C19H24F2N6O2/c20-15-2-1-3-16(21)14(15)10-24-19-25-11-17(27(28)29)18(26-19)23-9-13-6-4-12(8-22)5-7-13/h1-3,11-13H,4-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196930
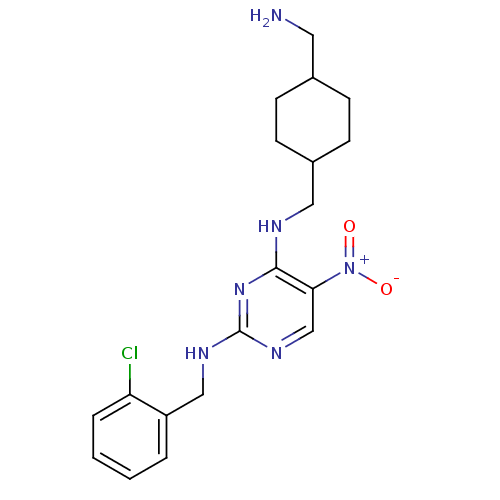
(CHEMBL246970 | N2-(2-chlorobenzyl)-N4-((4-(aminome...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3Cl)ncc2[N+]([O-])=O)CC1 |(35.48,-16.58,;35.49,-15.04,;34.15,-14.27,;32.82,-15.03,;31.49,-14.27,;31.5,-12.73,;30.16,-11.96,;30.16,-10.42,;28.82,-9.66,;27.49,-10.43,;26.15,-9.66,;24.82,-10.43,;23.48,-9.65,;22.15,-10.42,;20.82,-9.65,;19.49,-10.42,;19.48,-11.96,;20.83,-12.73,;22.15,-11.96,;23.49,-12.72,;26.15,-8.11,;27.48,-7.34,;28.82,-8.11,;30.15,-7.33,;31.49,-8.09,;30.15,-5.79,;32.82,-11.95,;34.16,-12.73,)| Show InChI InChI=1S/C19H25ClN6O2/c20-16-4-2-1-3-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-7-5-13(9-21)6-8-14/h1-4,12-14H,5-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196984
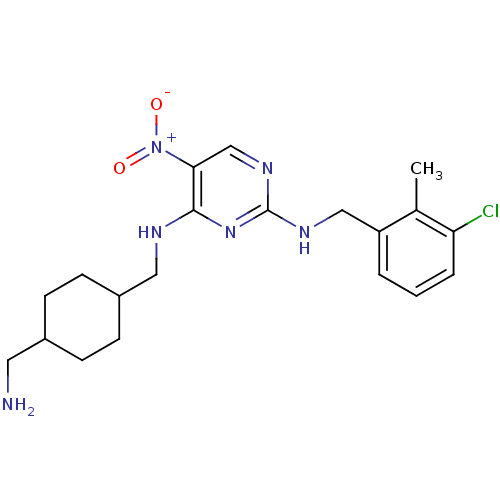
(CHEMBL246387 | N2-(3-chloro-2-methylbenzyl)-N4-((4...)Show SMILES Cc1c(Cl)cccc1CNc1ncc(c(NCC2CCC(CN)CC2)n1)[N+]([O-])=O |(8.85,.06,;7.52,.82,;6.19,.05,;6.19,-1.49,;4.85,.82,;4.85,2.36,;6.18,3.13,;7.51,2.36,;8.85,3.12,;10.18,2.35,;11.51,3.12,;11.52,4.67,;12.84,5.44,;14.18,4.67,;14.19,3.12,;15.52,2.36,;15.52,.82,;16.86,.05,;16.85,-1.49,;18.18,-2.25,;19.52,-1.49,;20.85,-2.26,;20.85,-3.8,;19.52,.05,;18.18,.83,;12.85,2.35,;15.52,5.45,;16.85,4.69,;15.51,6.99,)| Show InChI InChI=1S/C20H27ClN6O2/c1-13-16(3-2-4-17(13)21)11-24-20-25-12-18(27(28)29)19(26-20)23-10-15-7-5-14(9-22)6-8-15/h2-4,12,14-15H,5-11,22H2,1H3,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
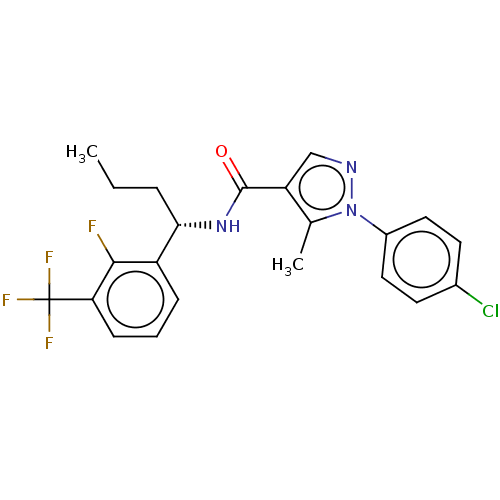
