

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human recombinant dopamine D2S receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50435127 (CHEMBL2392022) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Peptide YY from neuropeptide Y receptor type 2 in human KAN-TS cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM85035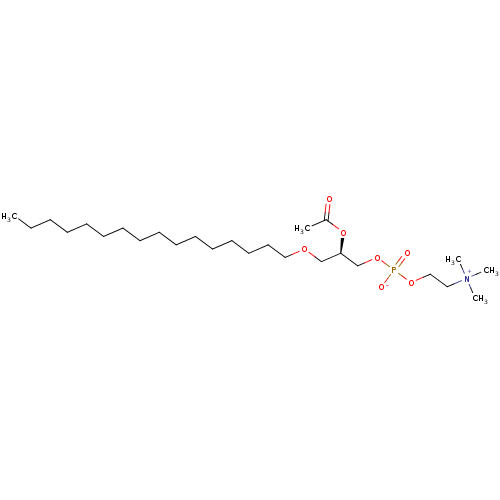 (CAS_65154-06-5 | PAF | bloodplatelet-activatingfac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]PAF from platelet activating factor receptor in human platelets after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490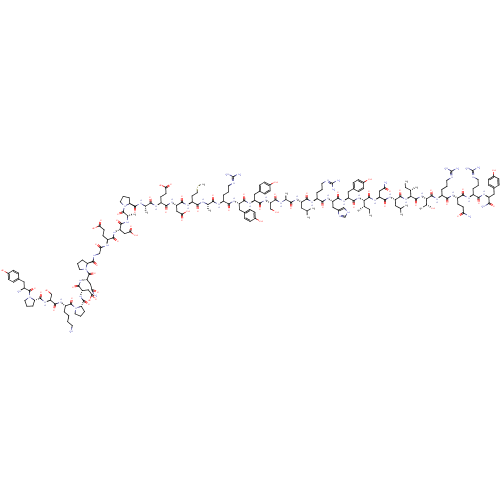 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Peptide YY from neuropeptide Y receptor type 1 in human SK-N-MC cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156454 (CHEMBL264100 | des-Arg10-Kallidin) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H](Des-Arg10)-Kallidin from bradykinin B1 receptor in human IMR90 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50049949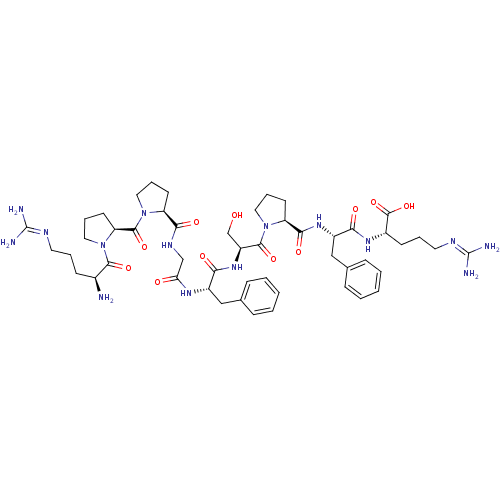 ((BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH | (b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Bradykinin from human recombinant bradykinin B2 receptor expressed in CHEM1 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Pyrilamine from human recombinant histamine H1 receptor expressed in CHOK1 cells after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Dexamethasone from glucocorticoid receptor in human HeLaS3 cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM82561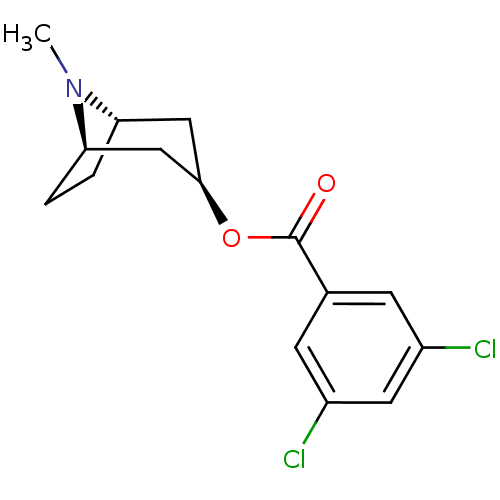 (CAS_40796-97-2 | TROPANYL 3,5-DICHLOROBENZOATE | T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from human recombinant 5HT3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50435126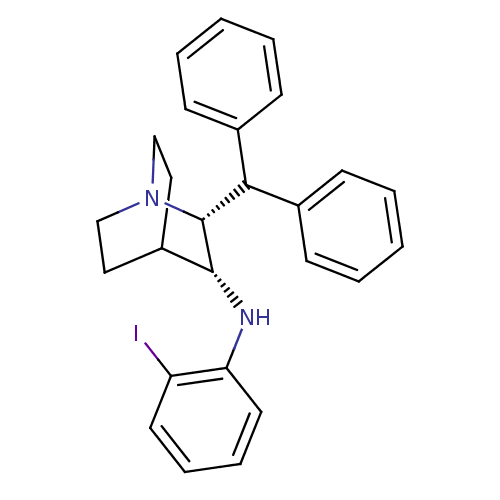 (CHEMBL2392023) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Substance P from human recombinant substance P receptor expressed in CHO cells after 90 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid type B receptor subunit 1 (Homo sapiens (Human)) | BDBM50435128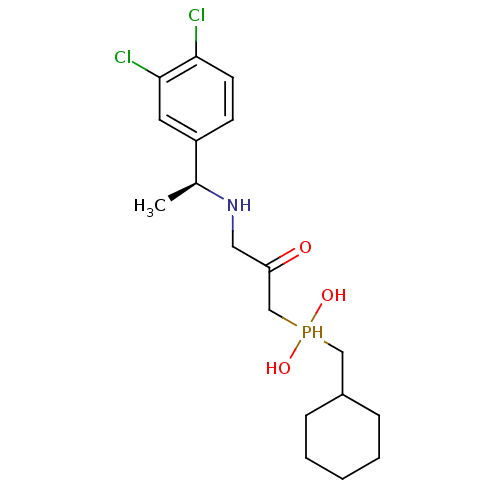 (CHEMBL2391908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]CGP54626 from human recombinant GABAB1A receptor expressed in CHO cells after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM8885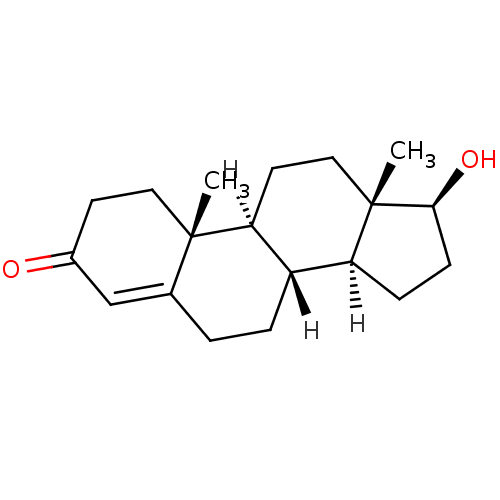 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Mibolerone from rat recombinant androgen receptor expressed in Escherichia coli after 4 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24732 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 32 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Haloperidol from sigma 1 receptor in human jurkat cells after 4 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24731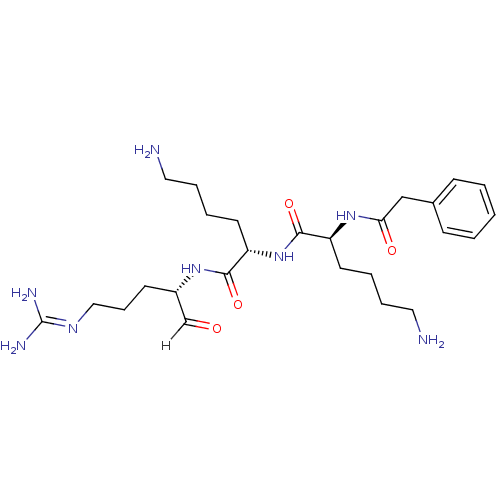 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | -47.8 | 51 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24734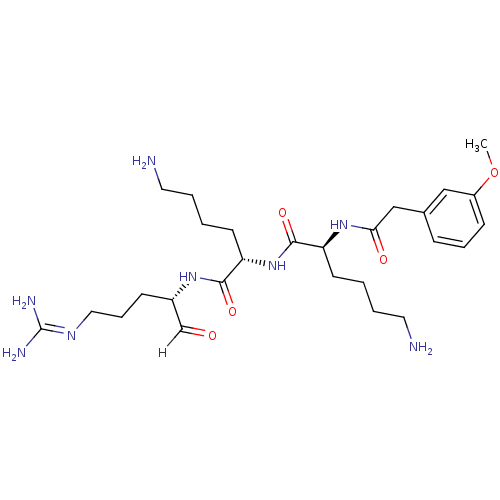 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | 60 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24746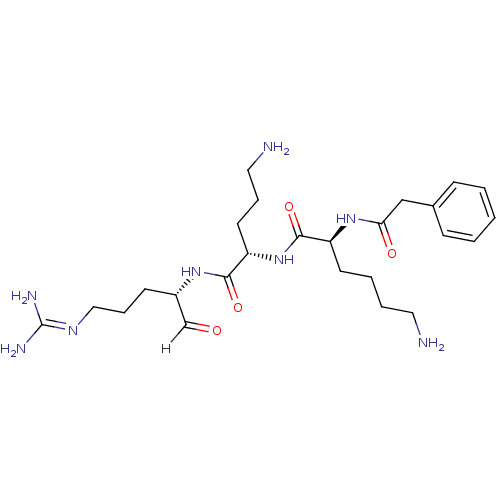 ((2S)-6-amino-N-[(1S)-4-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -46.8 | 73 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM22568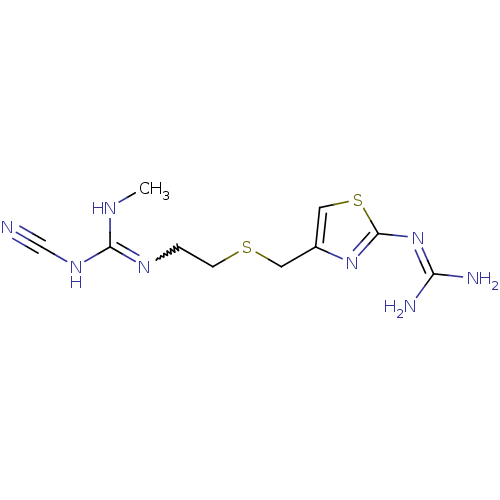 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Aminopotentidine from human recombinant histamine H2 receptor expressed in CHOK1 cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24739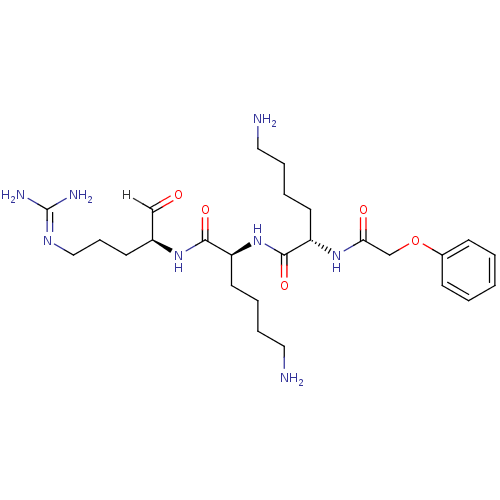 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -45.8 | 107 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24733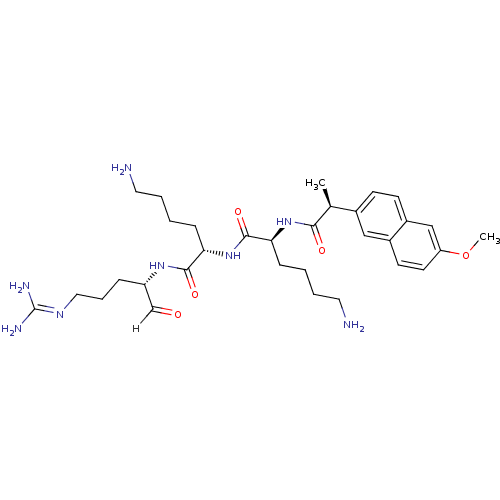 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.7 | 112 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24735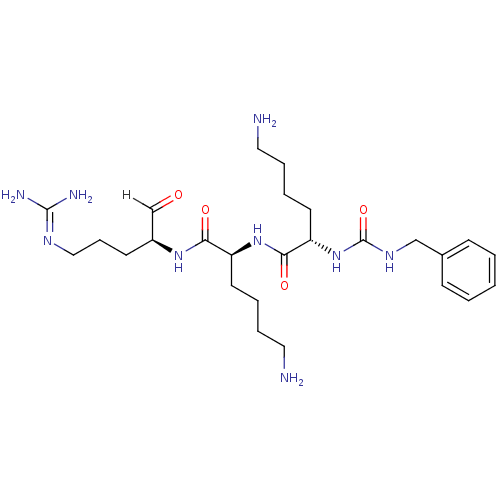 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -45.0 | 146 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24741 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -44.8 | 154 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24736 (Capped tripeptide aldehyde inhibitor, 24 | benzyl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -43.9 | 222 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24728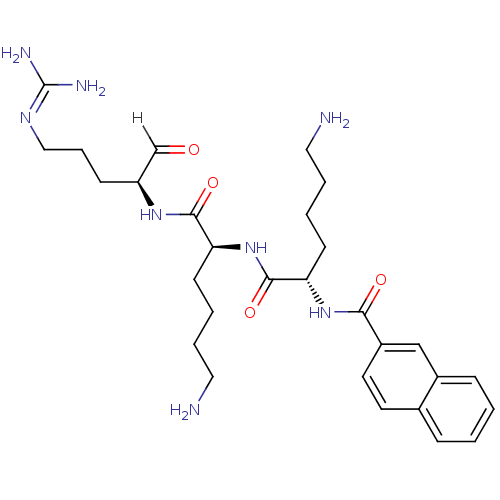 