

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM160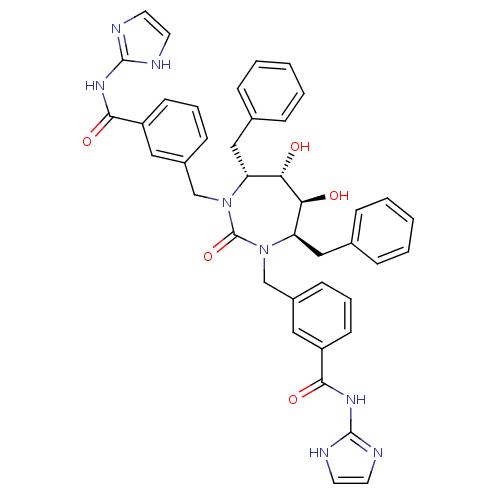 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450717 (CHEMBL317087) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065075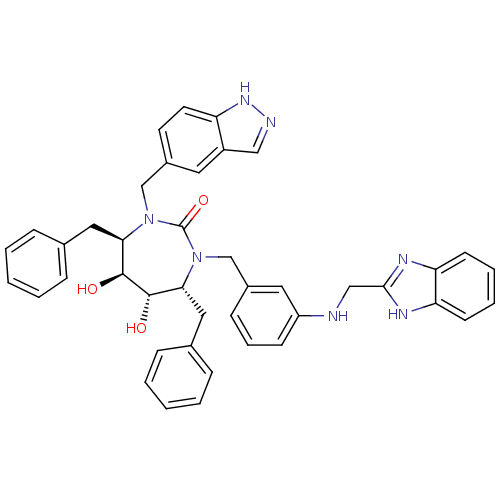 ((4R,5S,6S,7R)-1-{3-[(1H-Benzoimidazol-2-ylmethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065080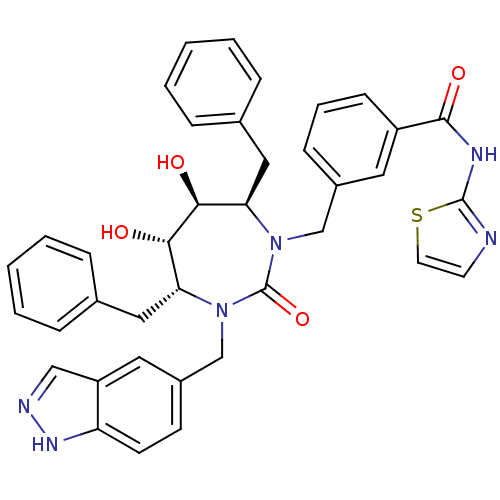 (3-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065089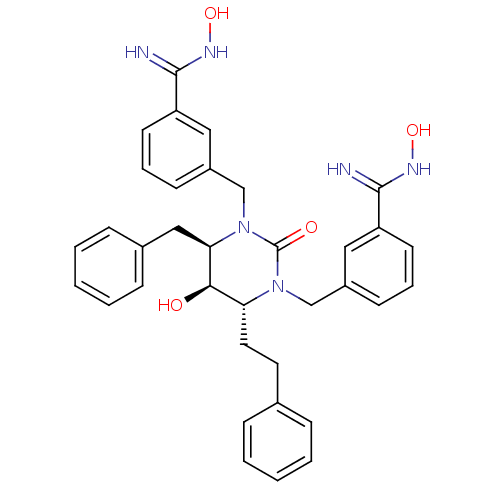 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamide oxime)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065078 ((4R,5S,6S,7R)-1-(3-Amino-4-fluoro-benzyl)-4,7-dibe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM161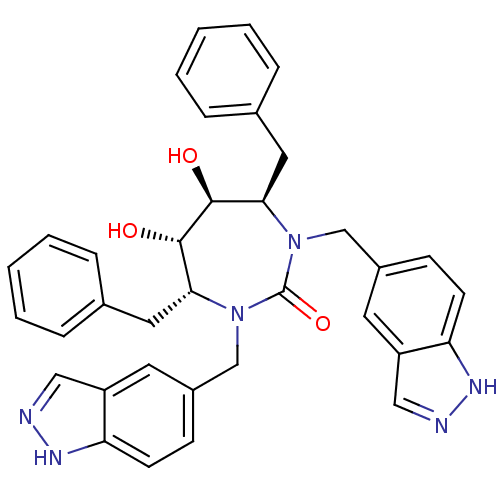 