 Found 1289 hits with Last Name = 'gunzner' and Initial = 'j'
Found 1289 hits with Last Name = 'gunzner' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363990
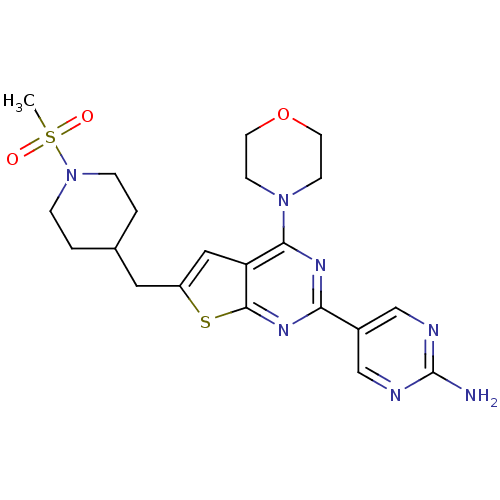
(CHEMBL1949915)Show SMILES CS(=O)(=O)N1CCC(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-4-2-14(3-5-28)10-16-11-17-19(27-6-8-31-9-7-27)25-18(26-20(17)32-16)15-12-23-21(22)24-13-15/h11-14H,2-10H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129179
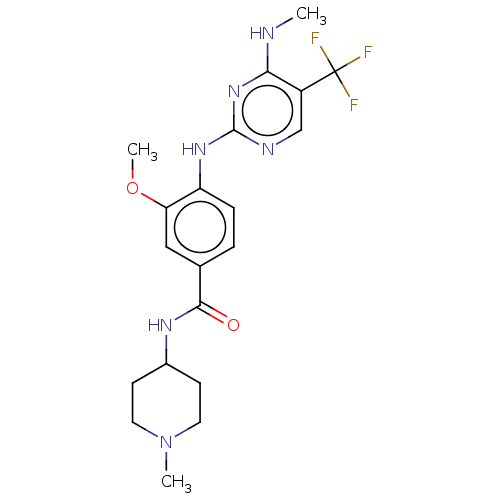
(US8802674, 282)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)NC2CCN(C)CC2)ncc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O2/c1-24-17-14(20(21,22)23)11-25-19(28-17)27-15-5-4-12(10-16(15)31-3)18(30)26-13-6-8-29(2)9-7-13/h4-5,10-11,13H,6-9H2,1-3H3,(H,26,30)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129199
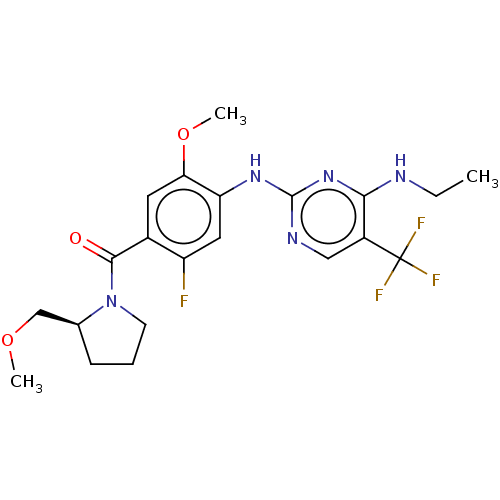
(US8802674, 306)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCC[C@H]2COC)ncc1C(F)(F)F |r| Show InChI InChI=1S/C21H25F4N5O3/c1-4-26-18-14(21(23,24)25)10-27-20(29-18)28-16-9-15(22)13(8-17(16)33-3)19(31)30-7-5-6-12(30)11-32-2/h8-10,12H,4-7,11H2,1-3H3,(H2,26,27,28,29)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129173
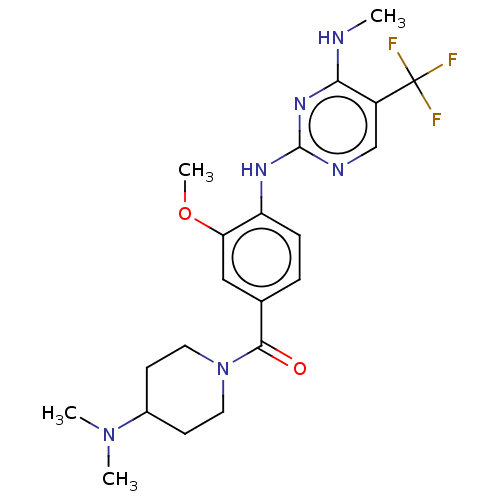
(US8802674, 276)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCC(CC2)N(C)C)ncc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O2/c1-25-18-15(21(22,23)24)12-26-20(28-18)27-16-6-5-13(11-17(16)32-4)19(31)30-9-7-14(8-10-30)29(2)3/h5-6,11-12,14H,7-10H2,1-4H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347087
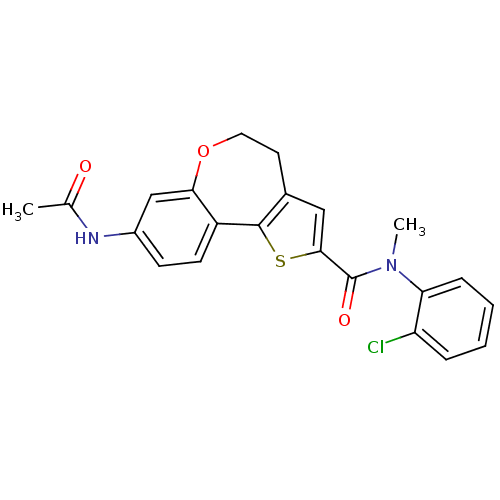
(CHEMBL1796273)Show SMILES CN(C(=O)c1cc2CCOc3cc(NC(C)=O)ccc3-c2s1)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-13(26)24-15-7-8-16-19(12-15)28-10-9-14-11-20(29-21(14)16)22(27)25(2)18-6-4-3-5-17(18)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419754
