

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50038163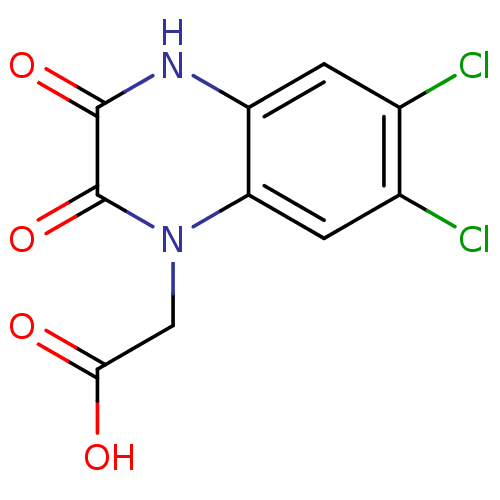 ((6,7-Dichloro-2,3-dioxo-3,4-dihydro-2H-quinoxalin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for glycine site-NMDA receptor was determined by the ability to displace [3H]glycine in rat cortical membranes | Bioorg Med Chem Lett 3: 2801-2804 (1993) Article DOI: 10.1016/S0960-894X(01)80768-X BindingDB Entry DOI: 10.7270/Q2Z89CWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50281275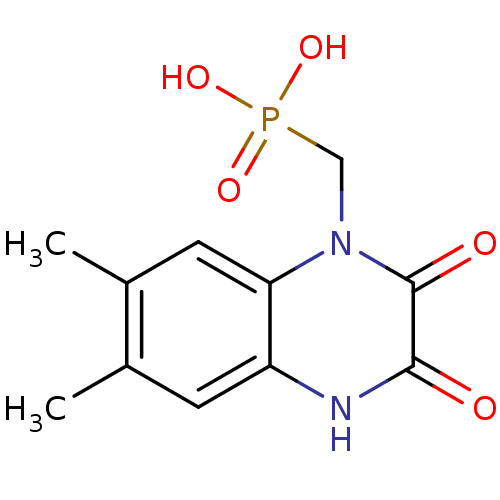 ((6,7-Dimethyl-2,3-dioxo-3,4-dihydro-2H-quinoxalin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 5.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for glycine site-NMDA receptor was determined by the ability to displace [3H]glycine in rat cortical membranes | Bioorg Med Chem Lett 3: 2801-2804 (1993) Article DOI: 10.1016/S0960-894X(01)80768-X BindingDB Entry DOI: 10.7270/Q2Z89CWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50281273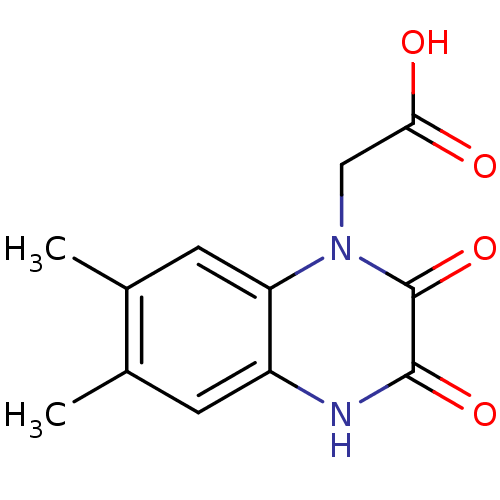 ((6,7-Dimethyl-2,3-dioxo-3,4-dihydro-2H-quinoxalin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for glycine site-NMDA receptor was determined by the ability to displace [3H]glycine in rat cortical membranes | Bioorg Med Chem Lett 3: 2801-2804 (1993) Article DOI: 10.1016/S0960-894X(01)80768-X BindingDB Entry DOI: 10.7270/Q2Z89CWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171275 