

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Wild-type human DHFR | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761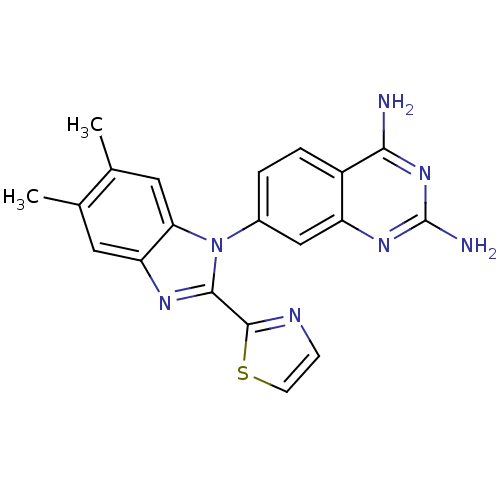 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757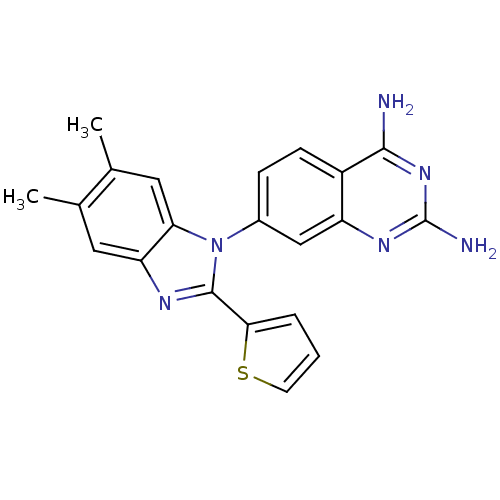 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131853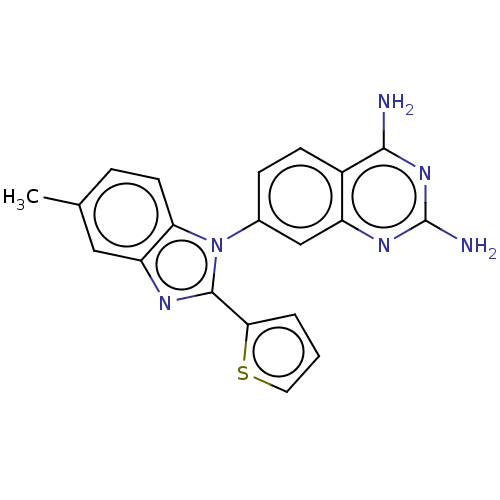 (US8835445, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131853 (US8835445, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50280055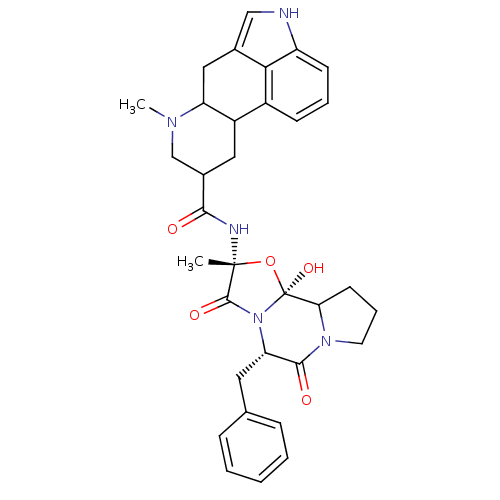 (50285557 | 7-Methyl-4,6,6a,7,8,9,10,10a-octahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by PDSP Ki Database | Biochem Biophys Res Commun 184: 752-9 (1992) Article DOI: 10.1016/0006-291x(92)90654-4 BindingDB Entry DOI: 10.7270/Q2ST7NB1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448758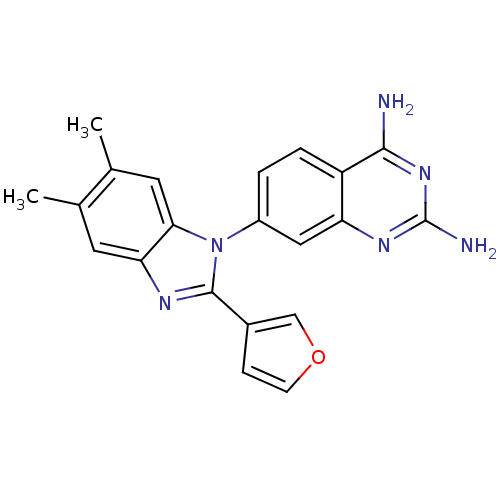 (CHEMBL3128024) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231459 (CHEMBL252818 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609451 (US11702418, Example 10-10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609446 (US11702418, Example 10-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609420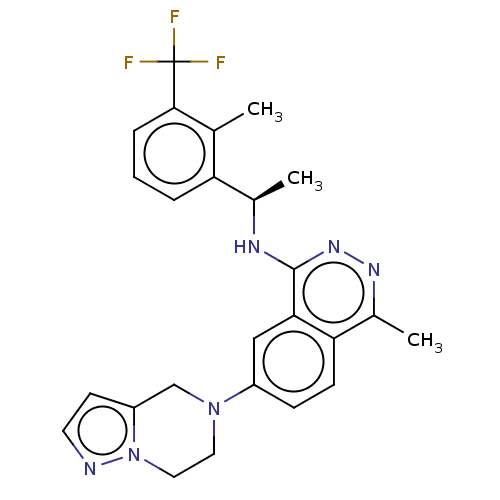 (US11702418, Example 6-10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609526 (US11702418, Example 11-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609525 (US11702418, Example 11-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609524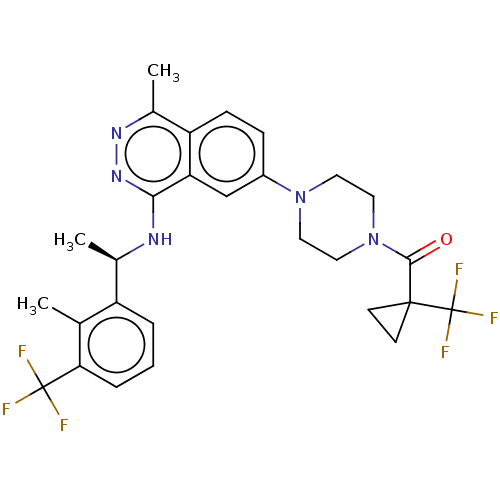 (US11702418, Example 11-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609523 (US11702418, Example 11-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609522 ((R)-(4-(1-methyl-4-((1-(2-methyl-3-(trifluoromethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448743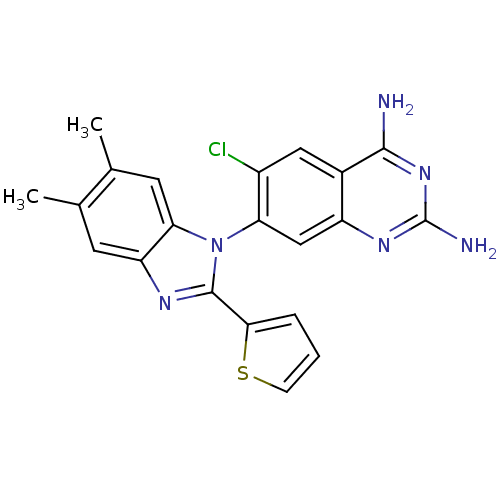 (CHEMBL3128015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609452 (US11702418, Example 10-11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231448 (CHEMBL253022 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50026273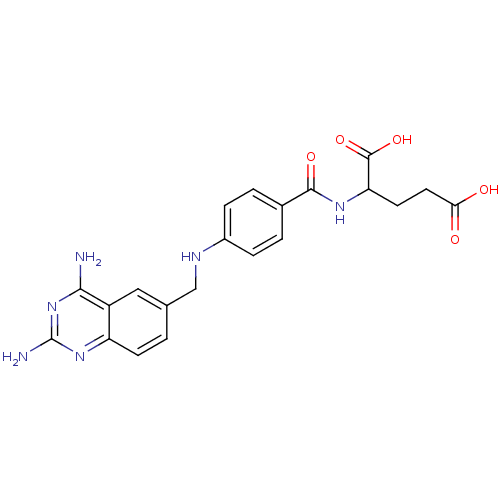 (2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50026273 (2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744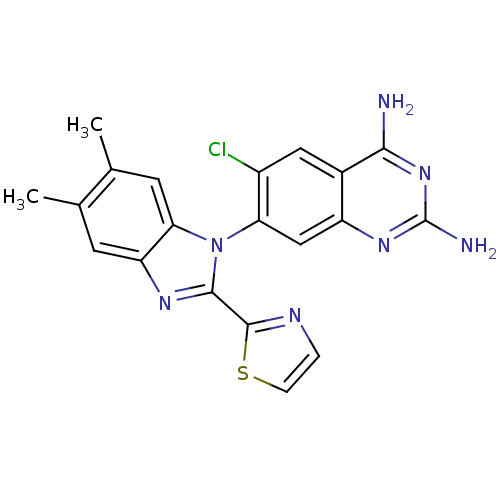 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131845 (US8835445, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131845 (US8835445, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448740 (CHEMBL3128018) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131846 (US8835445, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131846 (US8835445, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448741 (CHEMBL3128017) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175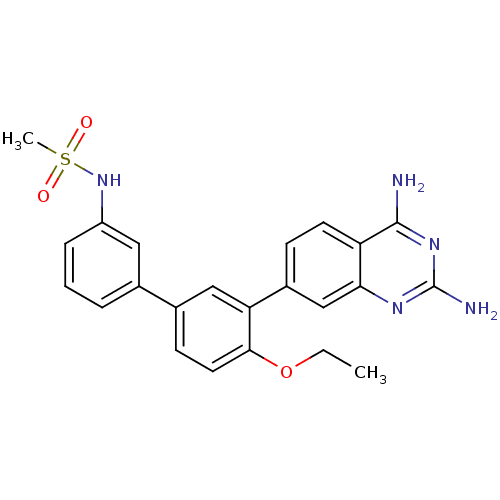 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM609413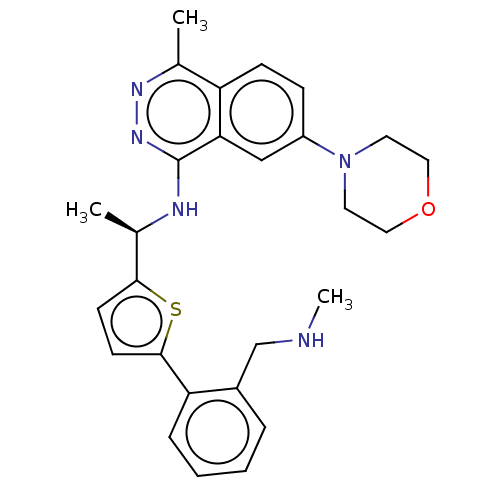 (US11702418, Example 6-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2C251J6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50378812 (CHEMBL1221411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Abl after 30 mins | Bioorg Med Chem 18: 6316-21 (2010) Article DOI: 10.1016/j.bmc.2010.07.021 BindingDB Entry DOI: 10.7270/Q2CZ384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50303233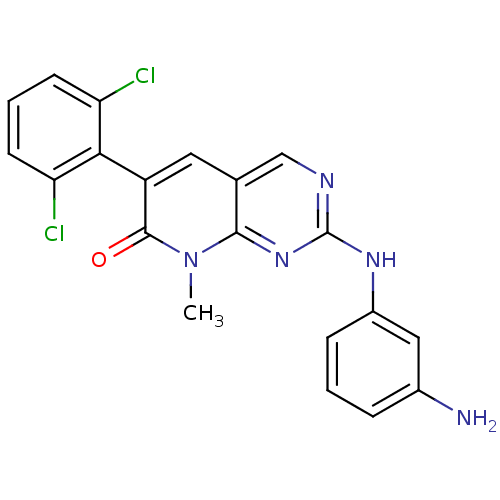 (2-(3-aminophenylamino)-6-(2,6-dichlorophenyl)-8-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Abl after 30 mins | Bioorg Med Chem 18: 6316-21 (2010) Article DOI: 10.1016/j.bmc.2010.07.021 BindingDB Entry DOI: 10.7270/Q2CZ384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 31874 total ) | Next | Last >> |