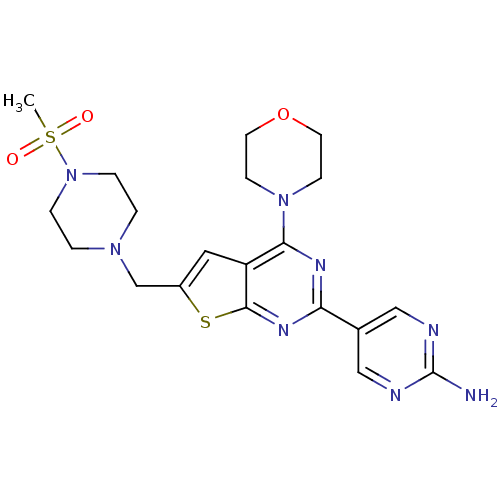
(CHEMBL1949910)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-18(27-6-8-31-9-7-27)24-17(25-19(16)32-15)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398676
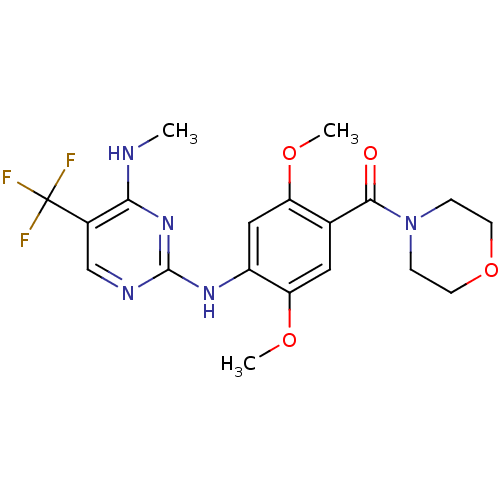
(CHEMBL2178125)Show SMILES CNc1nc(Nc2cc(OC)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O4/c1-23-16-12(19(20,21)22)10-24-18(26-16)25-13-9-14(29-2)11(8-15(13)30-3)17(28)27-4-6-31-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668
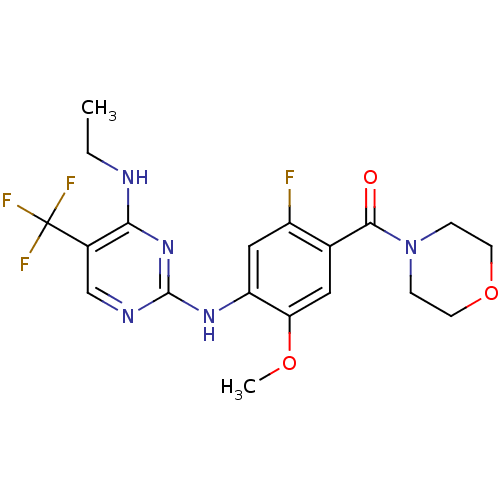
(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 |
J Med Chem 55: 5536-45 (2012)
Article DOI: 10.1021/jm300452p
BindingDB Entry DOI: 10.7270/Q2RR20CQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396150
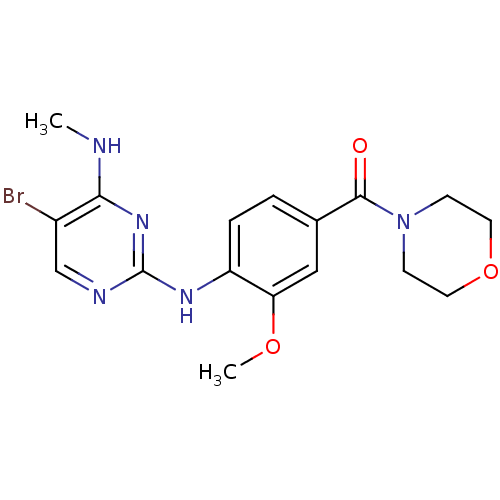
(CHEMBL2171743)Show InChI InChI=1S/C17H20BrN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 |
J Med Chem 55: 5536-45 (2012)
Article DOI: 10.1021/jm300452p
BindingDB Entry DOI: 10.7270/Q2RR20CQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419770
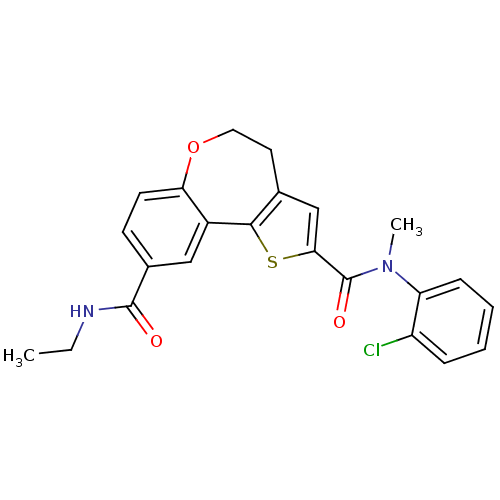
(CHEMBL1950035)Show SMILES CCNC(=O)c1ccc2OCCc3cc(sc3-c2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C23H21ClN2O3S/c1-3-25-22(27)15-8-9-19-16(12-15)21-14(10-11-29-19)13-20(30-21)23(28)26(2)18-7-5-4-6-17(18)24/h4-9,12-13H,3,10-11H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398662
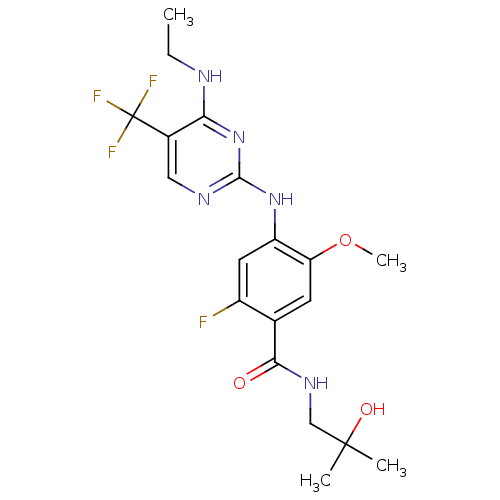
(CHEMBL2178140)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)NCC(C)(C)O)ncc1C(F)(F)F Show InChI InChI=1S/C19H23F4N5O3/c1-5-24-15-11(19(21,22)23)8-25-17(28-15)27-13-7-12(20)10(6-14(13)31-4)16(29)26-9-18(2,3)30/h6-8,30H,5,9H2,1-4H3,(H,26,29)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419769