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065086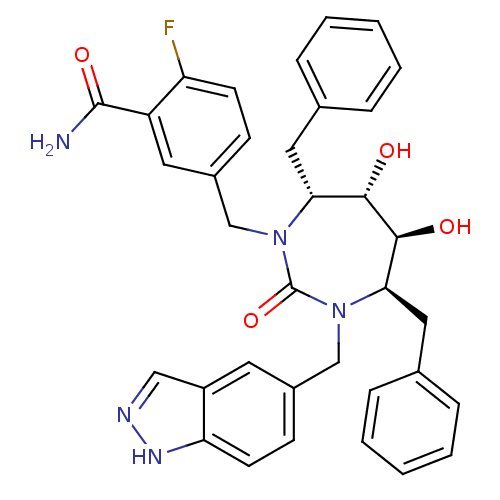 (5-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450726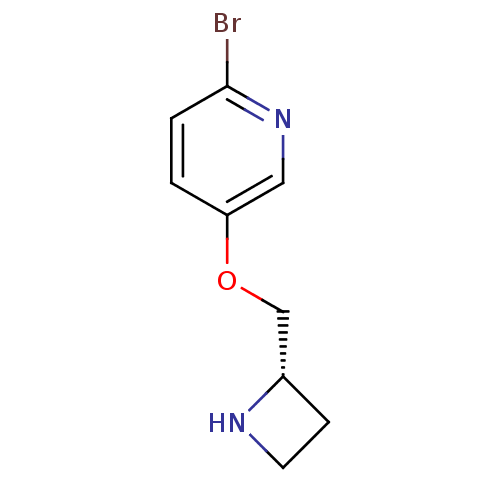 (CHEMBL407258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065087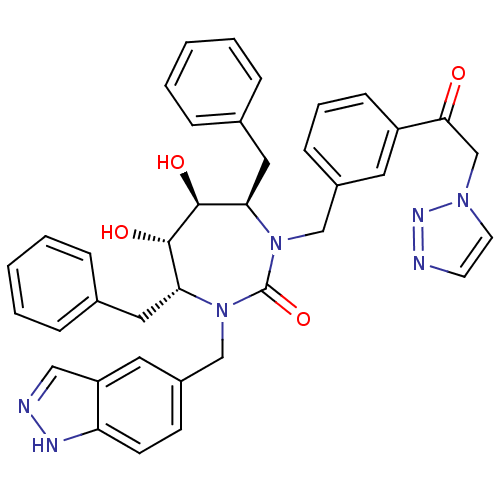 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124714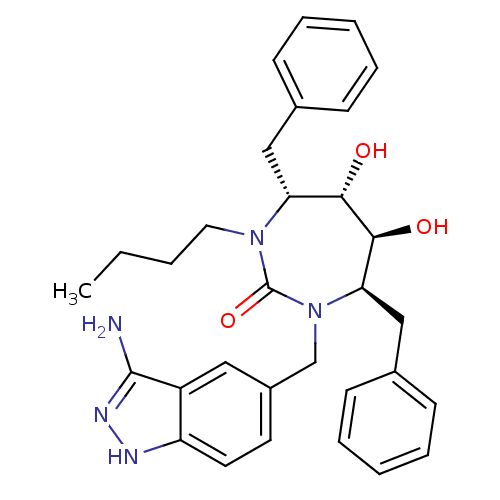 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065071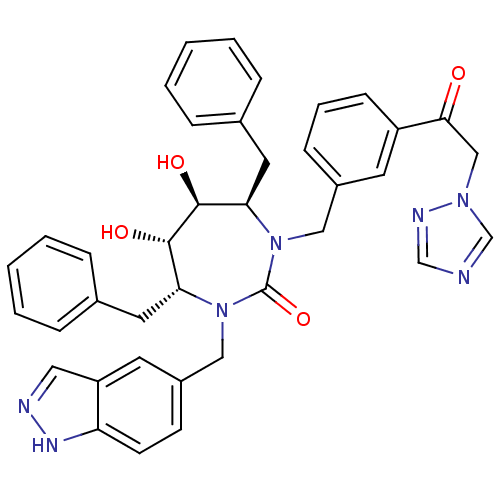 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450734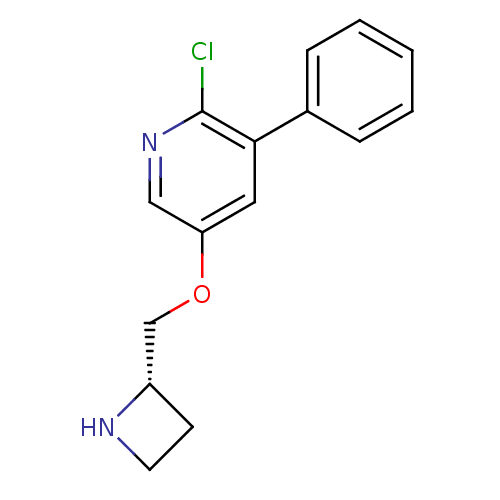 (CHEMBL318869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM164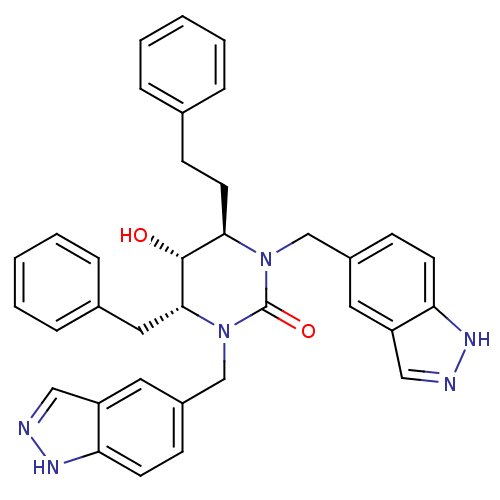 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis(1H-indazol-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450710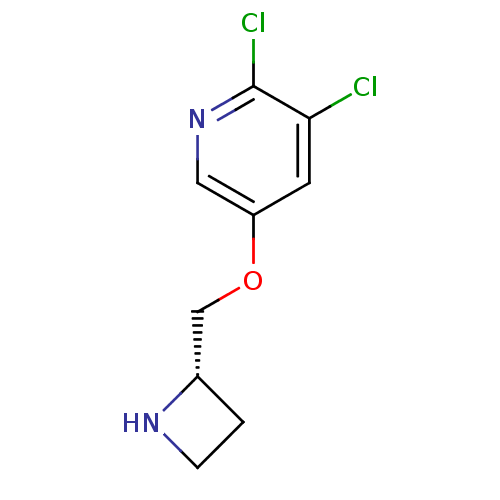 (CHEMBL97555) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM155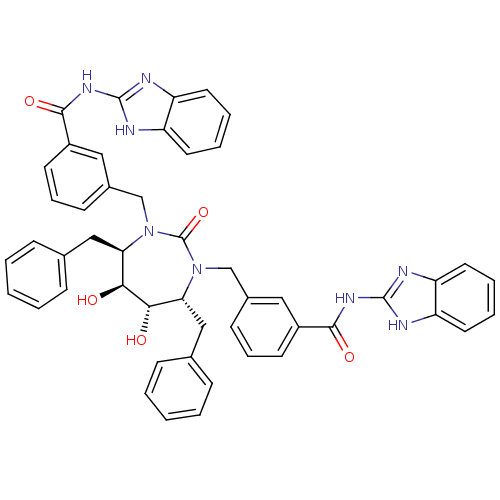 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065060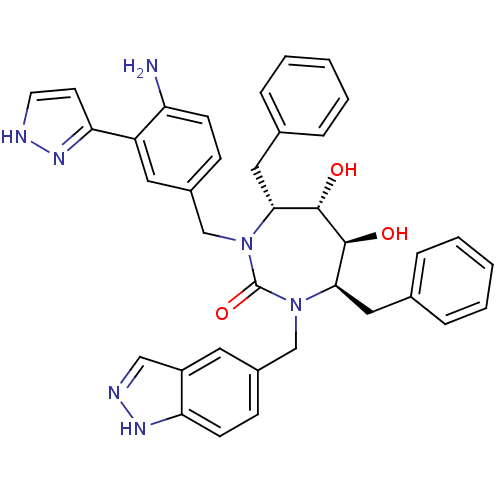 ((4R,5S,6S,7R)-1-[4-Amino-3-(2H-pyrazol-3-yl)-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450720 (CHEMBL94683) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065079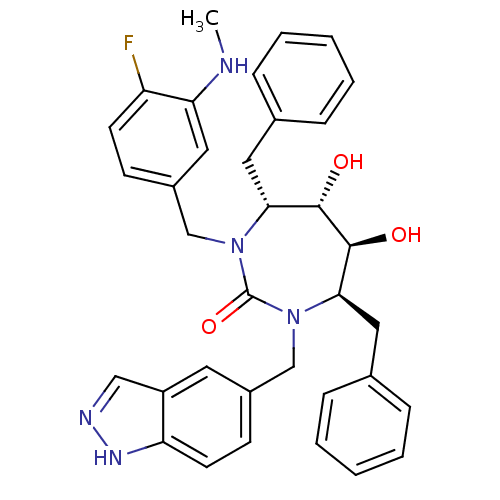 ((4R,5S,6S,7R)-4,7-Dibenzyl-1-(4-fluoro-3-methylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM154 