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50518187

(CHEMBL4583870)Show SMILES CCCC(NC(=O)c1cnn(c1C)-c1ccc(Cl)cc1)c1ccc(nc1)S(C)(=O)=O Show InChI InChI=1S/C21H23ClN4O3S/c1-4-5-19(15-6-11-20(23-12-15)30(3,28)29)25-21(27)18-13-24-26(14(18)2)17-9-7-16(22)8-10-17/h6-13,19H,4-5H2,1-3H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196985
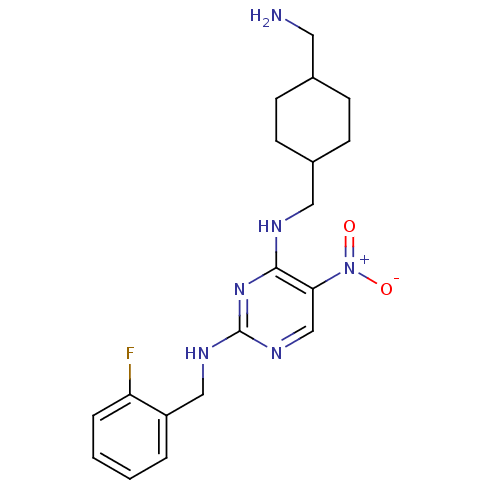
(CHEMBL395689 | N2-(2-fluorobenzyl)-N4-((4-(aminome...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3F)ncc2[N+]([O-])=O)CC1 |(36.12,-26.9,;36.12,-25.36,;34.79,-24.59,;33.46,-25.35,;32.13,-24.59,;32.13,-23.05,;30.8,-22.28,;30.8,-20.74,;29.46,-19.98,;28.12,-20.75,;26.79,-19.98,;25.46,-20.75,;24.12,-19.98,;22.79,-20.74,;21.46,-19.97,;20.12,-20.74,;20.12,-22.28,;21.46,-23.05,;22.79,-22.28,;24.13,-23.04,;26.79,-18.43,;28.12,-17.66,;29.46,-18.43,;30.79,-17.65,;32.13,-18.41,;30.78,-16.11,;33.46,-22.27,;34.79,-23.05,)| Show InChI InChI=1S/C19H25FN6O2/c20-16-4-2-1-3-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-7-5-13(9-21)6-8-14/h1-4,12-14H,5-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196931
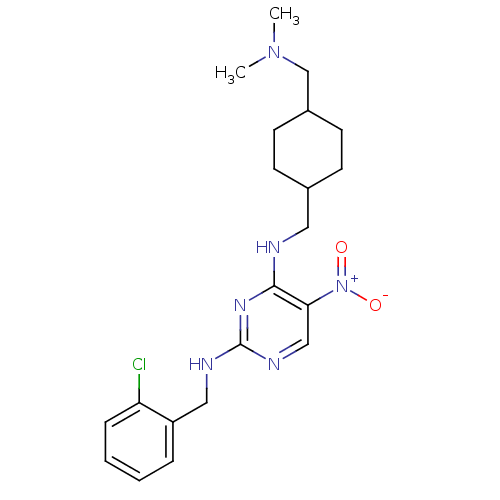
(CHEMBL246160 | N2-(2-chlorobenzyl)-N4-((4-((dimeth...)Show SMILES CN(C)CC1CCC(CNc2nc(NCc3ccccc3Cl)ncc2[N+]([O-])=O)CC1 |(6.51,-38.8,;5.18,-38.02,;3.85,-38.79,;5.18,-36.48,;3.85,-35.71,;2.52,-36.48,;1.19,-35.71,;1.19,-34.17,;-.14,-33.41,;-.14,-31.87,;-1.48,-31.1,;-2.82,-31.87,;-4.15,-31.1,;-5.48,-31.87,;-6.82,-31.1,;-8.15,-31.87,;-9.48,-31.09,;-10.82,-31.86,;-10.82,-33.4,;-9.48,-34.17,;-8.15,-33.4,;-6.81,-34.17,;-4.15,-29.56,;-2.82,-28.78,;-1.48,-29.55,;-.15,-28.77,;1.19,-29.54,;-.16,-27.23,;2.52,-33.39,;3.85,-34.17,)| Show InChI InChI=1S/C21H29ClN6O2/c1-27(2)14-16-9-7-15(8-10-16)11-23-20-19(28(29)30)13-25-21(26-20)24-12-17-5-3-4-6-18(17)22/h3-6,13,15-16H,7-12,14H2,1-2H3,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196937

(CHEMBL391610 | N2-(3-fluorophenethyl)-N4-((4-(amin...)Show SMILES NCC1CCC(CNc2nc(NCCc3cccc(F)c3)ncc2[N+]([O-])=O)CC1 |(6.52,-16.97,;6.53,-15.43,;5.19,-14.65,;3.86,-15.42,;2.54,-14.65,;2.54,-13.11,;1.2,-12.35,;1.2,-10.81,;-.14,-10.04,;-1.46,-10.81,;-2.8,-10.06,;-4.13,-10.83,;-5.47,-10.07,;-6.8,-10.85,;-8.13,-10.09,;-9.45,-10.87,;-10.79,-10.11,;-10.8,-8.57,;-9.47,-7.79,;-9.48,-6.25,;-8.14,-8.55,;-2.81,-8.51,;-1.48,-7.73,;-.14,-8.5,;1.2,-7.73,;2.53,-8.49,;1.19,-6.19,;3.86,-12.34,;5.19,-13.11,)| Show InChI InChI=1S/C20H27FN6O2/c21-17-3-1-2-14(10-17)8-9-23-20-25-13-18(27(28)29)19(26-20)24-12-16-6-4-15(11-22)5-7-16/h1-3,10,13,15-16H,4-9,11-12,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398338

(CHEMBL1628706)Show SMILES CC(C)(O)CC[C@H](C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O |r| Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... |
Bioorg Med Chem Lett 29: 435-440 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.015
BindingDB Entry DOI: 10.7270/Q2D79FSV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196965
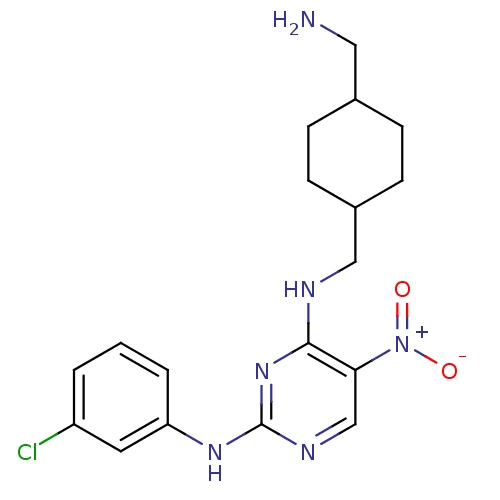
(CHEMBL396924 | N4-((4-(aminomethyl)cyclohexyl)meth...)Show SMILES NCC1CCC(CNc2nc(Nc3cccc(Cl)c3)ncc2[N+]([O-])=O)CC1 |(32.49,-5.1,;32.49,-3.56,;31.16,-2.79,;29.82,-3.55,;28.5,-2.79,;28.5,-1.24,;27.17,-.48,;27.16,1.06,;25.83,1.83,;24.49,1.06,;23.16,1.83,;21.82,1.06,;20.49,1.83,;19.17,1.06,;17.84,1.83,;17.83,3.37,;19.17,4.14,;19.17,5.68,;20.5,3.37,;23.16,3.38,;24.49,4.15,;25.82,3.38,;27.15,4.16,;28.49,3.39,;27.14,5.7,;29.83,-.47,;31.16,-1.25,)| Show InChI InChI=1S/C18H23ClN6O2/c19-14-2-1-3-15(8-14)23-18-22-11-16(25(26)27)17(24-18)21-10-13-6-4-12(9-20)5-7-13/h1-3,8,11-13H,4-7,9-10,20H2,(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196968
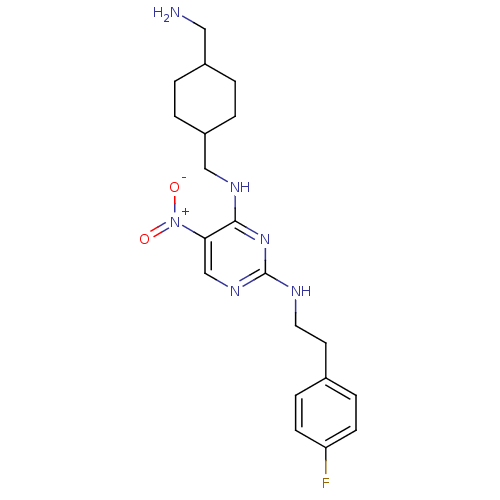
(CHEMBL247377 | N2-(4-fluorophenethyl)-N4-((4-(amin...)Show SMILES NCC1CCC(CNc2nc(NCCc3ccc(F)cc3)ncc2[N+]([O-])=O)CC1 |(22.9,-17.09,;22.91,-15.55,;21.57,-14.78,;20.24,-15.54,;18.92,-14.78,;18.92,-13.24,;17.58,-12.47,;17.58,-10.93,;16.24,-10.17,;14.92,-10.94,;13.58,-10.18,;12.25,-10.96,;10.91,-10.2,;9.58,-10.98,;8.25,-10.21,;8.24,-8.67,;6.91,-7.91,;5.58,-8.69,;4.24,-7.93,;5.59,-10.24,;6.93,-10.99,;13.57,-8.63,;14.9,-7.86,;16.24,-8.62,;17.58,-7.85,;18.91,-8.62,;17.57,-6.31,;20.24,-12.46,;21.57,-13.24,)| Show InChI InChI=1S/C20H27FN6O2/c21-17-7-5-14(6-8-17)9-10-23-20-25-13-18(27(28)29)19(26-20)24-12-16-3-1-15(11-22)2-4-16/h5-8,13,15-16H,1-4,9-12,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196944
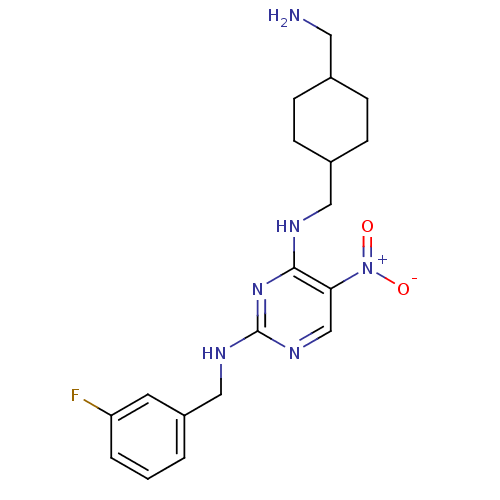
(CHEMBL245757 | N2-(3-fluorobenzyl)-N4-((4-(aminome...)Show SMILES NCC1CCC(CNc2nc(NCc3cccc(F)c3)ncc2[N+]([O-])=O)CC1 |(5.48,-36.28,;5.49,-34.74,;4.16,-33.97,;2.82,-34.73,;1.49,-33.97,;1.5,-32.43,;.16,-31.66,;.16,-30.12,;-1.18,-29.36,;-2.51,-30.13,;-3.85,-29.36,;-5.18,-30.13,;-6.51,-29.36,;-7.85,-30.12,;-9.18,-29.35,;-10.51,-30.12,;-10.52,-31.66,;-9.17,-32.43,;-9.17,-33.97,;-7.85,-31.66,;-3.84,-27.81,;-2.52,-27.04,;-1.18,-27.81,;.15,-27.03,;1.49,-27.79,;.15,-25.49,;2.82,-31.65,;4.16,-32.43,)| Show InChI InChI=1S/C19H25FN6O2/c20-16-3-1-2-15(8-16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-6-4-13(9-21)5-7-14/h1-3,8,12-14H,4-7,9-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196934
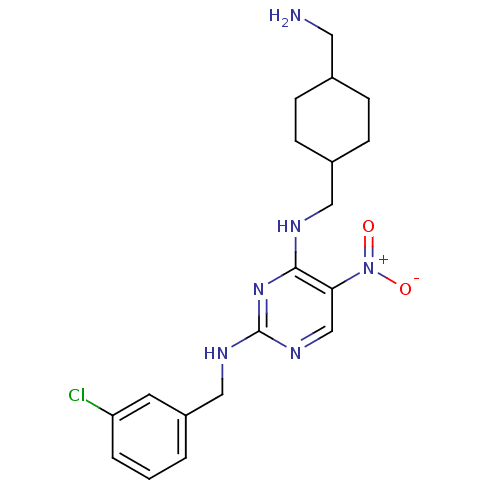
(CHEMBL437941 | N2-(3-chlorobenzyl)-N4-((4-(aminome...)Show SMILES NCC1CCC(CNc2nc(NCc3cccc(Cl)c3)ncc2[N+]([O-])=O)CC1 |(5.19,-26.14,;5.19,-24.6,;3.86,-23.83,;2.52,-24.59,;1.2,-23.82,;1.2,-22.29,;-.13,-21.52,;-.14,-19.98,;-1.47,-19.21,;-2.81,-19.99,;-4.14,-19.22,;-5.48,-19.98,;-6.81,-19.21,;-8.14,-19.98,;-9.48,-19.21,;-10.81,-19.97,;-10.81,-21.52,;-9.47,-22.29,;-9.46,-23.83,;-8.14,-21.52,;-4.14,-17.67,;-2.81,-16.9,;-1.48,-17.66,;-.14,-16.89,;1.2,-17.65,;-.15,-15.35,;2.53,-21.51,;3.86,-22.28,)| Show InChI InChI=1S/C19H25ClN6O2/c20-16-3-1-2-15(8-16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-6-4-13(9-21)5-7-14/h1-3,8,12-14H,4-7,9-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196943
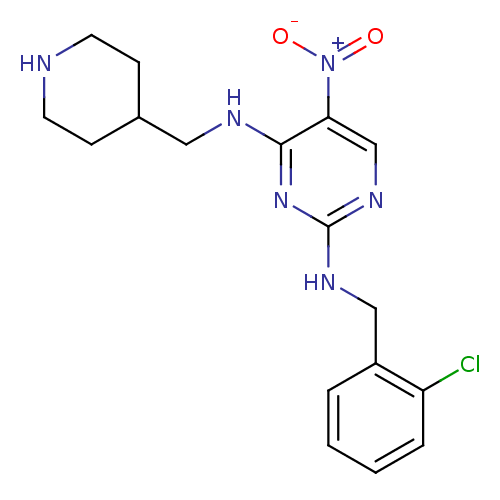
(CHEMBL246161 | N2-(2-chlorobenzyl)-5-nitro-N4-(pip...)Show SMILES [O-][N+](=O)c1cnc(NCc2ccccc2Cl)nc1NCC1CCNCC1 Show InChI InChI=1S/C17H21ClN6O2/c18-14-4-2-1-3-13(14)10-21-17-22-11-15(24(25)26)16(23-17)20-9-12-5-7-19-8-6-12/h1-4,11-12,19H,5-10H2,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196942

(CHEMBL391609 | N4-((4-(aminomethyl)cyclohexyl)meth...)Show SMILES NCC1CCC(CNc2nc(NCCCCc3ccccc3)ncc2[N+]([O-])=O)CC1 |(5.66,-5.07,;5.66,-3.53,;4.33,-2.75,;3,-3.52,;1.67,-2.75,;1.67,-1.21,;.34,-.45,;.33,1.09,;-1,1.86,;-2.33,1.09,;-3.66,1.84,;-4.99,1.07,;-6.33,1.83,;-7.66,1.05,;-9,1.81,;-10.33,1.03,;-11.67,1.79,;-11.68,3.33,;-13.01,4.09,;-14.33,3.31,;-14.33,1.77,;-13,1.01,;-3.67,3.39,;-2.34,4.17,;-1,3.4,;.33,4.17,;1.67,3.41,;.33,5.71,;3,-.44,;4.33,-1.21,)| Show InChI InChI=1S/C22H32N6O2/c23-14-18-9-11-19(12-10-18)15-25-21-20(28(29)30)16-26-22(27-21)24-13-5-4-8-17-6-2-1-3-7-17/h1-3,6-7,16,18-19H,4-5,8-15,23H2,(H2,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196963
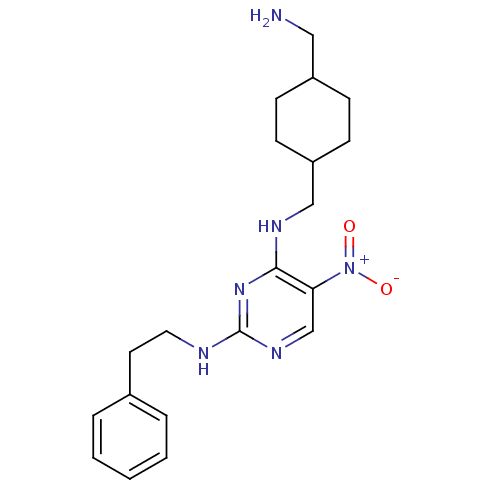
(CHEMBL247573 | N4-((4-(aminomethyl)cyclohexyl)meth...)Show SMILES NCC1CCC(CNc2nc(NCCc3ccccc3)ncc2[N+]([O-])=O)CC1 |(20.36,-49.58,;20.37,-48.04,;19.04,-47.27,;17.7,-48.03,;16.37,-47.27,;16.37,-45.73,;15.03,-44.96,;15.03,-43.42,;13.7,-42.66,;12.36,-43.43,;11.02,-42.66,;9.69,-43.43,;8.36,-42.66,;7.02,-43.42,;5.69,-42.65,;5.7,-41.11,;4.37,-40.34,;3.03,-41.11,;3.04,-42.66,;4.37,-43.42,;11.03,-41.11,;12.36,-40.34,;13.69,-41.11,;15.03,-40.33,;16.36,-41.09,;15.02,-38.79,;17.7,-44.95,;19.04,-45.73,)| Show InChI InChI=1S/C20H28N6O2/c21-12-16-6-8-17(9-7-16)13-23-19-18(26(27)28)14-24-20(25-19)22-11-10-15-4-2-1-3-5-15/h1-5,14,16-17H,6-13,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196974
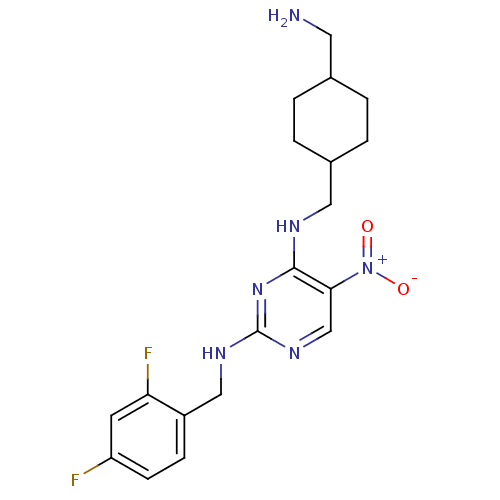
(CHEMBL245982 | N2-(2,4-difluorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3ccc(F)cc3F)ncc2[N+]([O-])=O)CC1 |(22.46,-36.56,;22.47,-35.02,;21.13,-34.25,;19.8,-35.01,;18.47,-34.25,;18.48,-32.71,;17.14,-31.94,;17.14,-30.4,;15.8,-29.64,;14.47,-30.41,;13.13,-29.64,;11.8,-30.41,;10.46,-29.64,;9.13,-30.4,;7.8,-29.63,;6.47,-30.4,;6.46,-31.94,;5.13,-32.71,;7.81,-32.71,;9.13,-31.94,;10.47,-32.7,;13.13,-28.09,;14.46,-27.32,;15.8,-28.09,;17.13,-27.31,;18.47,-28.07,;17.13,-25.77,;19.8,-31.93,;21.14,-32.71,)| Show InChI InChI=1S/C19H24F2N6O2/c20-15-6-5-14(16(21)7-15)10-24-19-25-11-17(27(28)29)18(26-19)23-9-13-3-1-12(8-22)2-4-13/h5-7,11-13H,1-4,8-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data