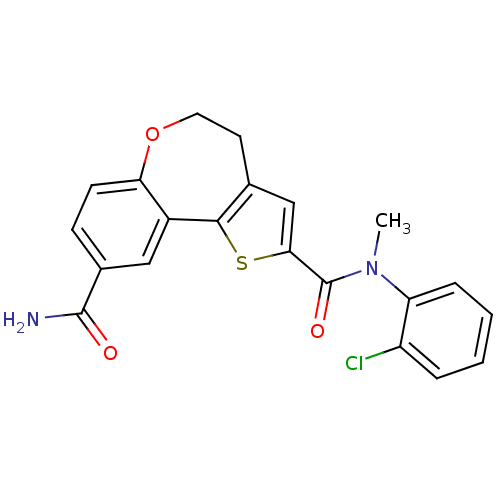
(CHEMBL1950034)Show SMILES CN(C(=O)c1cc2CCOc3ccc(cc3-c2s1)C(N)=O)c1ccccc1Cl Show InChI InChI=1S/C21H17ClN2O3S/c1-24(16-5-3-2-4-15(16)22)21(26)18-11-12-8-9-27-17-7-6-13(20(23)25)10-14(17)19(12)28-18/h2-7,10-11H,8-9H2,1H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347090
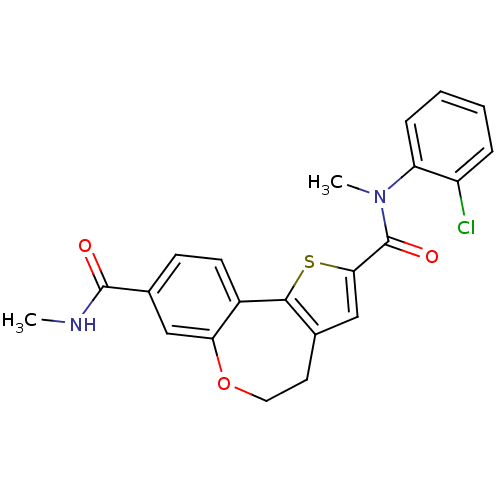
(CHEMBL1796276)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-24-21(26)14-7-8-15-18(11-14)28-10-9-13-12-19(29-20(13)15)22(27)25(2)17-6-4-3-5-16(17)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419761

(CHEMBL1949919)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cc2c(nc(nc2s1)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O3S2/c1-32(28,29)15-4-2-3-13(9-15)17-10-16-19(27-5-7-30-8-6-27)25-18(26-20(16)31-17)14-11-23-21(22)24-12-14/h2-4,9-12H,5-8H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419759
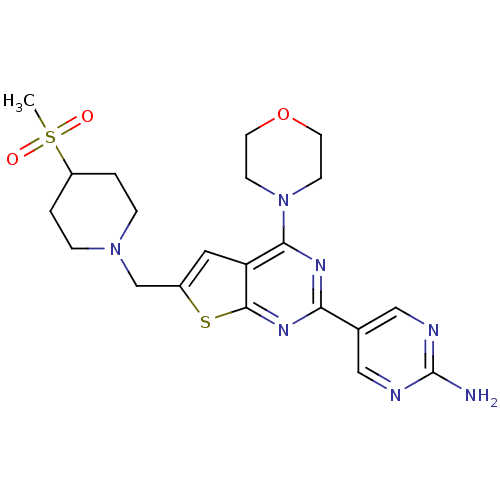
(CHEMBL1949916)Show SMILES CS(=O)(=O)C1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)16-2-4-27(5-3-16)13-15-10-17-19(28-6-8-31-9-7-28)25-18(26-20(17)32-15)14-11-23-21(22)24-12-14/h10-12,16H,2-9,13H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398667

(CHEMBL2178135)Show SMILES COc1cc(C(=O)N2CCOCC2)c(F)cc1Nc1ncc(c(NC2CC2)n1)C(F)(F)F Show InChI InChI=1S/C20H21F4N5O3/c1-31-16-8-12(18(30)29-4-6-32-7-5-29)14(21)9-15(16)27-19-25-10-13(20(22,23)24)17(28-19)26-11-2-3-11/h8-11H,2-7H2,1H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129180
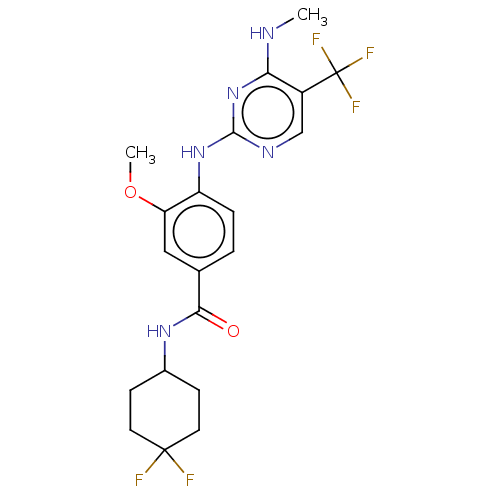
(US8802674, 283)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)NC2CCC(F)(F)CC2)ncc1C(F)(F)F Show InChI InChI=1S/C20H22F5N5O2/c1-26-16-13(20(23,24)25)10-27-18(30-16)29-14-4-3-11(9-15(14)32-2)17(31)28-12-5-7-19(21,22)8-6-12/h3-4,9-10,12H,5-8H2,1-2H3,(H,28,31)(H2,26,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129139