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065069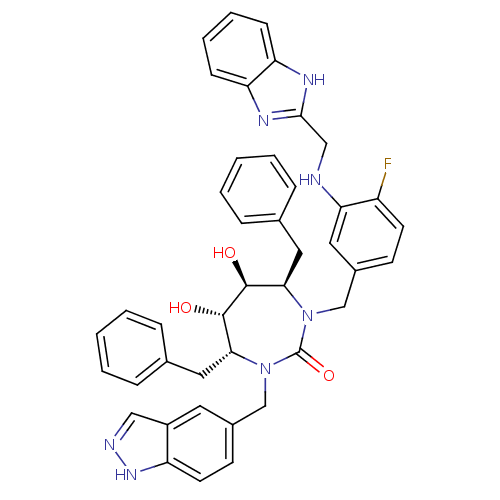 ((4R,5S,6S,7R)-1-{3-[(1H-Benzoimidazol-2-ylmethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065067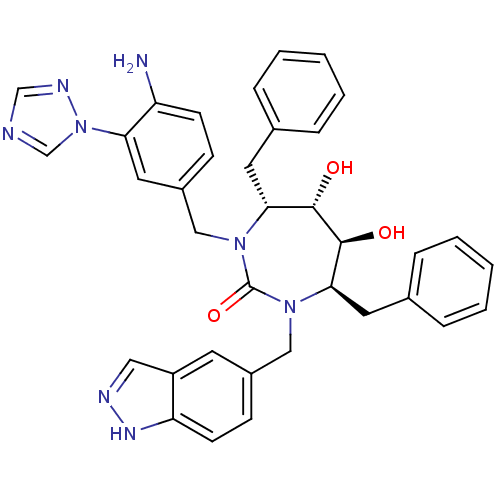 ((4R,5S,6S,7R)-1-(4-Amino-3-[1,2,4]triazol-1-yl-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50143784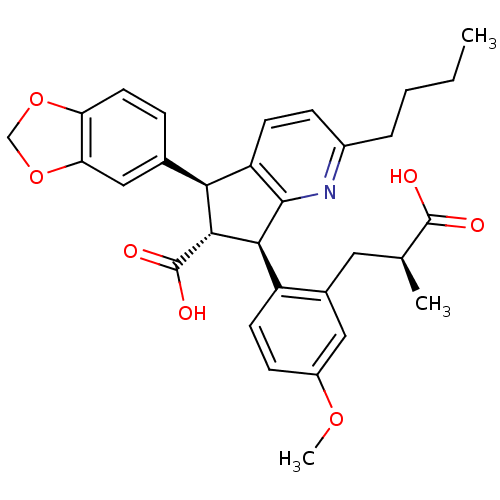 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1262-70 (1999) BindingDB Entry DOI: 10.7270/Q2Q52N5Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065064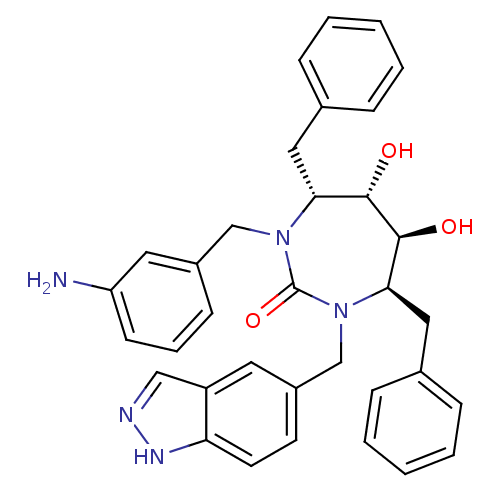 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065082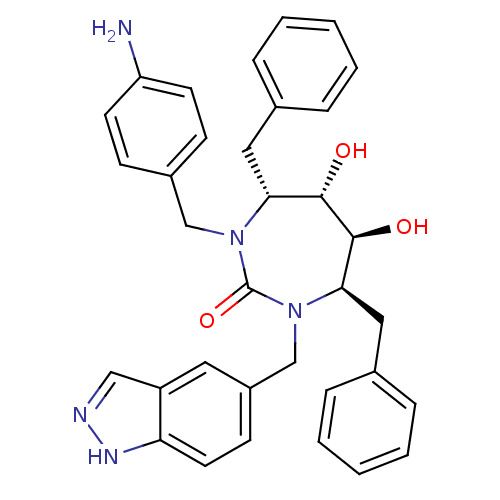 ((4R,5S,6S,7R)-1-(4-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7026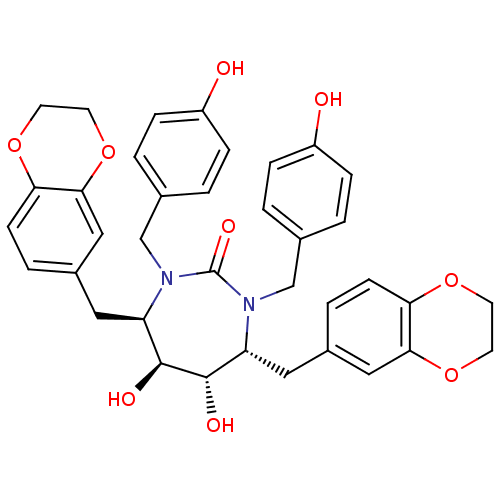 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC1 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124721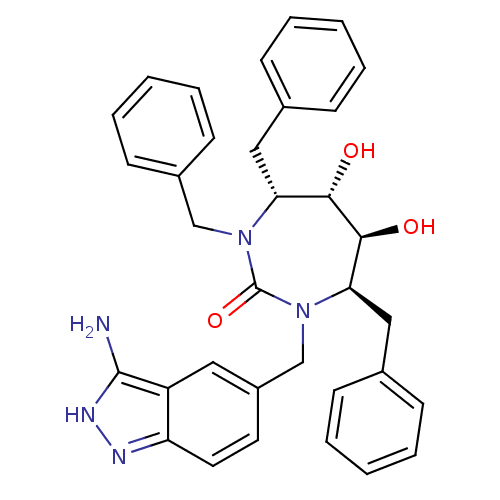 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065074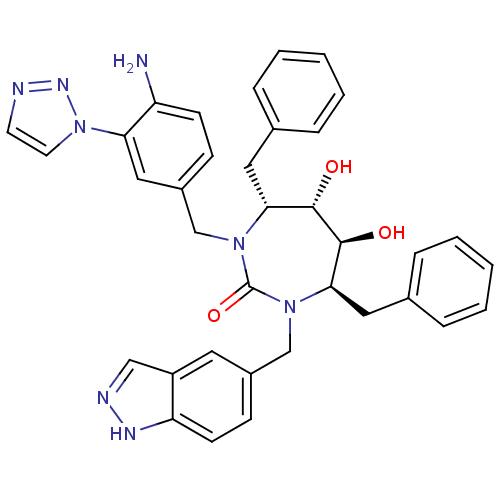 ((4R,5S,6S,7R)-1-(4-Amino-3-[1,2,3]triazol-1-yl-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065091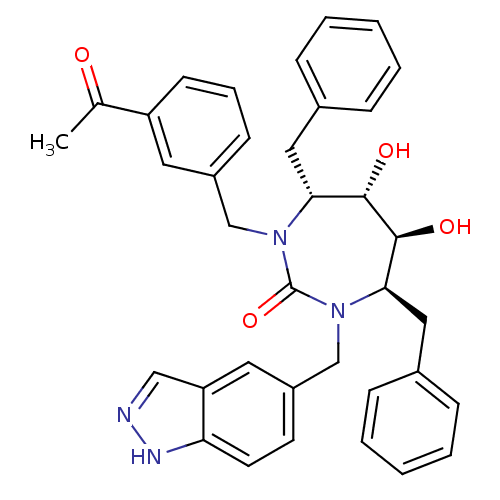 ((4R,5S,6S,7R)-1-(3-Acetyl-benzyl)-4,7-dibenzyl-5,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065068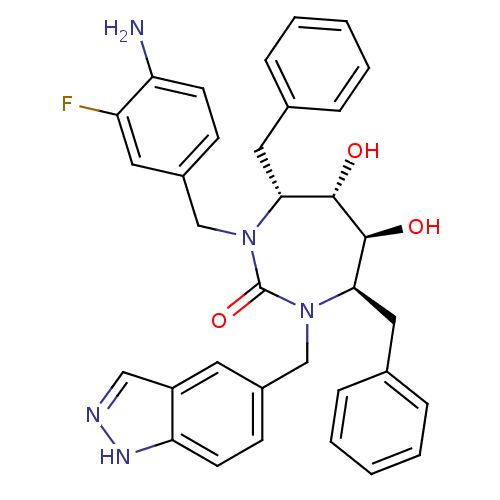 ((4R,5S,6S,7R)-1-(4-Amino-3-fluoro-benzyl)-4,7-dibe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124716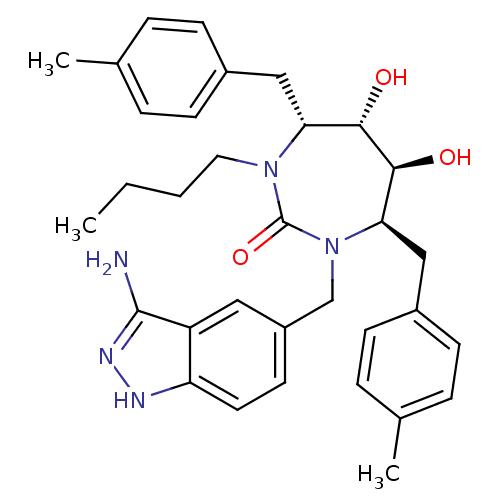 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450712 (CHEMBL96604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450713 (CHEMBL96837) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM156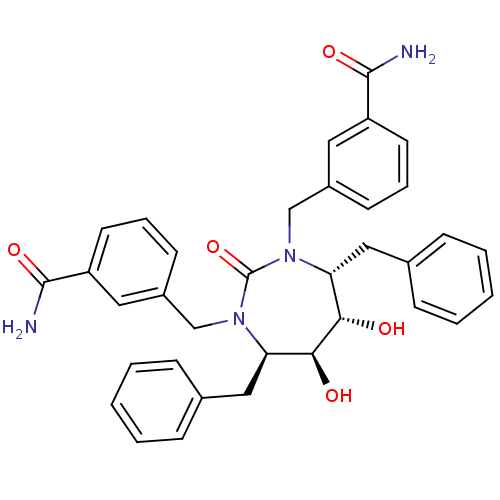 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-3-[(3-carbamoylphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065065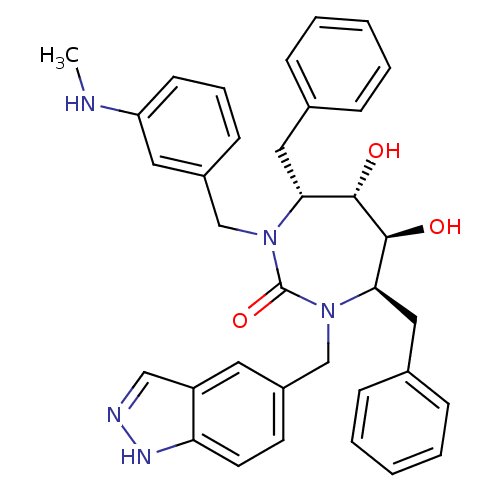 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7019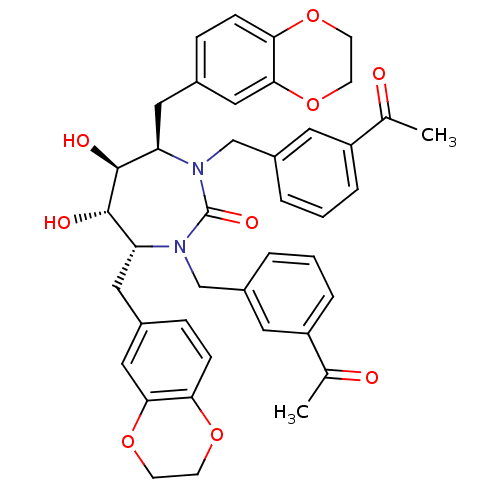 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065073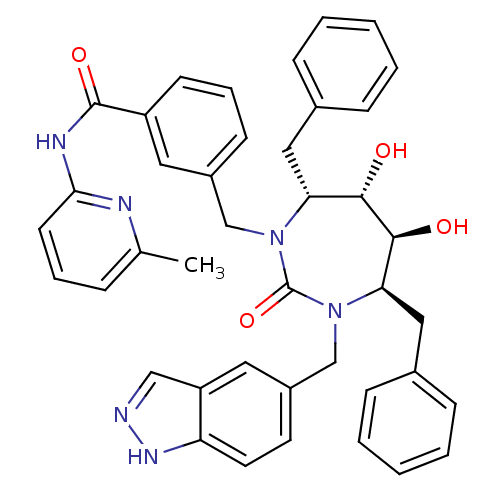 (3-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50062639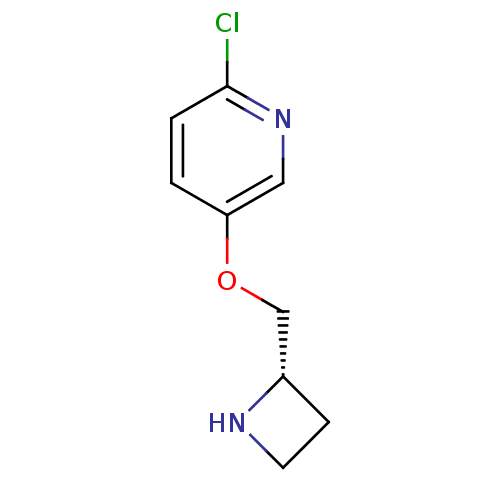 (5-((S)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7024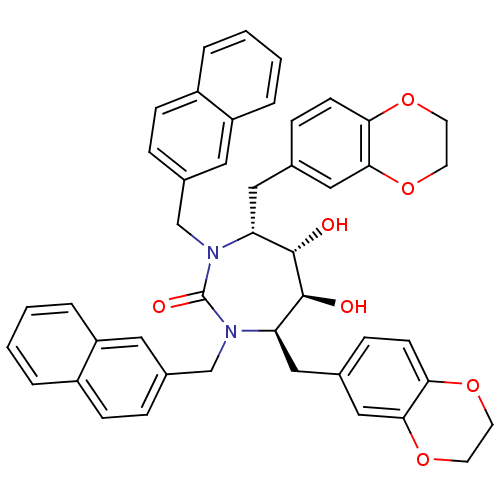 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7020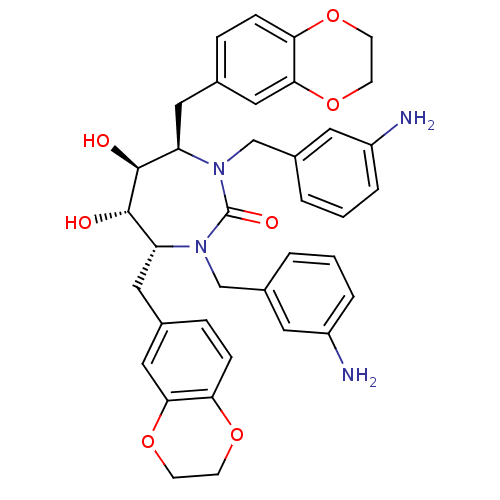 ((4R,5S,6S,7R)-1,3-bis[(3-aminophenyl)methyl]-4,7-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50062641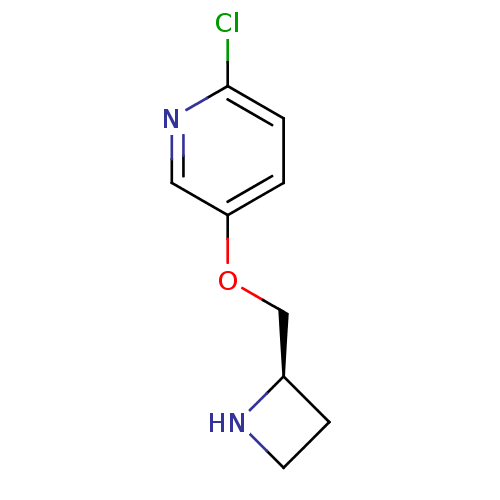 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50173133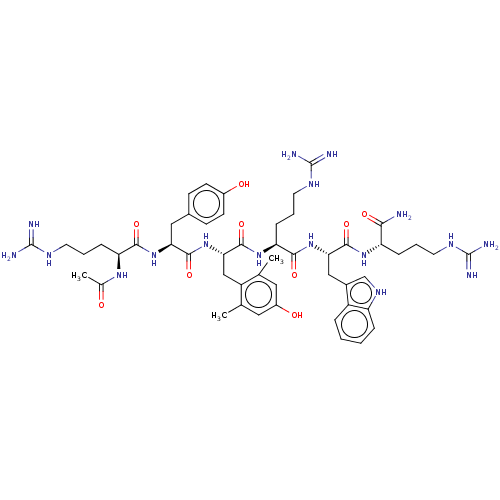 (CHEMBL3810319) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counti... | J Med Chem 59: 3777-92 (2016) Article DOI: 10.1021/acs.jmedchem.5b01976 BindingDB Entry DOI: 10.7270/Q2T72KC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065083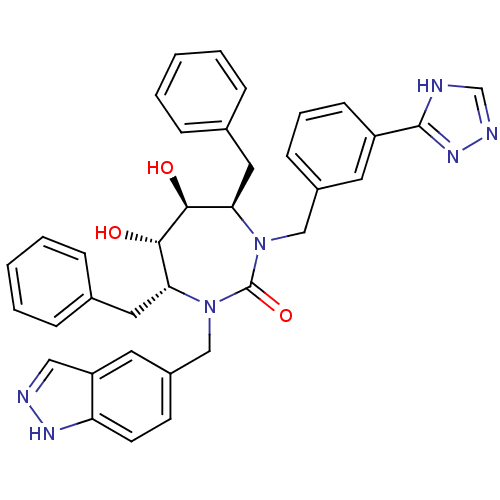 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450723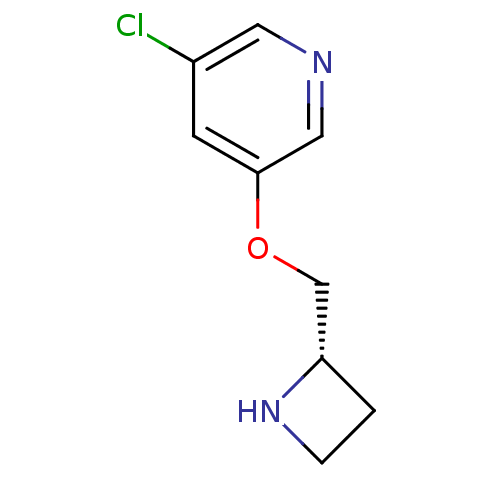 (CHEMBL95113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM159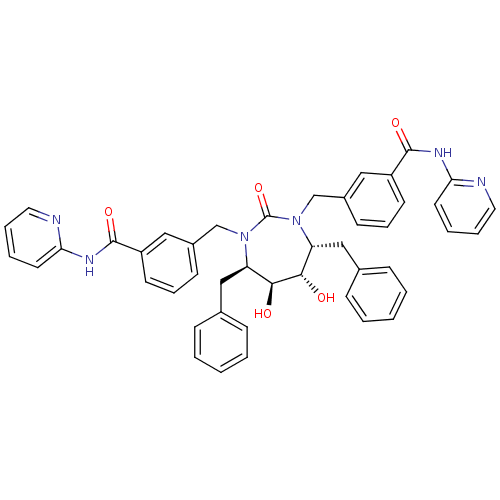 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065092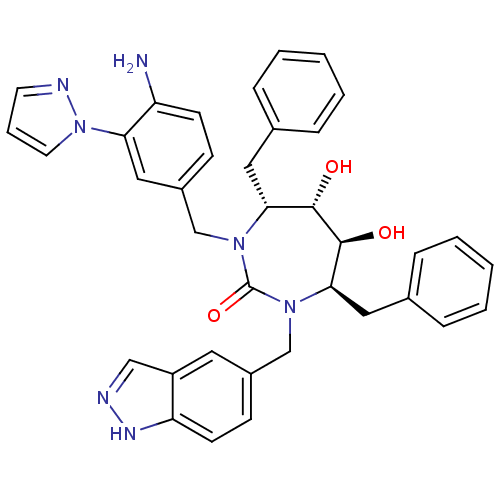 ((4R,5S,6S,7R)-1-(4-Amino-3-pyrazol-1-yl-benzyl)-4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM168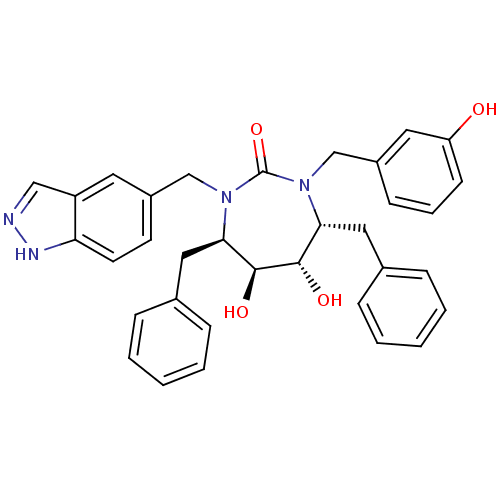 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-[(3-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124715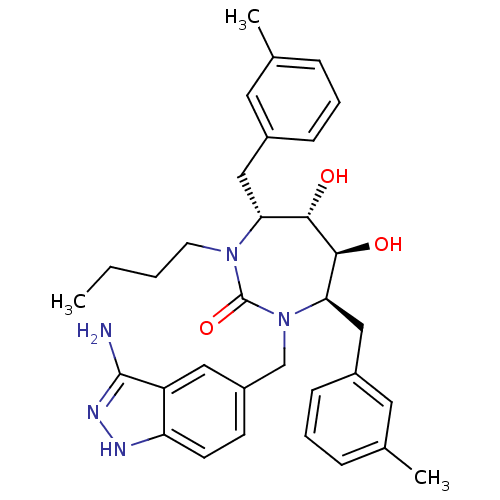 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065085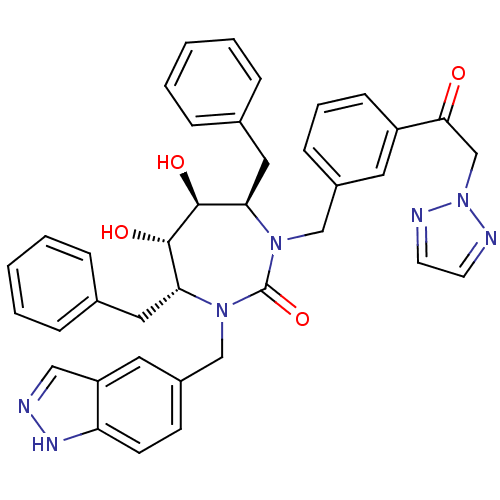 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 24273 total ) | Next | Last >> |