

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199123 (N-(4-(1-(4,6-bis((4-hydroxyphenyl)amino)-1,3,5-tri...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073116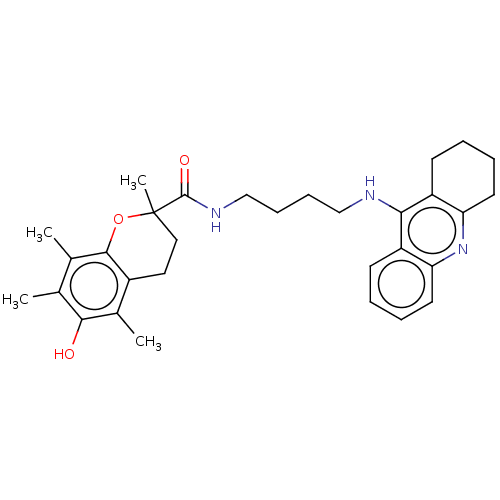 (CHEMBL3410952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199123 (N-(4-(1-(4,6-bis((4-hydroxyphenyl)amino)-1,3,5-tri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.12 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073114 (CHEMBL3410954) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073115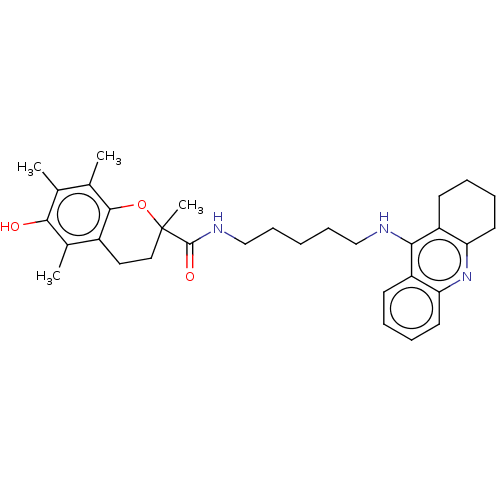 (CHEMBL3410953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199124 (N-(4-(1-(4,6-bis((4-methoxyphenyl)amino)-1,3,5-tri...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073116 (CHEMBL3410952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199124 (N-(4-(1-(4,6-bis((4-methoxyphenyl)amino)-1,3,5-tri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073113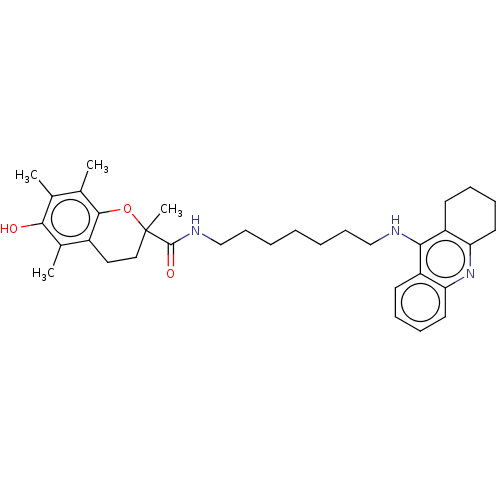 (CHEMBL3410955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073114 (CHEMBL3410954) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199128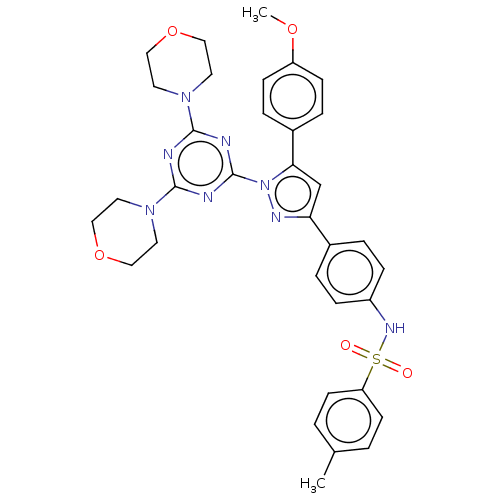 (N-(4-(1-(4,6-dimorpholino-1,3,5-triazin-2-yl)-5-(4...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31.2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073115 (CHEMBL3410953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073112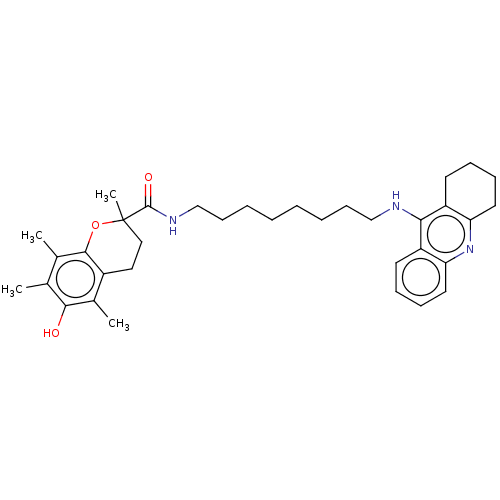 (CHEMBL3410956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073117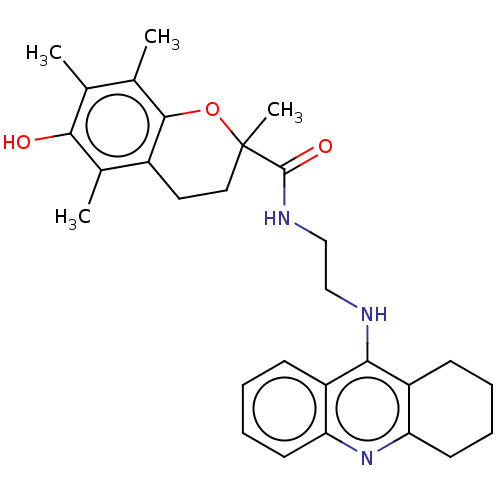 (CHEMBL3410951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199128 (N-(4-(1-(4,6-dimorpholino-1,3,5-triazin-2-yl)-5-(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42.3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199127 (N-(4-(1-(4,6-di(piperazin-1-yl)-1,3,5-triazin-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47.1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199127 (N-(4-(1-(4,6-di(piperazin-1-yl)-1,3,5-triazin-2-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55.3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073117 (CHEMBL3410951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199125 (N-(4-(1-(4,6-bis(o-tolylamino)-1,3,5-triazin-2-yl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58.2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073113 (CHEMBL3410955) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199125 (N-(4-(1-(4,6-bis(o-tolylamino)-1,3,5-triazin-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67.1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199126 (N-(4-(1-(4,6-bis((4-nitrophenyl)amino)-1,3,5-triaz...