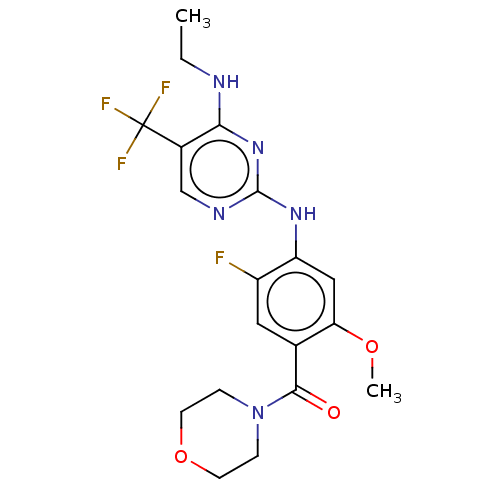
(US8802674, 238)Show SMILES CCNc1nc(Nc2cc(OC)c(cc2F)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-15(30-2)11(8-13(14)20)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129200
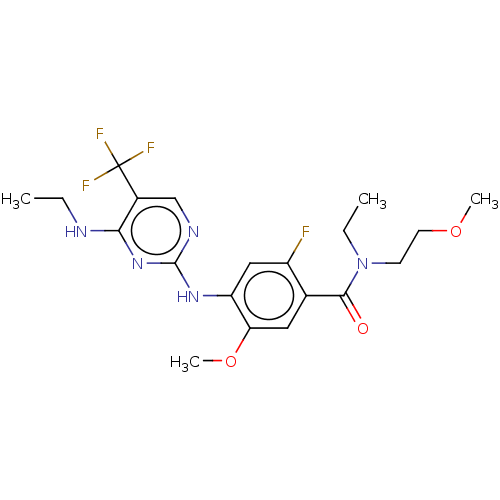
(US8802674, 307)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N(CC)CCOC)ncc1C(F)(F)F Show InChI InChI=1S/C20H25F4N5O3/c1-5-25-17-13(20(22,23)24)11-26-19(28-17)27-15-10-14(21)12(9-16(15)32-4)18(30)29(6-2)7-8-31-3/h9-11H,5-8H2,1-4H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129167
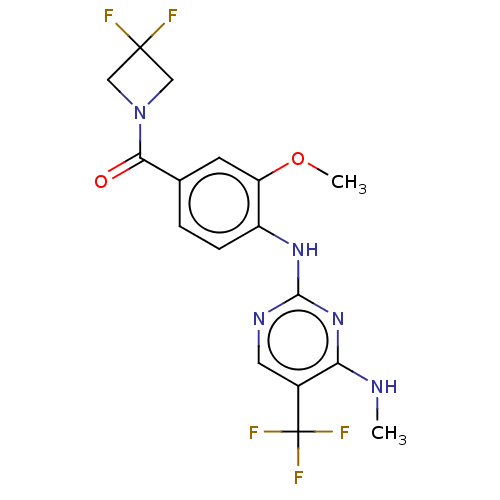
(US8802674, 270)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CC(F)(F)C2)ncc1C(F)(F)F Show InChI InChI=1S/C17H16F5N5O2/c1-23-13-10(17(20,21)22)6-24-15(26-13)25-11-4-3-9(5-12(11)29-2)14(28)27-7-16(18,19)8-27/h3-6H,7-8H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129089
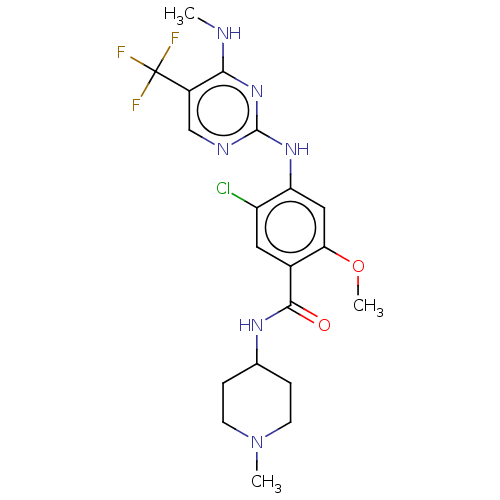
(US8802674, 173)Show SMILES CNc1nc(Nc2cc(OC)c(cc2Cl)C(=O)NC2CCN(C)CC2)ncc1C(F)(F)F Show InChI InChI=1S/C20H24ClF3N6O2/c1-25-17-13(20(22,23)24)10-26-19(29-17)28-15-9-16(32-3)12(8-14(15)21)18(31)27-11-4-6-30(2)7-5-11/h8-11H,4-7H2,1-3H3,(H,27,31)(H2,25,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129047
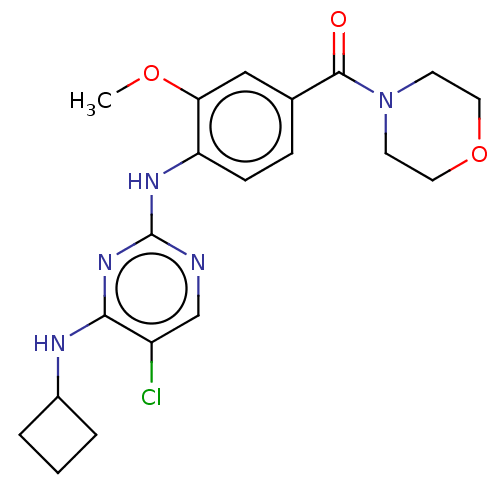
(US8802674, 81)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(NC2CCC2)n1)C(=O)N1CCOCC1 Show InChI InChI=1S/C20H24ClN5O3/c1-28-17-11-13(19(27)26-7-9-29-10-8-26)5-6-16(17)24-20-22-12-15(21)18(25-20)23-14-3-2-4-14/h5-6,11-12,14H,2-4,7-10H2,1H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129197
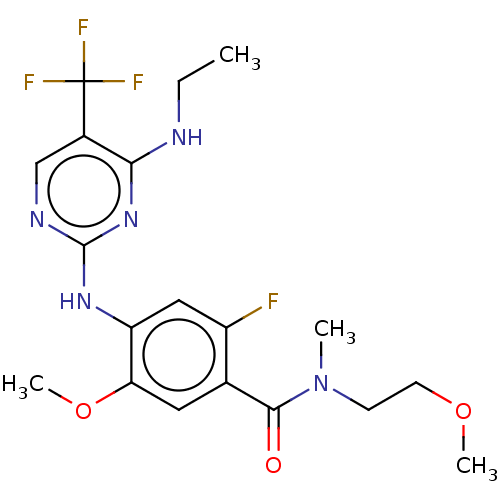
(US8802674, 304)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N(C)CCOC)ncc1C(F)(F)F Show InChI InChI=1S/C19H23F4N5O3/c1-5-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)31-4)17(29)28(2)6-7-30-3/h8-10H,5-7H2,1-4H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129177
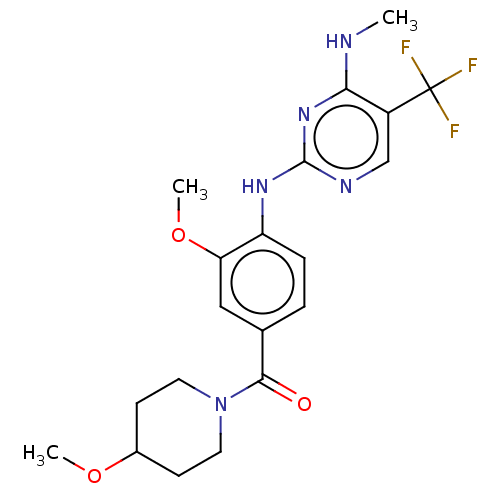
(US8802674, 280)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCC(CC2)OC)ncc1C(F)(F)F Show InChI InChI=1S/C20H24F3N5O3/c1-24-17-14(20(21,22)23)11-25-19(27-17)26-15-5-4-12(10-16(15)31-3)18(29)28-8-6-13(30-2)7-9-28/h4-5,10-11,13H,6-9H2,1-3H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129175
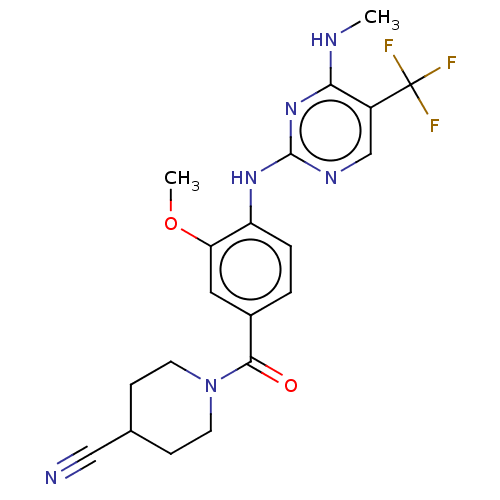
(US8802674, 278)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCC(CC2)C#N)ncc1C(F)(F)F Show InChI InChI=1S/C20H21F3N6O2/c1-25-17-14(20(21,22)23)11-26-19(28-17)27-15-4-3-13(9-16(15)31-2)18(30)29-7-5-12(10-24)6-8-29/h3-4,9,11-12H,5-8H2,1-2H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129169
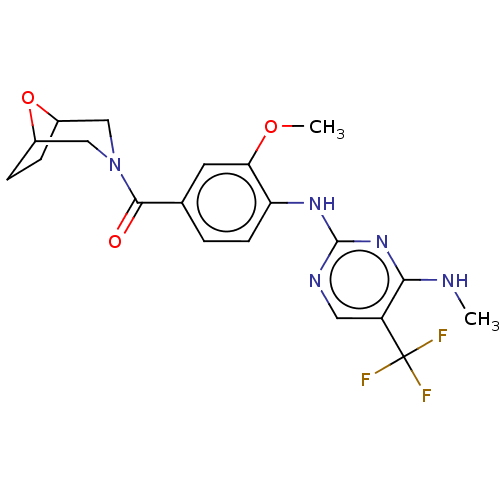
(US8802674, 272)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CC3CCC(C2)O3)ncc1C(F)(F)F Show InChI InChI=1S/C20H22F3N5O3/c1-24-17-14(20(21,22)23)8-25-19(27-17)26-15-6-3-11(7-16(15)30-2)18(29)28-9-12-4-5-13(10-28)31-12/h3,6-8,12-13H,4-5,9-10H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129058
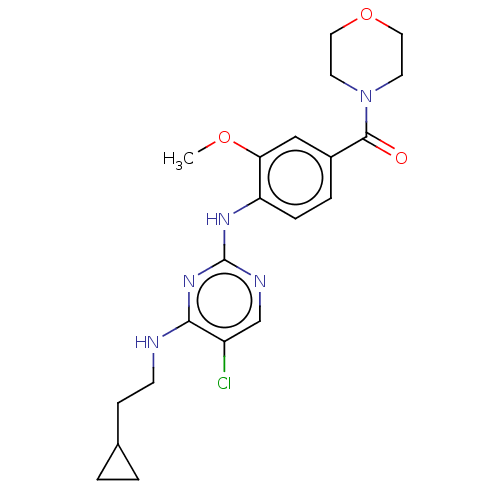
(US8802674, 104)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(NCCC2CC2)n1)C(=O)N1CCOCC1 Show InChI InChI=1S/C21H26ClN5O3/c1-29-18-12-15(20(28)27-8-10-30-11-9-27)4-5-17(18)25-21-24-13-16(22)19(26-21)23-7-6-14-2-3-14/h4-5,12-14H,2-3,6-11H2,1H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
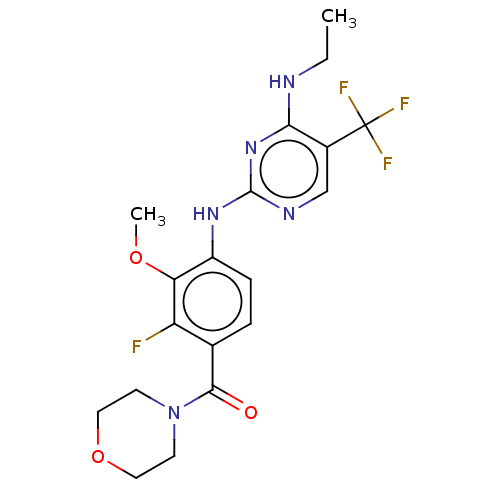
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129141
(US8802674, 241)Show SMILES CCNc1nc(Nc2ccc(C(=O)N3CCOCC3)c(F)c2OC)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-13-5-4-11(14(20)15(13)30-2)17(29)28-6-8-31-9-7-28/h4-5,10H,3,6-9H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
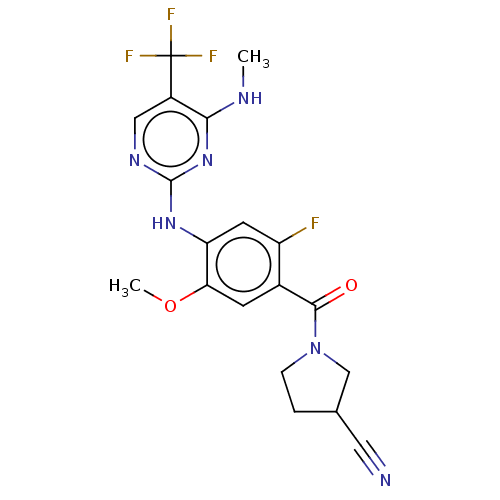
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129094
(US8802674, 178)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCC(C2)C#N)ncc1C(F)(F)F Show InChI InChI=1S/C19H18F4N6O2/c1-25-16-12(19(21,22)23)8-26-18(28-16)27-14-6-13(20)11(5-15(14)31-2)17(30)29-4-3-10(7-24)9-29/h5-6,8,10H,3-4,9H2,1-2H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668

(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
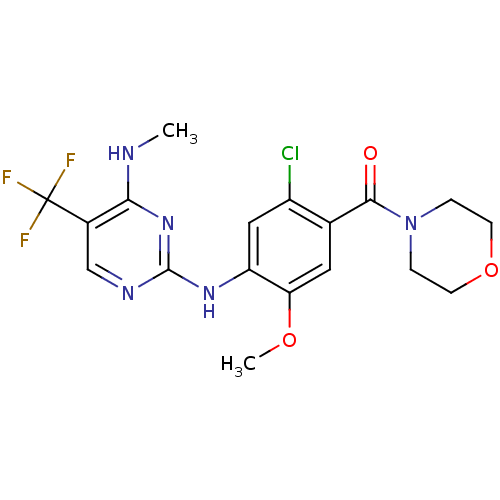
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398677
(CHEMBL2178124 | US8802674, 292)Show SMILES CNc1nc(Nc2cc(Cl)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-12(19)10(7-14(13)29-2)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129176
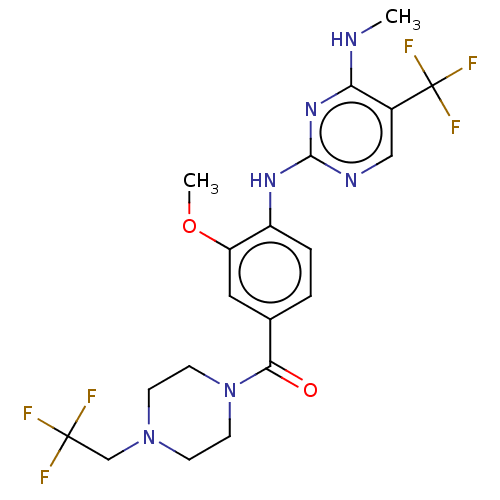
(US8802674, 279)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCN(CC(F)(F)F)CC2)ncc1C(F)(F)F Show InChI InChI=1S/C20H22F6N6O2/c1-27-16-13(20(24,25)26)10-28-18(30-16)29-14-4-3-12(9-15(14)34-2)17(33)32-7-5-31(6-8-32)11-19(21,22)23/h3-4,9-10H,5-8,11H2,1-2H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129152
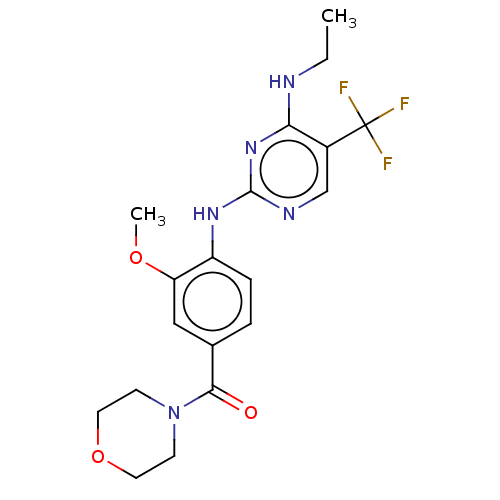
(US8802674, 257)Show SMILES CCNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O3/c1-3-23-16-13(19(20,21)22)11-24-18(26-16)25-14-5-4-12(10-15(14)29-2)17(28)27-6-8-30-9-7-27/h4-5,10-11H,3,6-9H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398675
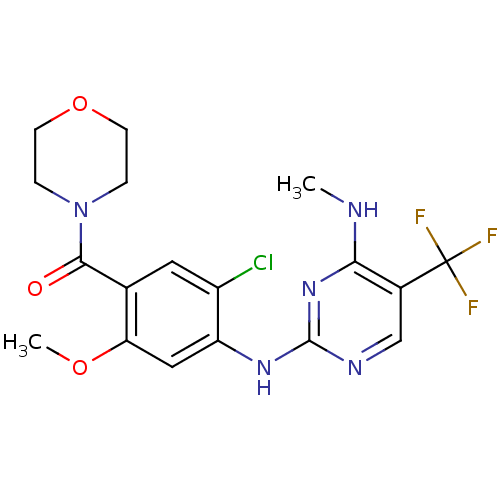
(CHEMBL2178126 | US8802674, 296)Show SMILES CNc1nc(Nc2cc(OC)c(cc2Cl)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-14(29-2)10(7-12(13)19)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419760
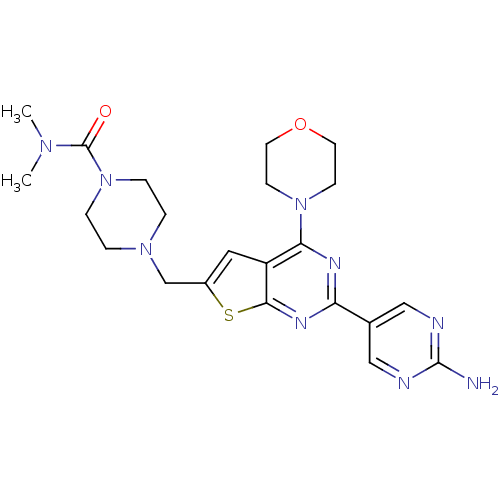
(CHEMBL1949918)Show SMILES CN(C)C(=O)N1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C22H29N9O2S/c1-28(2)22(32)31-5-3-29(4-6-31)14-16-11-17-19(30-7-9-33-10-8-30)26-18(27-20(17)34-16)15-12-24-21(23)25-13-15/h11-13H,3-10,14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129032
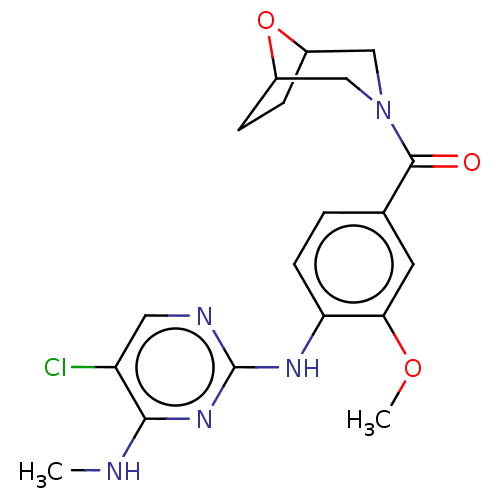
(US8802674, 63)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CC3CCC(C2)O3)ncc1Cl Show InChI InChI=1S/C19H22ClN5O3/c1-21-17-14(20)8-22-19(24-17)23-15-6-3-11(7-16(15)27-2)18(26)25-9-12-4-5-13(10-25)28-12/h3,6-8,12-13H,4-5,9-10H2,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419773
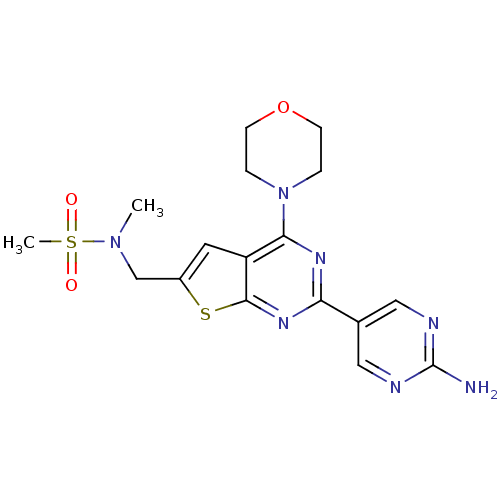
(CHEMBL1949917)Show SMILES CN(Cc1cc2c(nc(nc2s1)-c1cnc(N)nc1)N1CCOCC1)S(C)(=O)=O Show InChI InChI=1S/C17H21N7O3S2/c1-23(29(2,25)26)10-12-7-13-15(24-3-5-27-6-4-24)21-14(22-16(13)28-12)11-8-19-17(18)20-9-11/h7-9H,3-6,10H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129124
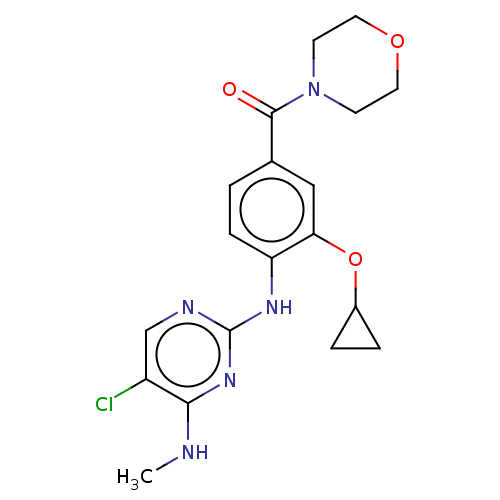
(US8802674, 217)Show SMILES CNc1nc(Nc2ccc(cc2OC2CC2)C(=O)N2CCOCC2)ncc1Cl Show InChI InChI=1S/C19H22ClN5O3/c1-21-17-14(20)11-22-19(24-17)23-15-5-2-12(10-16(15)28-13-3-4-13)18(26)25-6-8-27-9-7-25/h2,5,10-11,13H,3-4,6-9H2,1H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398665
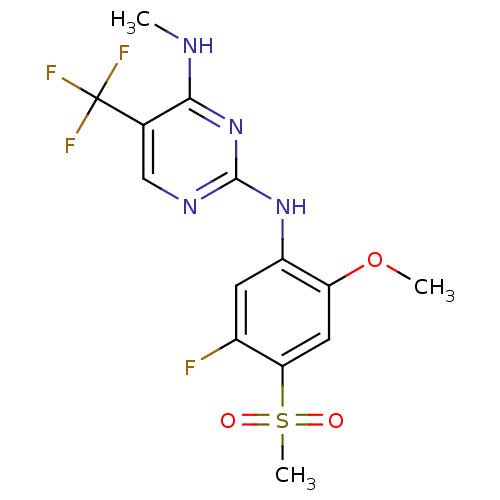
(CHEMBL2178137 | US9145402, 15)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C14H14F4N4O3S/c1-19-12-7(14(16,17)18)6-20-13(22-12)21-9-4-8(15)11(26(3,23)24)5-10(9)25-2/h4-6H,1-3H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129028
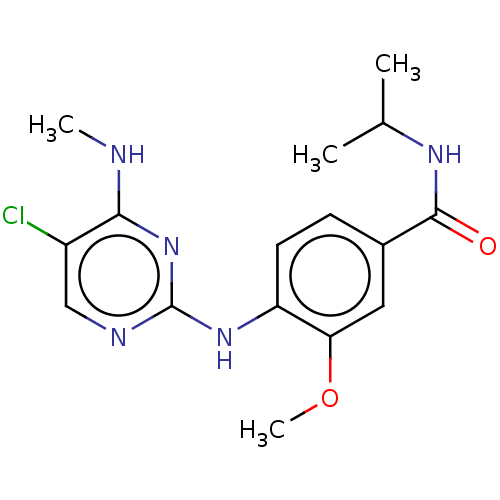
(US8802674, 53)Show InChI InChI=1S/C16H20ClN5O2/c1-9(2)20-15(23)10-5-6-12(13(7-10)24-4)21-16-19-8-11(17)14(18-3)22-16/h5-9H,1-4H3,(H,20,23)(H2,18,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419758

(CHEMBL1949914)Show SMILES Nc1ncc(cn1)-c1nc(N2CCOCC2)c2cc(CC3CCN(CC(F)(F)F)CC3)sc2n1 Show InChI InChI=1S/C22H26F3N7OS/c23-22(24,25)13-31-3-1-14(2-4-31)9-16-10-17-19(32-5-7-33-8-6-32)29-18(30-20(17)34-16)15-11-27-21(26)28-12-15/h10-12,14H,1-9,13H2,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396150

(CHEMBL2171743)Show InChI InChI=1S/C17H20BrN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398677

(CHEMBL2178124 | US8802674, 292)Show SMILES CNc1nc(Nc2cc(Cl)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-12(19)10(7-14(13)29-2)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
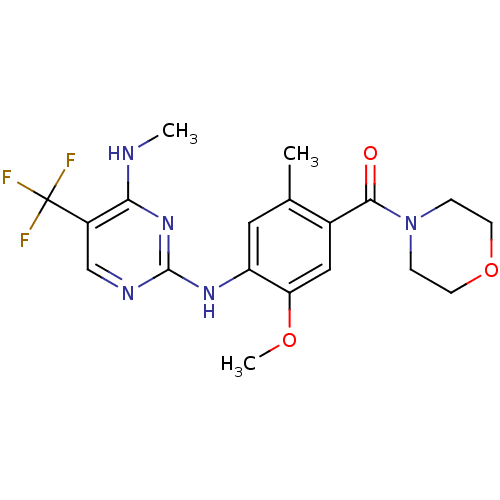
(Homo sapiens (Human)) | BDBM50398672
(CHEMBL2178130)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O3/c1-11-8-14(25-18-24-10-13(19(20,21)22)16(23-2)26-18)15(29-3)9-12(11)17(28)27-4-6-30-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129056
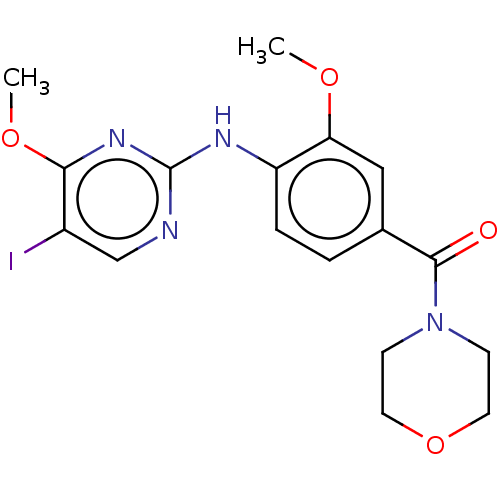
(US8802674, 101)Show InChI InChI=1S/C17H19IN4O4/c1-24-14-9-11(16(23)22-5-7-26-8-6-22)3-4-13(14)20-17-19-10-12(18)15(21-17)25-2/h3-4,9-10H,5-8H2,1-2H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129202
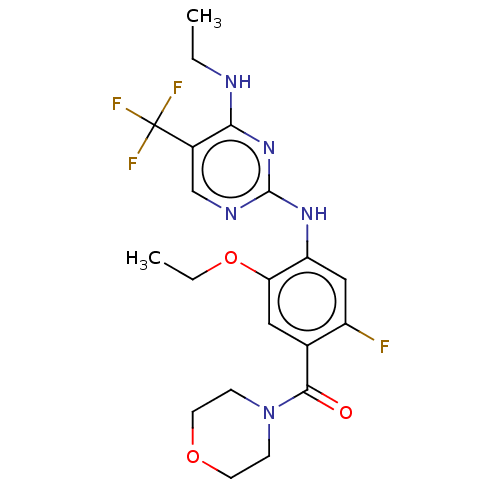
(US8802674, 309)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OCC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C20H23F4N5O3/c1-3-25-17-13(20(22,23)24)11-26-19(28-17)27-15-10-14(21)12(9-16(15)32-4-2)18(30)29-5-7-31-8-6-29/h9-11H,3-8H2,1-2H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129036
(US8802674, 70)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCN(CC2)C(=O)C2CC2)ncc1Cl Show InChI InChI=1S/C21H25ClN6O3/c1-23-18-15(22)12-24-21(26-18)25-16-6-5-14(11-17(16)31-2)20(30)28-9-7-27(8-10-28)19(29)13-3-4-13/h5-6,11-13H,3-4,7-10H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data