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 41 | -43.9 | 231 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24744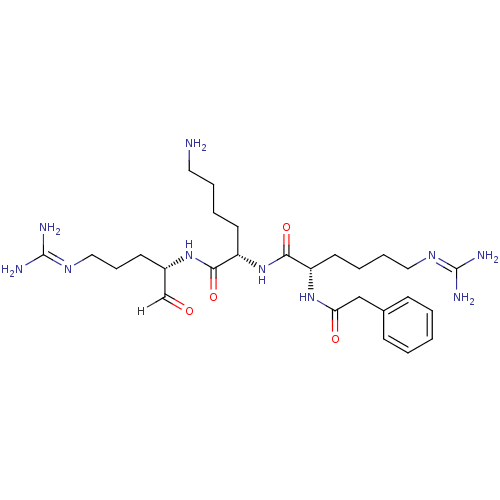 ((2S)-N-[(1S)-5-amino-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | -43.7 | 245 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24742 ((2S)-6-amino-2-[(2S)-5-amino-2-(1-phenylacetamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | -43.6 | 255 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24737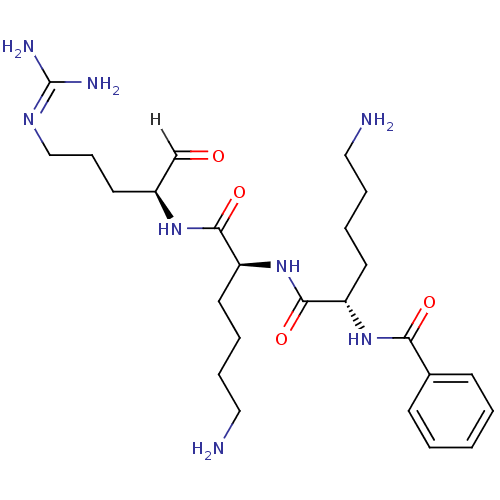 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | -43.4 | 271 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24748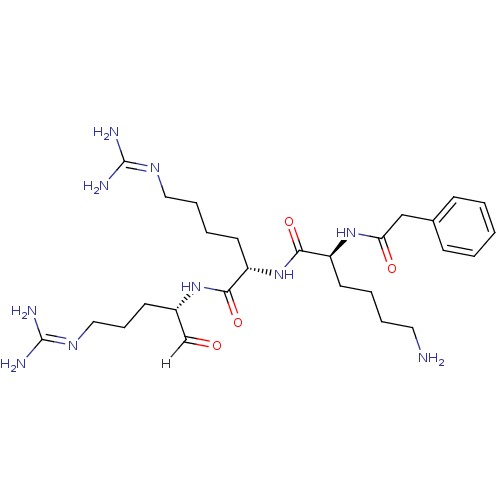 ((2S)-2-[(2S)-6-amino-2-(1-phenylacetamido)hexanami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | -43.2 | 297 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24745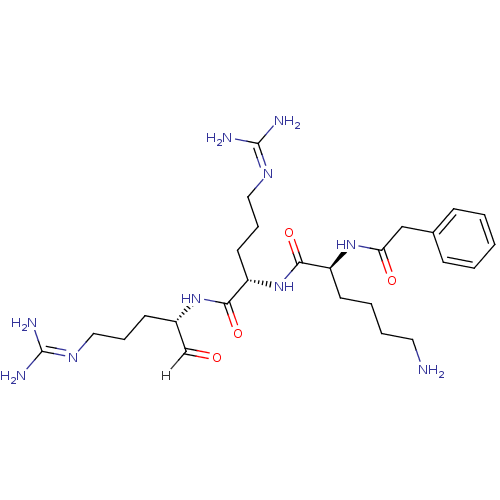 ((2S)-6-amino-N-[(1S)-4-carbamimidamido-1-{[(2S)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 58 | -43.0 | 325 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24738 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | -42.1 | 454 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24730 ((2S)-6-amino-2-[(2S)-6-amino-2-[(2E)-3-phenylprop-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 104 | -41.5 | 580 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24743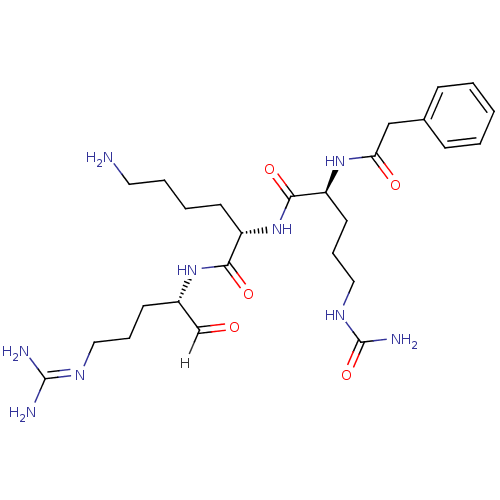 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 111 | -41.3 | 619 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24740 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -40.3 | 891 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24747 ((2S)-6-amino-N-[(1S)-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.07E+3 | -33.7 | 1.16E+4 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24749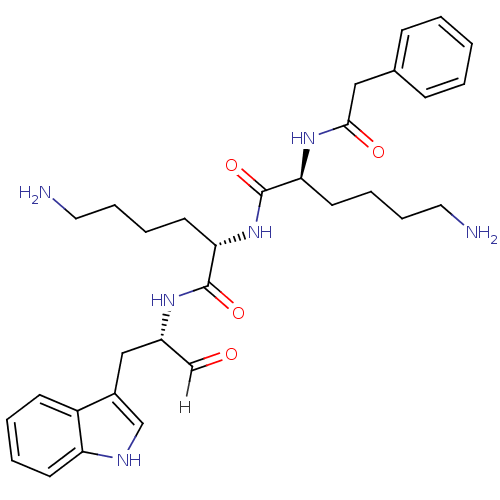 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-1-(1H-indol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | -31.7 | 2.57E+4 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM181062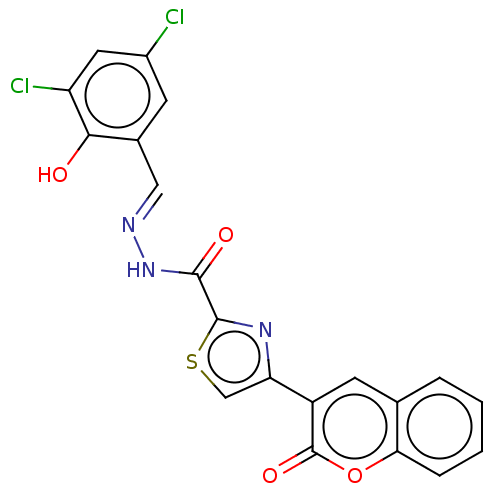 ((E)-N'-(3,5-Dichloro-2-hydroxybenzylidene)-4-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Babasaheb Bhimrao Ambedkar University (A Central University) Curated by ChEMBL | Assay Description Non-competitive inhibition of alpha-glucosidase (unknown origin) using p-nitro phenyl glucopyranoside as substrate preincubated for 10 mins followed ... | Eur J Med Chem 176: 343-377 (2019) Article DOI: 10.1016/j.ejmech.2019.04.025 BindingDB Entry DOI: 10.7270/Q2N87F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50203367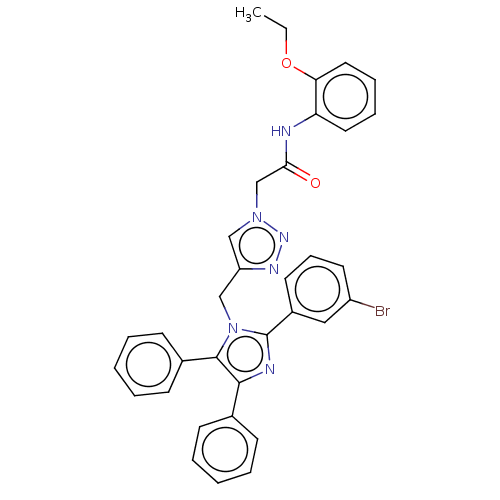 (CHEMBL3891396) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Babasaheb Bhimrao Ambedkar University (A Central University) Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) | Eur J Med Chem 176: 343-377 (2019) Article DOI: 10.1016/j.ejmech.2019.04.025 BindingDB Entry DOI: 10.7270/Q2N87F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50502047 (CHEMBL4459976) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Babasaheb Bhimrao Ambedkar University (A Central University) Curated by ChEMBL | Assay Description Competitive inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl-alpha-d-glucopyranoside as substrate preincubated for 10 mins follow... | Eur J Med Chem 176: 343-377 (2019) Article DOI: 10.1016/j.ejmech.2019.04.025 BindingDB Entry DOI: 10.7270/Q2N87F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM93118 (PTP1B Inhibitor, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 2.50E+4 | n/a | 6.27E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a | |
Central Drug Research Institute, | Assay Description The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... | Medicinal Chemistry Research 17: 123-136 (2008) BindingDB Entry DOI: 10.7270/Q2JQ0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182133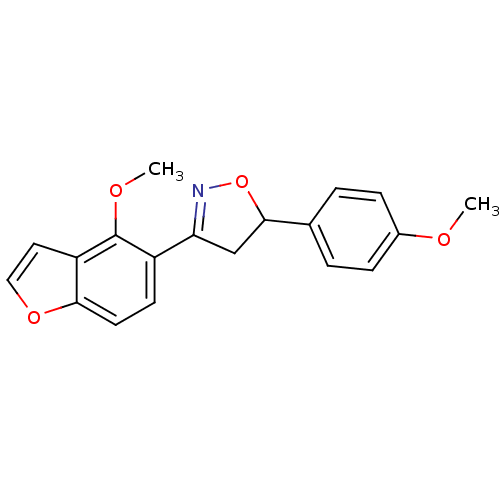 (3-(4-methoxybenzofuran-5-yl)-5-(4-methoxyphenyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM93109 (PTP1B Inhibitor, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 3.00E+4 | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a | |
Central Drug Research Institute, | Assay Description The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... | Medicinal Chemistry Research 17: 123-136 (2008) BindingDB Entry DOI: 10.7270/Q2JQ0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182134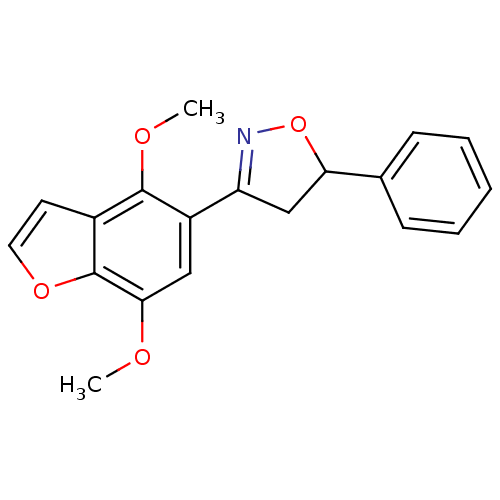 (3-(4,7-dimethoxybenzofuran-5-yl)-5-phenyl-4,5-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182131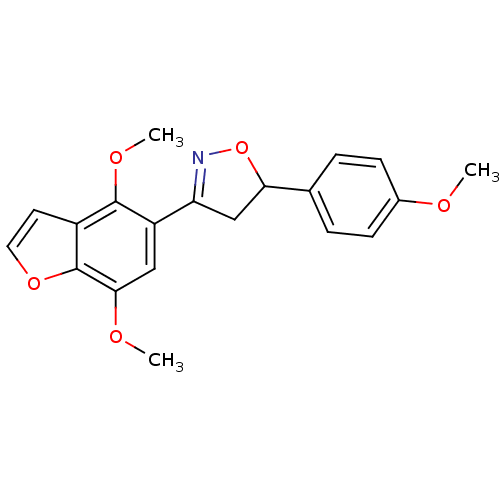 (3-(4,7-dimethoxybenzofuran-5-yl)-5-(4-methoxypheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM93111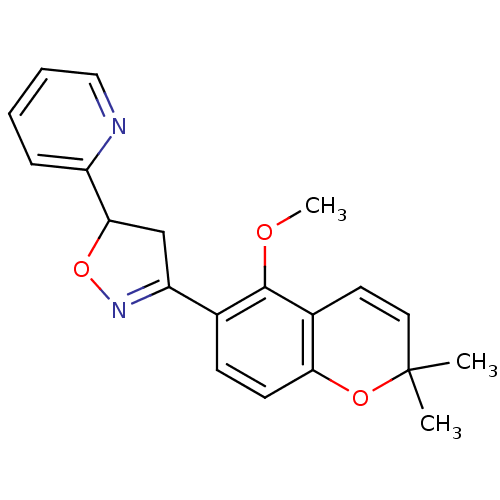 (PTP1B Inhibitor, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 3.40E+4 | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a | |
Central Drug Research Institute, | Assay Description The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... | Medicinal Chemistry Research 17: 123-136 (2008) BindingDB Entry DOI: 10.7270/Q2JQ0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182135 (3-(4-methoxybenzofuran-5-yl)-5-phenyl-4,5-dihydroi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM93113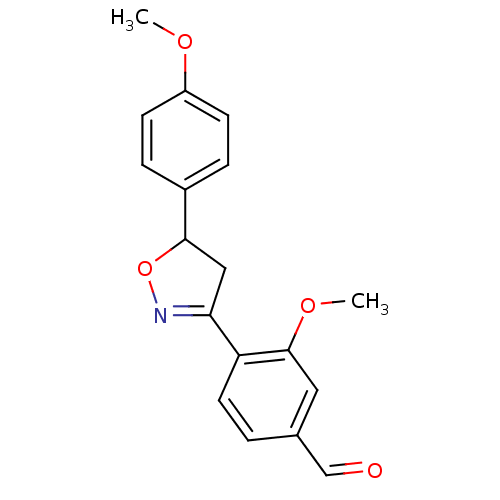 (PTP1B Inhibitor, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 4.80E+4 | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a | |
Central Drug Research Institute, | Assay Description The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... | Medicinal Chemistry Research 17: 123-136 (2008) BindingDB Entry DOI: 10.7270/Q2JQ0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM93112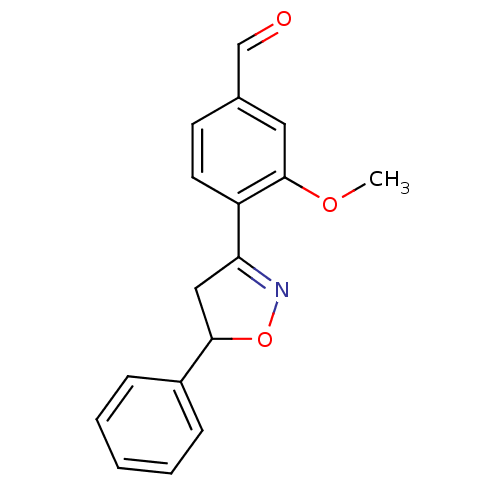 (PTP1B Inhibitor, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 4.80E+4 | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a | |
Central Drug Research Institute, | Assay Description The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... | Medicinal Chemistry Research 17: 123-136 (2008) BindingDB Entry DOI: 10.7270/Q2JQ0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50487368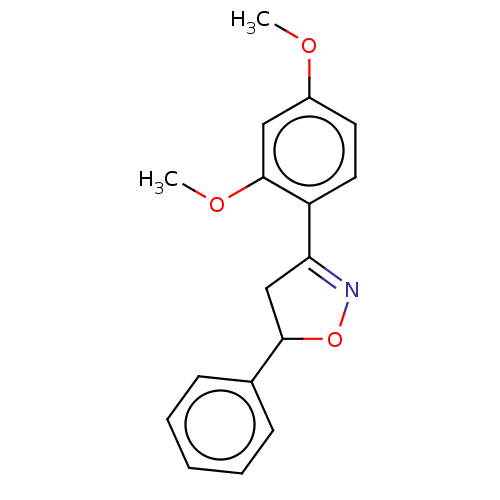 (CHEMBL2259740) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Central Drug Research Institute, | Assay Description The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... | Medicinal Chemistry Research 17: 123-136 (2008) BindingDB Entry DOI: 10.7270/Q2JQ0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50487364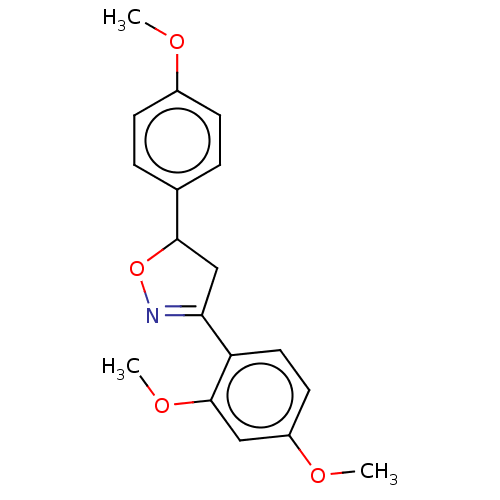 (CHEMBL2259741) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Central Drug Research Institute, | Assay Description The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... | Medicinal Chemistry Research 17: 123-136 (2008) BindingDB Entry DOI: 10.7270/Q2JQ0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM93110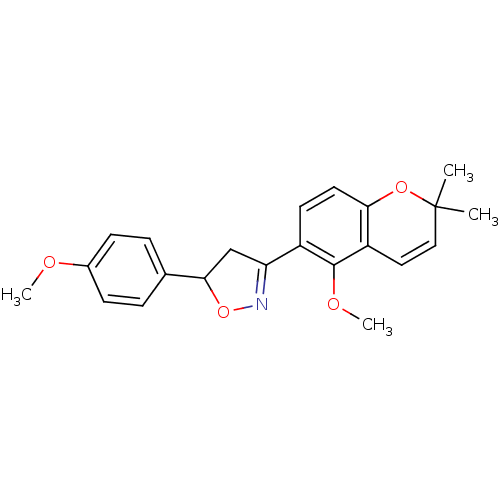 (PTP1B Inhibitor, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 5.60E+4 | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a | |
Central Drug Research Institute, | Assay Description The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... | Medicinal Chemistry Research 17: 123-136 (2008) BindingDB Entry DOI: 10.7270/Q2JQ0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1214 total ) | Next | Last >> |