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50241125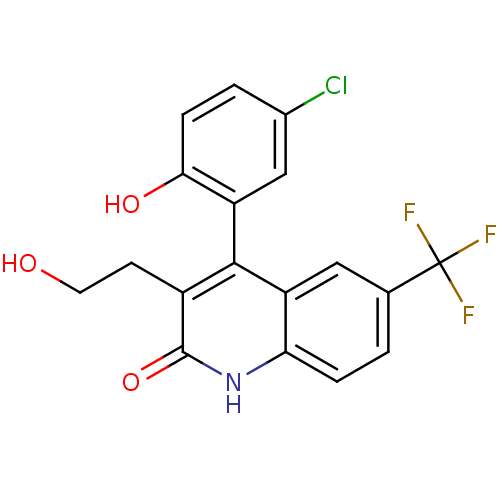 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 prepared from baculovirus-infected insect cells using 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171277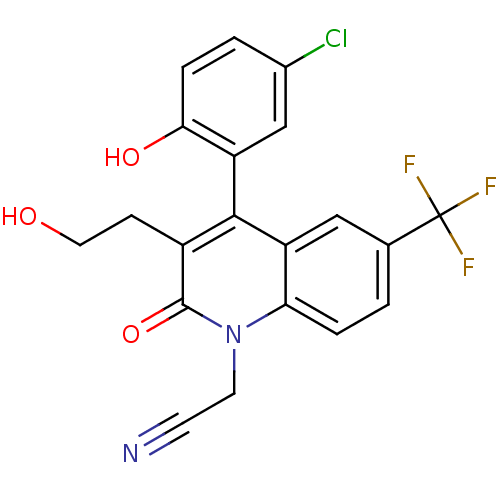 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 prepared from baculovirus-infected insect cells using 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C19 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171284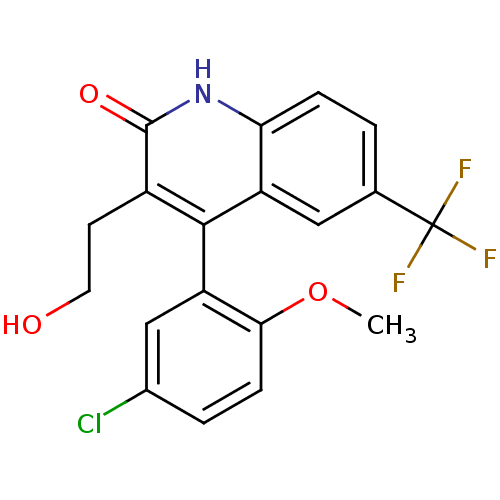 (4-(5-Chloro-2-methoxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using 7-benzyloxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50171282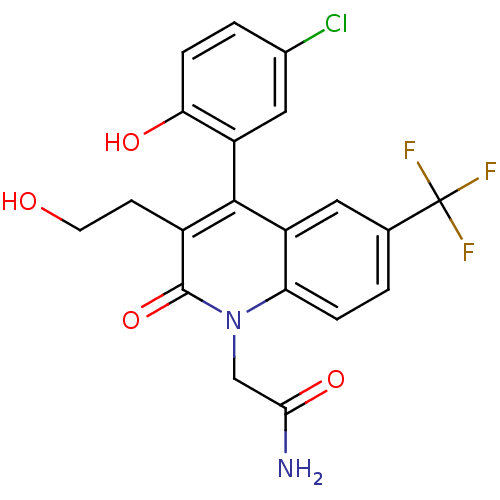 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C19 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50171280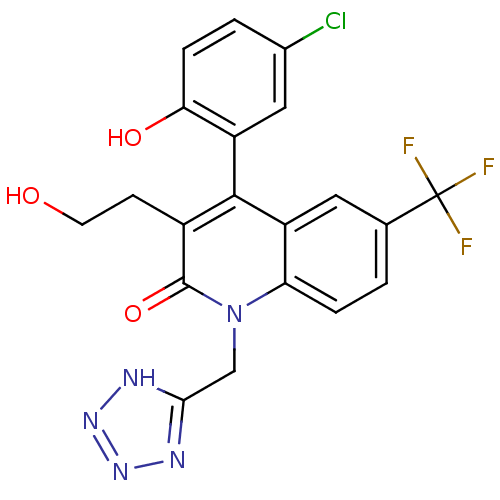 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C19 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171278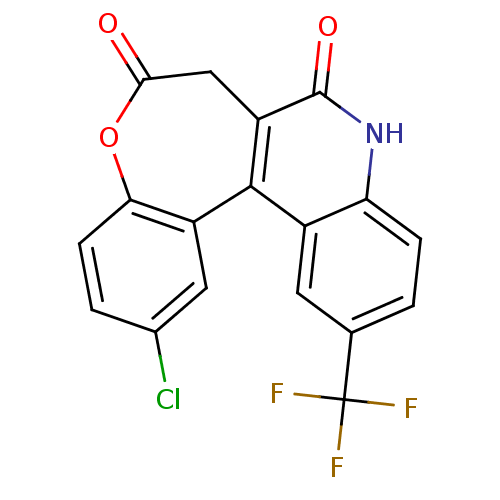 (12-Chloro-2-trifluoromethyl-5,7-dihydro-9-oxa-5-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using 7-benzyloxy- 4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using resorufin benzyl ether | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 prepared from baculovirus-infected insect cells using 