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199126 (N-(4-(1-(4,6-bis((4-nitrophenyl)amino)-1,3,5-triaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76.6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073113 (CHEMBL3410955) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199122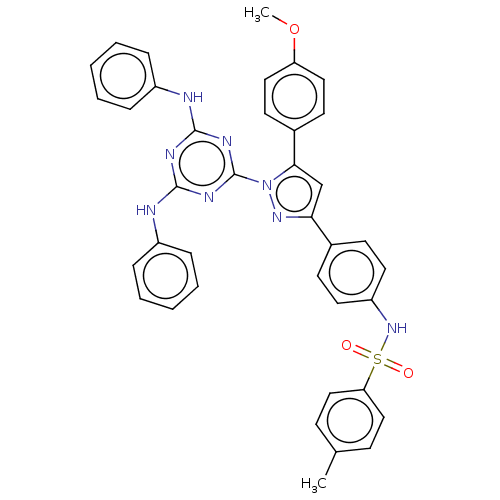 (N-(4-(1-(4,6-bis(phenylamino)-1,3,5-triazin-2-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073142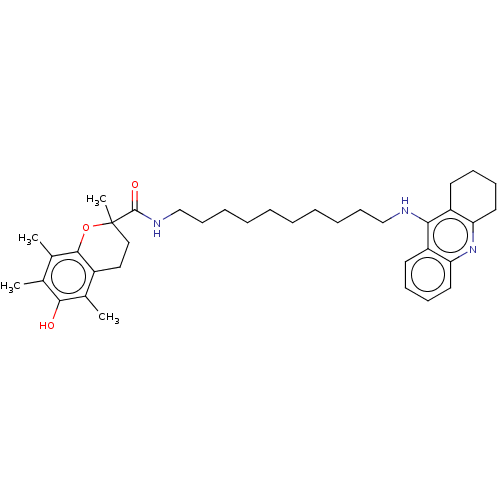 (CHEMBL3410957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199122 (N-(4-(1-(4,6-bis(phenylamino)-1,3,5-triazin-2-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073113 (CHEMBL3410955) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073117 (CHEMBL3410951) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073112 (CHEMBL3410956) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199121 (N-(4-(1-(4,6-bis((3-aminopropyl)amino)-1,3,5-triaz...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199121 (N-(4-(1-(4,6-bis((3-aminopropyl)amino)-1,3,5-triaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073115 (CHEMBL3410953) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073112 (CHEMBL3410956) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073112 (CHEMBL3410956) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073115 (CHEMBL3410953) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073142 (CHEMBL3410957) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073117 (CHEMBL3410951) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM199120 (N-(4-(1-(4,6-bis(ethylamino)-1,3,5-triazin-2-yl)-5...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 302 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Rattus norvegicus (Rat)) | BDBM199120 (N-(4-(1-(4,6-bis(ethylamino)-1,3,5-triazin-2-yl)-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 332 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Zhejiang Hospital | Assay Description where a pro-fluorescing peptide is used as substrate, and the fluorogenic activity of its cleavage product is measured after co-incubation with the a... | Chem Biol Drug Des 88: 756-765 (2016) Article DOI: 10.1111/cbdd.12807 BindingDB Entry DOI: 10.7270/Q2ZW1JR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073142 (CHEMBL3410957) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 435 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073142 (CHEMBL3410957) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 453 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073116 (CHEMBL3410952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 535 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073116 (CHEMBL3410952) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 558 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |