

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24732 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 32 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24731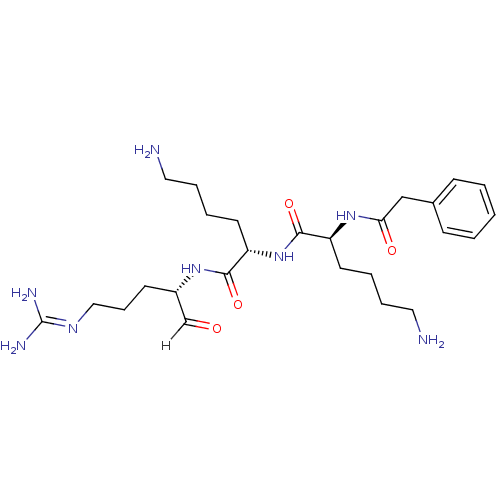 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | -47.8 | 51 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24734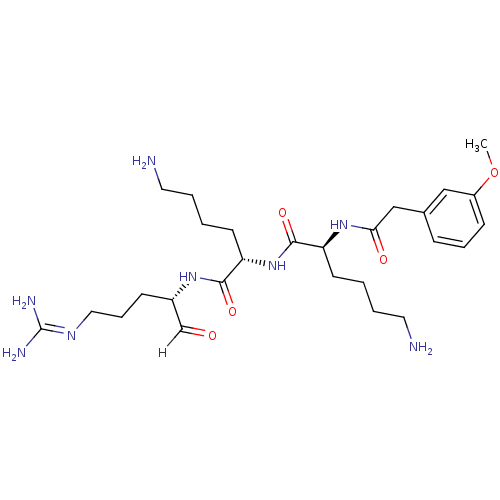 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | 60 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24746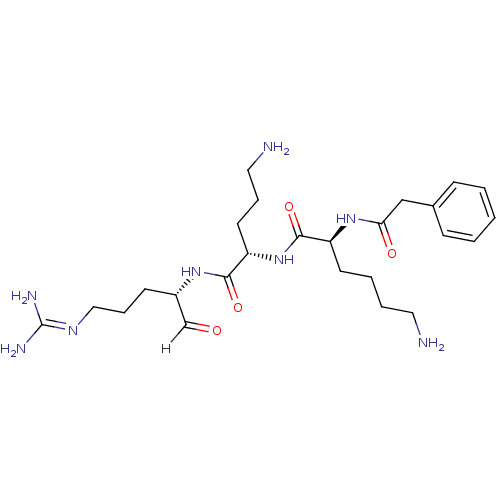 ((2S)-6-amino-N-[(1S)-4-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -46.8 | 73 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24739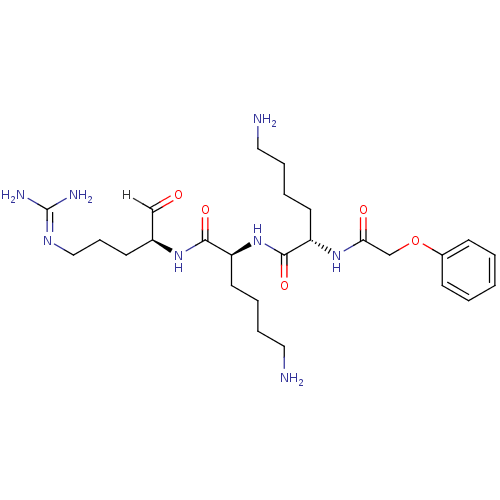 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -45.8 | 107 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24733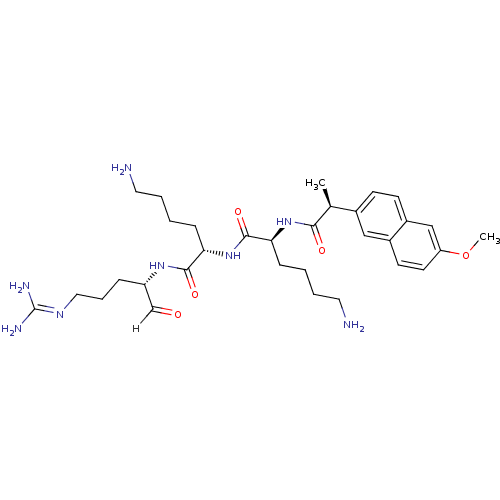 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.7 | 112 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24735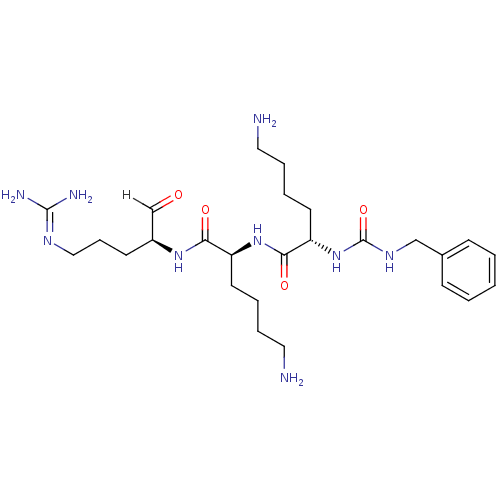 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -45.0 | 146 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24741 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -44.8 | 154 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24736 (Capped tripeptide aldehyde inhibitor, 24 | benzyl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -43.9 | 222 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24728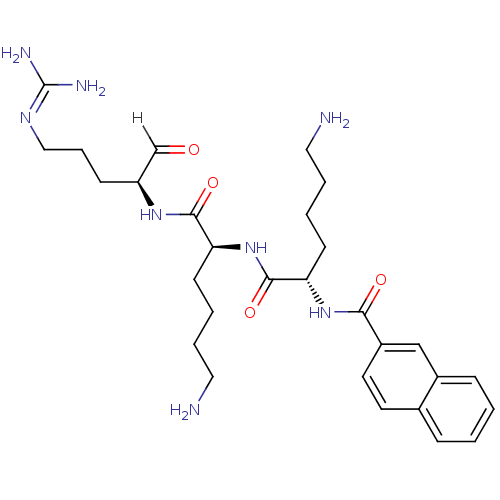 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 41 | -43.9 | 231 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24744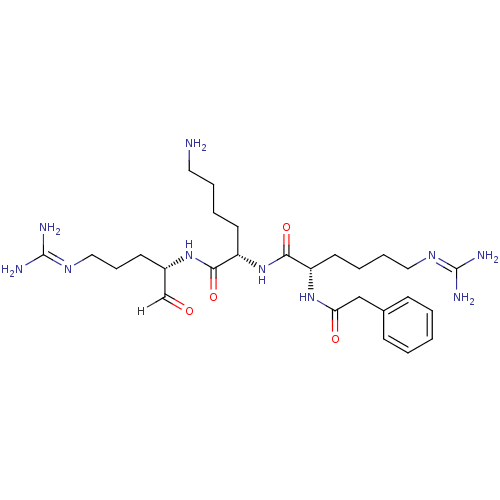 ((2S)-N-[(1S)-5-amino-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | -43.7 | 245 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24742 ((2S)-6-amino-2-[(2S)-5-amino-2-(1-phenylacetamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | -43.6 | 255 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24737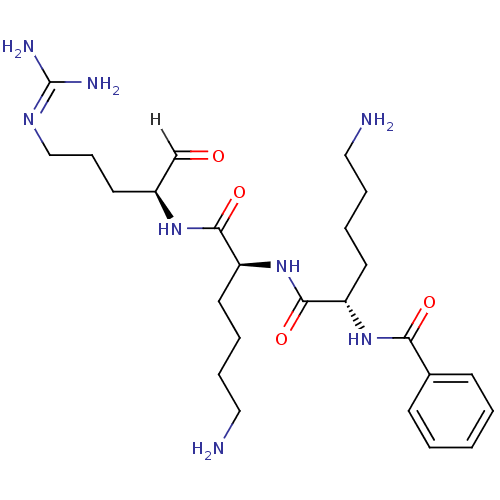 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | -43.4 | 271 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24748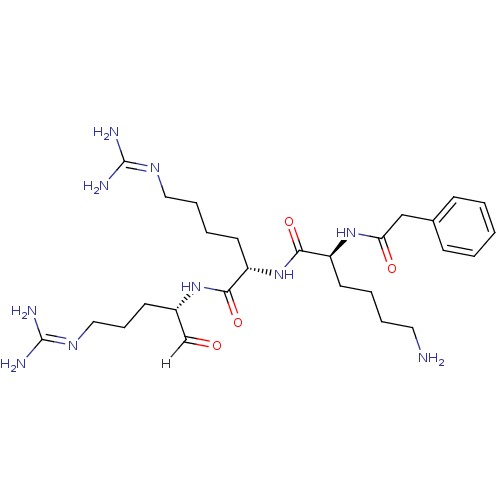 ((2S)-2-[(2S)-6-amino-2-(1-phenylacetamido)hexanami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | -43.2 | 297 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24745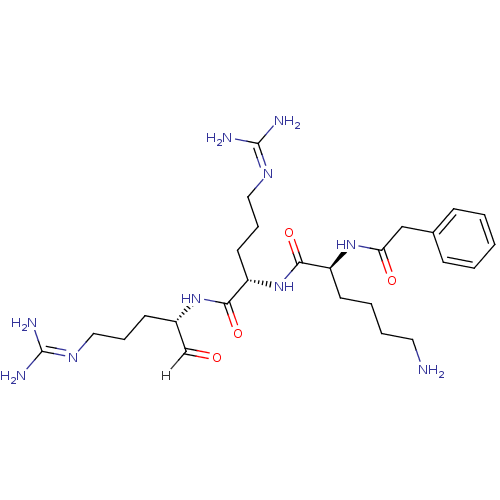 ((2S)-6-amino-N-[(1S)-4-carbamimidamido-1-{[(2S)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 58 | -43.0 | 325 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24738 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | -42.1 | 454 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24730 ((2S)-6-amino-2-[(2S)-6-amino-2-[(2E)-3-phenylprop-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 104 | -41.5 | 580 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24743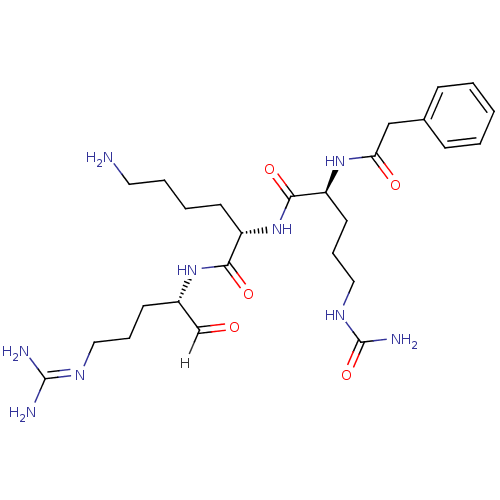 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 111 | -41.3 | 619 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24740 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -40.3 | 891 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24747 ((2S)-6-amino-N-[(1S)-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.07E+3 | -33.7 | 1.16E+4 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24749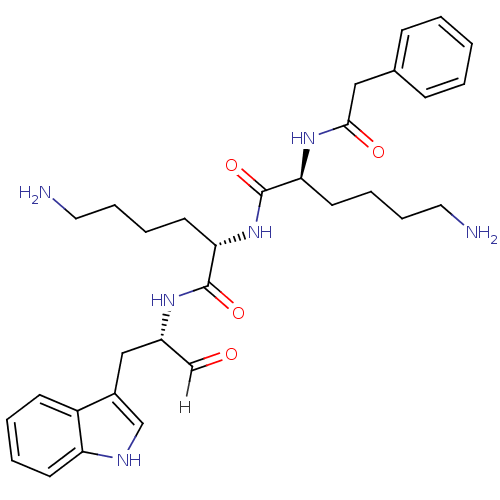 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-1-(1H-indol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | -31.7 | 2.57E+4 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371616 ((R)-4-(2-(6-Fluoropyridin-3-yl)-4-(trifluoromethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130865 (CHEMBL3634853) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130760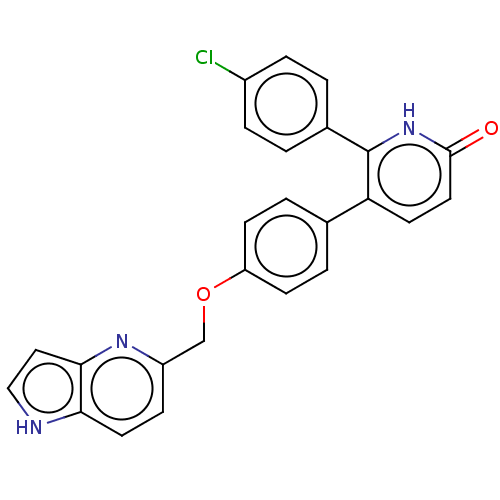 (CHEMBL3634855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50048260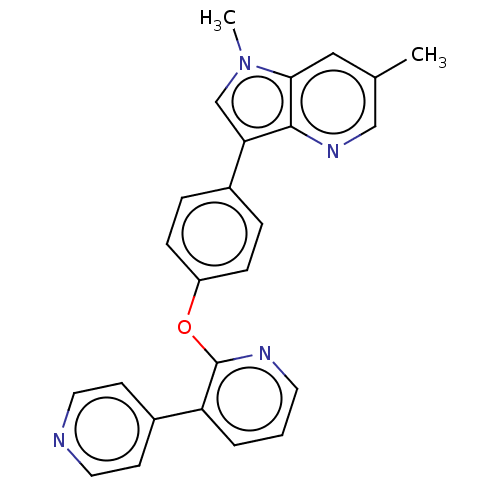 (CHEMBL3315045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay | Bioorg Med Chem Lett 24: 3238-42 (2014) Article DOI: 10.1016/j.bmcl.2014.06.028 BindingDB Entry DOI: 10.7270/Q25D8TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130759 (CHEMBL3634854) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545552 (CHEMBL4646742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545560 (CHEMBL4634421) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545558 (CHEMBL4642748) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545563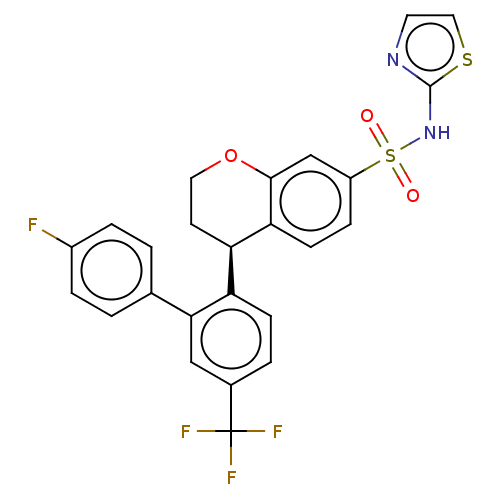 (CHEMBL4636838) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130761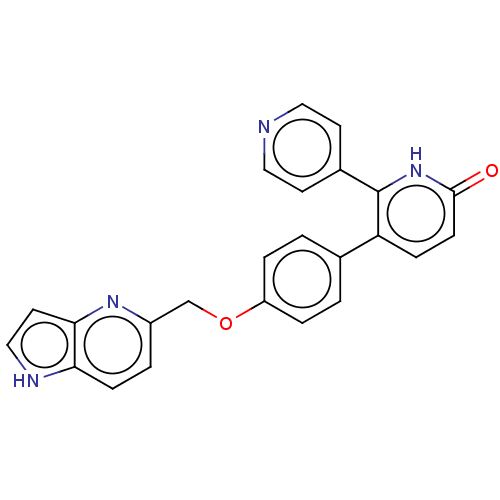 (CHEMBL3634856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371523 ((R)-N-(6-Fluoropyridin-2-yl)-4-(2-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130763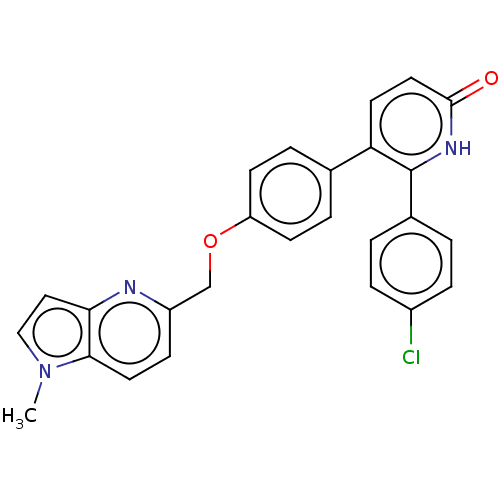 (CHEMBL3634858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545567 (CHEMBL4649638) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM50048260 (CHEMBL3315045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-tagged PDE10A using [3H]-cAMP as substrate by scintillation proximity assay | Bioorg Med Chem Lett 24: 3238-42 (2014) Article DOI: 10.1016/j.bmcl.2014.06.028 BindingDB Entry DOI: 10.7270/Q25D8TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130849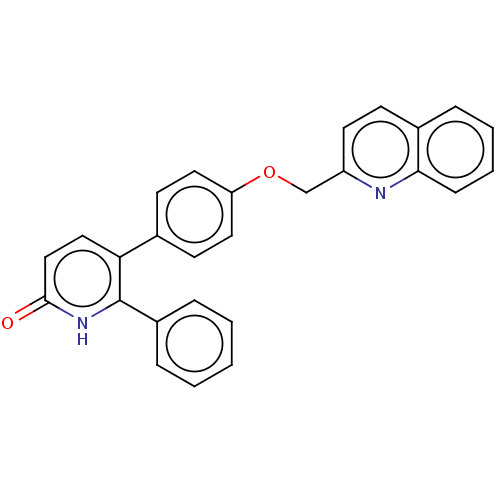 (CHEMBL3634744) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130850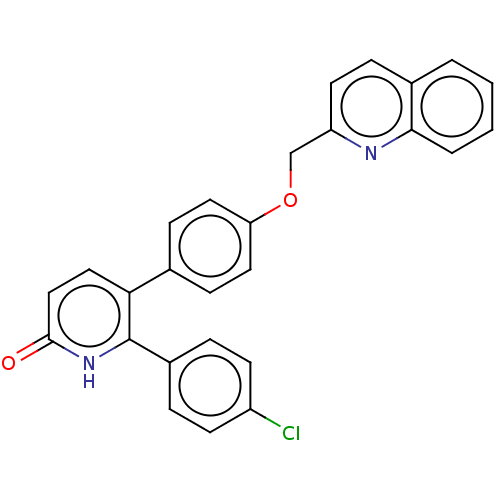 (CHEMBL3634746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371635 ((R)-4-(2-(1H-1,2,3-Triazol-1-yl)-4-(trifluoromethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130860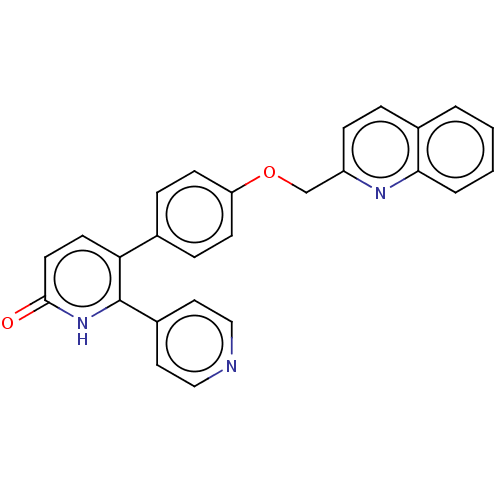 (CHEMBL3634847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Mus musculus) | BDBM50048260 (CHEMBL3315045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of mouse recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay | Bioorg Med Chem Lett 24: 3238-42 (2014) Article DOI: 10.1016/j.bmcl.2014.06.028 BindingDB Entry DOI: 10.7270/Q25D8TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130853 (CHEMBL3634748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50048261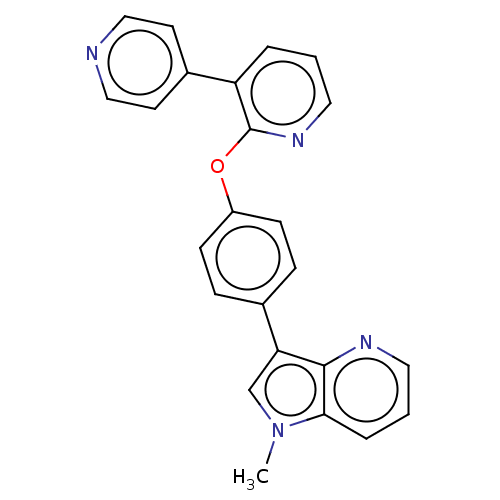 (CHEMBL3315044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay | Bioorg Med Chem Lett 24: 3238-42 (2014) Article DOI: 10.1016/j.bmcl.2014.06.028 BindingDB Entry DOI: 10.7270/Q25D8TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371776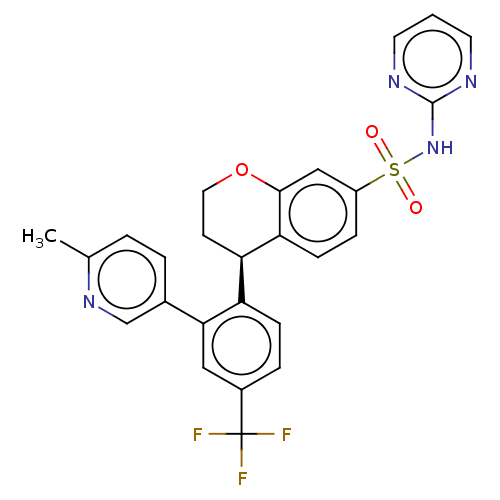 ((R)-4-(2-(6-methylpyridin-3-yl)-4-(trifluoromethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130854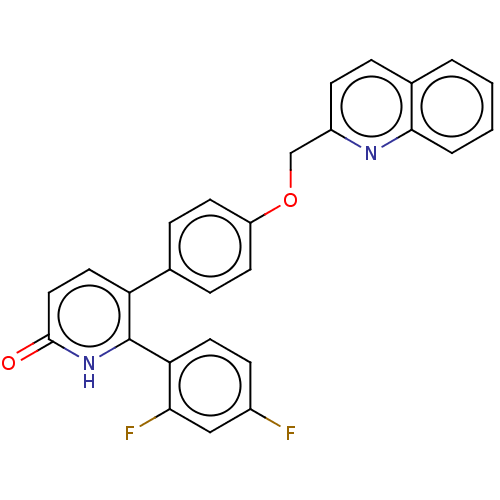 (CHEMBL3634749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545554 (CHEMBL4635247) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371648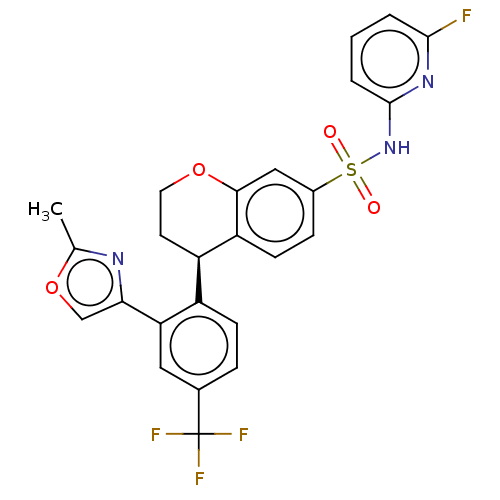 ((R)-N-(6-Fluoropyridin-2-yl)-4-(2-(2-methyloxazol-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130841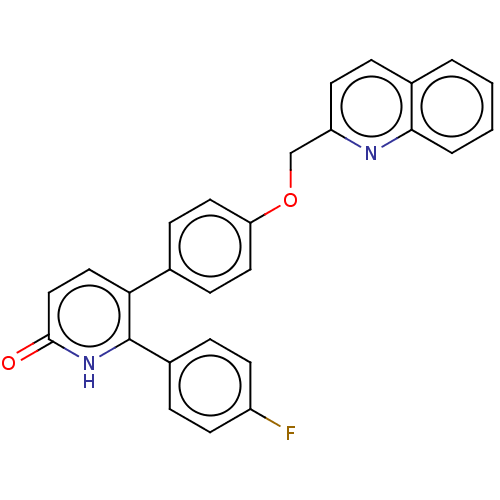 (CHEMBL3634745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130762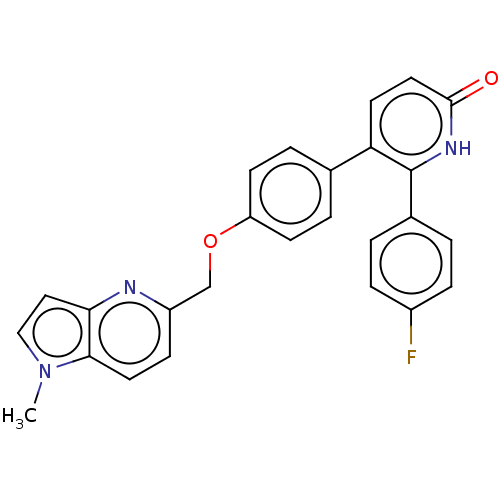 (CHEMBL3634857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371652 ((R)-4-(2-(2-methyloxazol-4-yl)-4-(trifluoromethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50130858 (CHEMBL3634845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 320 total ) | Next | Last >> |