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using resorufin benzyl ether | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 prepared from baculovirus-infected insect cells using 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171283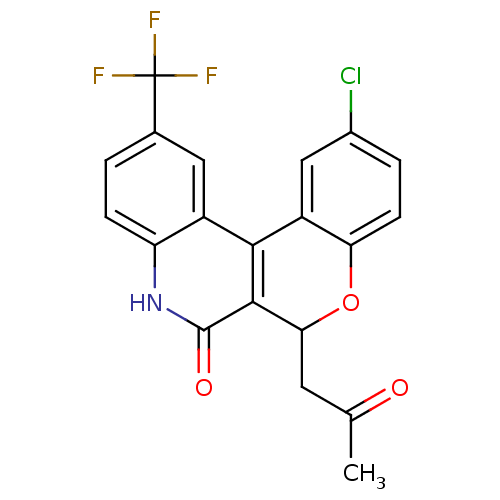 (2-Chloro-6-(2-oxo-propyl)-11-trifluoromethyl-6,8-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2D6 prepared from baculovirus-infected insect cells using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7- methoxy-4-meth... | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C19 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using 7-benzyloxy- 4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using 7-benzyloxy- 4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 1A2 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2D6 prepared from baculovirus-infected insect cells using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methy... | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171279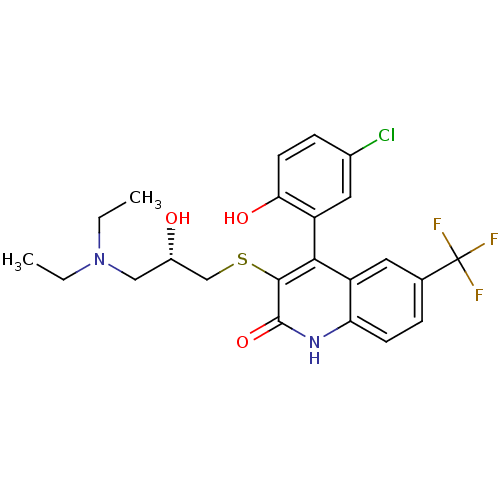 (4-(5-Chloro-2-hydroxy-phenyl)-3-[3-((S)-diethyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 1A2 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using resorufin benzyl ether | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171276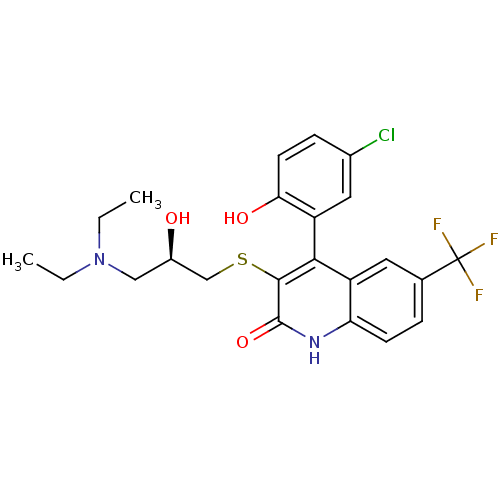 (4-(5-Chloro-2-hydroxy-phenyl)-3-[3-((R)-diethyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 1A2 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using resorufin benzylether | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 1A2 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171281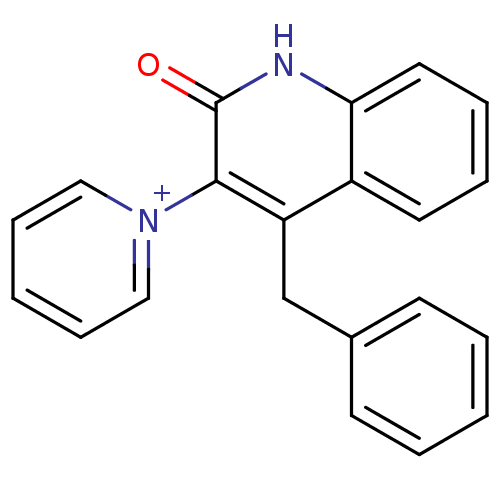 (1-(4-Benzyl-2-oxo-1,2-dihydro-quinolin-3-yl)-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2D6 prepared from baculovirus-infected insect cells using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7- methoxy-4-meth... | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2D6 prepared from baculovirus-infected insect cells using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7- methoxy-4-meth... | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50442470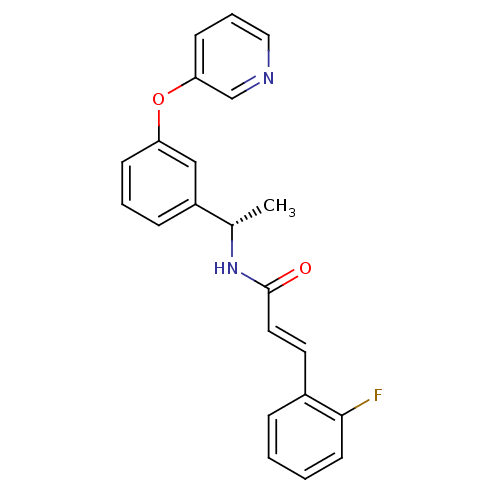 (CHEMBL401942) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50410081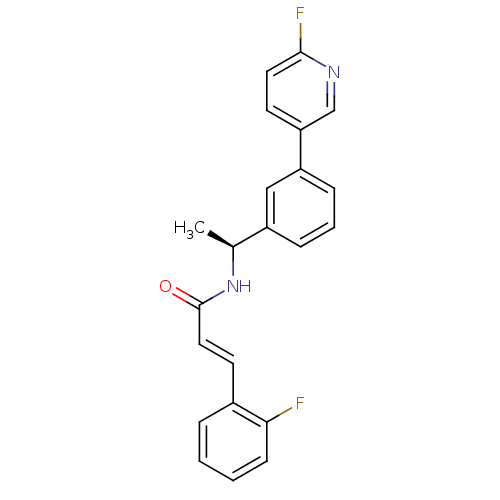 (CHEMBL361184) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50410077 (CHEMBL180886) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143560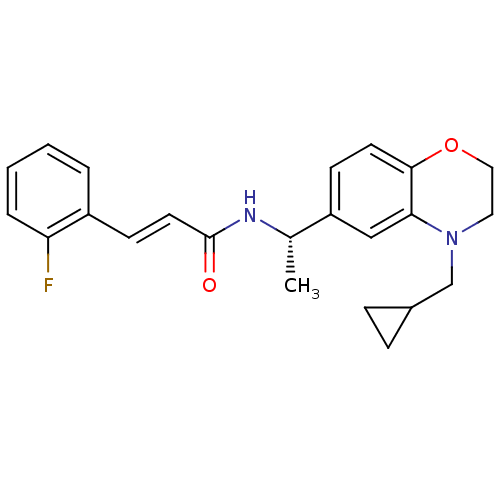 ((E)-N-[(S)-1-(4-Cyclopropylmethyl-3,4-dihydro-2H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143558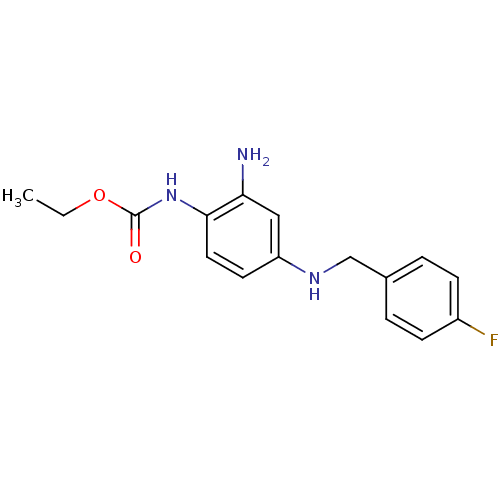 (CHEMBL41355 | EZOGABINE | N-(2-amino-4-(4-fluorobe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50130610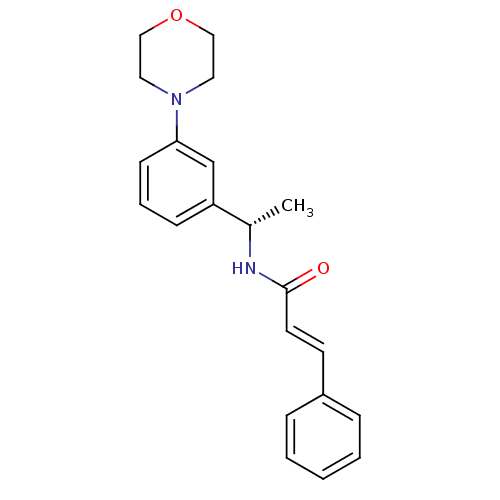 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50442469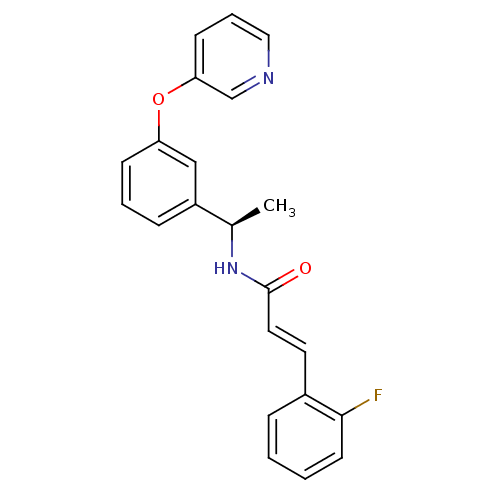 (CHEMBL2440362) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50442468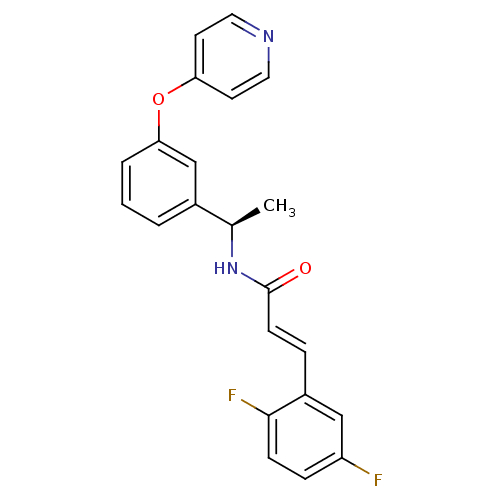 (CHEMBL2440364) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50142346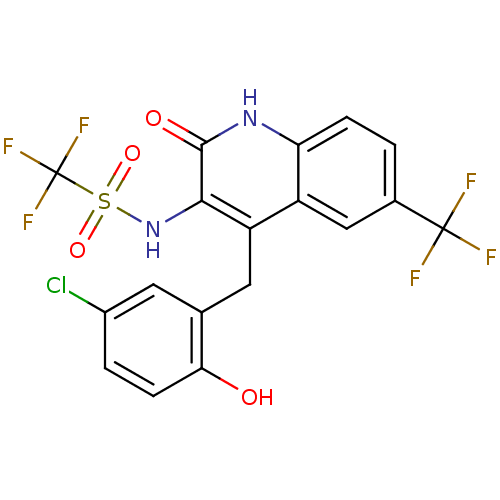 (CHEMBL41078 | N-[4-(5-Chloro-2-hydroxy-benzyl)-2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 663 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Increased outward current at -40 mV mediated by mouse KCNQ2 channel expressed in Xenopus laevis oocytes | Bioorg Med Chem Lett 14: 1615-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.073 BindingDB Entry DOI: 10.7270/Q20V8C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143561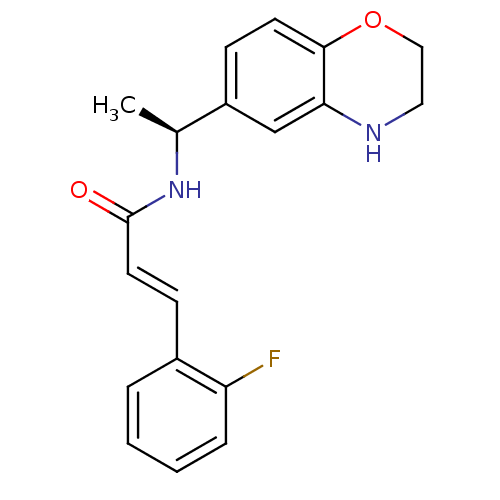 ((E)-N-[(S)-1-(3,4-Dihydro-2H-benzo[1,4]oxazin-6-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143559 ((E)-N-[1-((S)-4-Ethyl-3,4-dihydro-2H-benzo[1,4]oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143560 ((E)-N-[(S)-1-(4-Cyclopropylmethyl-3,4-dihydro-2H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143558 (CHEMBL41355 | EZOGABINE | N-(2-amino-4-(4-fluorobe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143557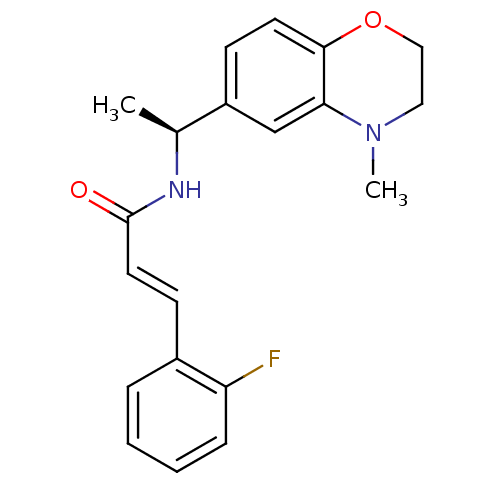 ((E)-3-(2-Fluoro-phenyl)-N-[1-((S)-4-methyl-3,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 940 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |