

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1166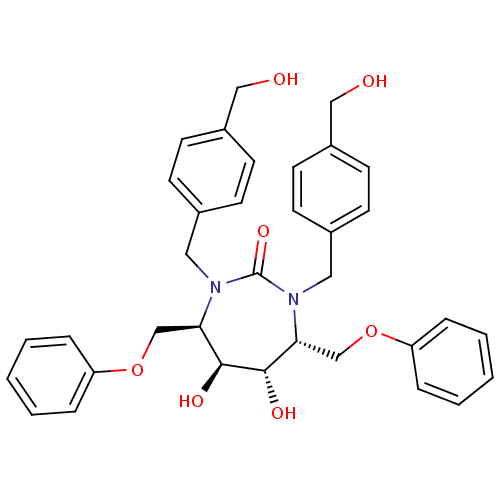 ((4R,5S,6S,7R)-1,3-Bis{[4-(hydroxymethyl)phenyl]met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-53.4 | 10 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM150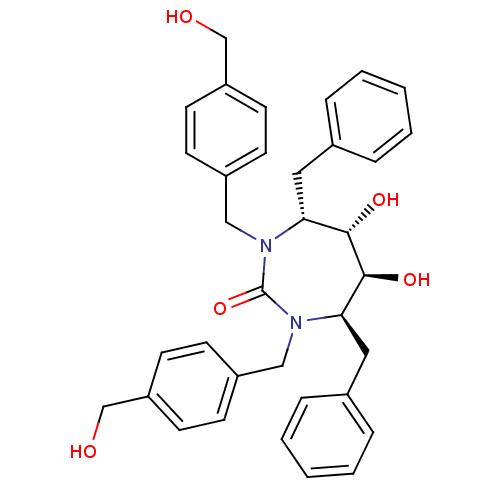 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <1 | <-53.4 | 15 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50031942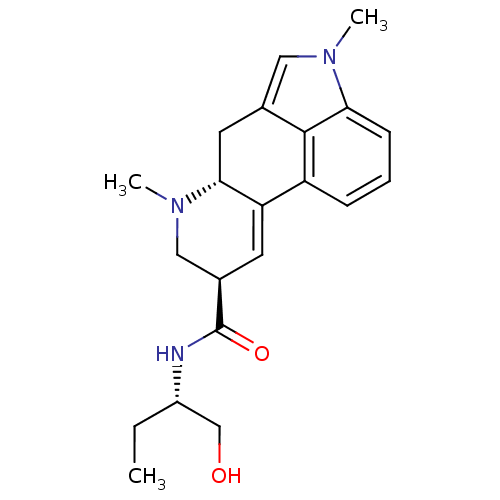 ((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT2C receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50031942 ((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT2A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1157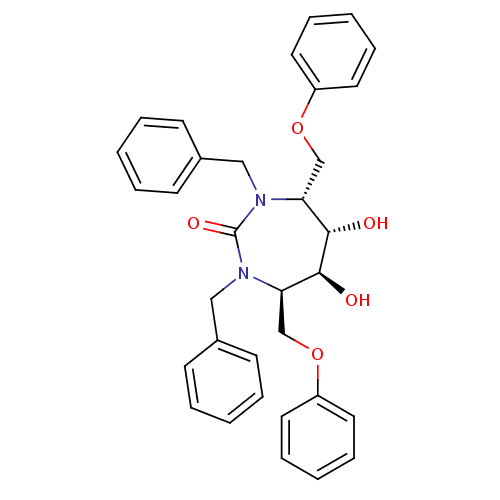 ((4R,5S,6S,7R)-1,3-Dibenzyl-4,7-bis(phenoxymethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 12.2 | -47.0 | 80 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1164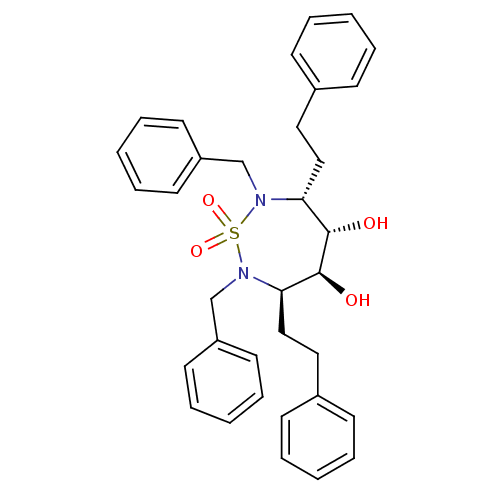 ((3R,4S,5S,6R)-2,7-Dibenzyl-3,6-bis(2-phenylethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14.7 | -46.5 | 200 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1163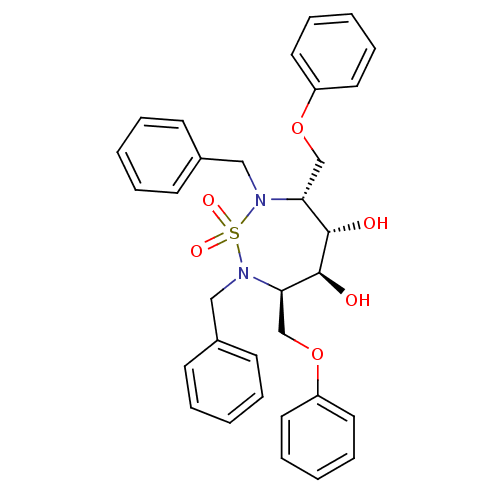 ((3R,4S,5S,6R)-2,7-Dibenzyl-3,6-bis(phenoxymethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 19.1 | -45.8 | 200 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT2C receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50240618 ((2E)-2-[(5-methoxy-1H-indol-3-yl)methylene]-N-pent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1161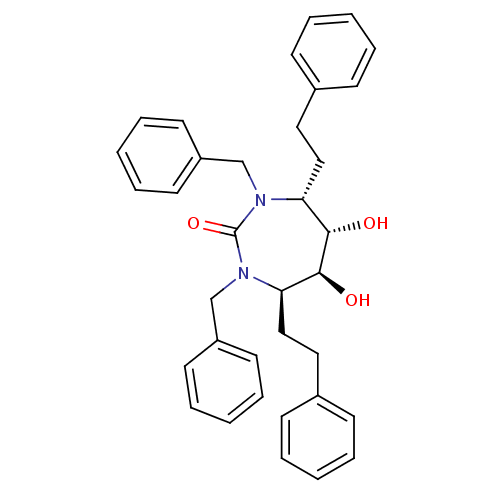 ((4R,5S,6S,7R)-1,3-Dibenzyl-4,7-bis(2-phenylethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 214 | -39.6 | 4.00E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT2C receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1162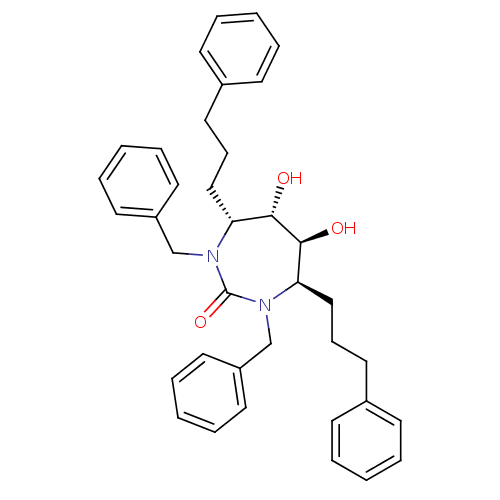 ((4R,5S,6S,7R)-1,3-Dibenzyl-4,7-bis(3-phenylpropyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 570 | -37.1 | 1.00E+4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT2A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT2A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50241836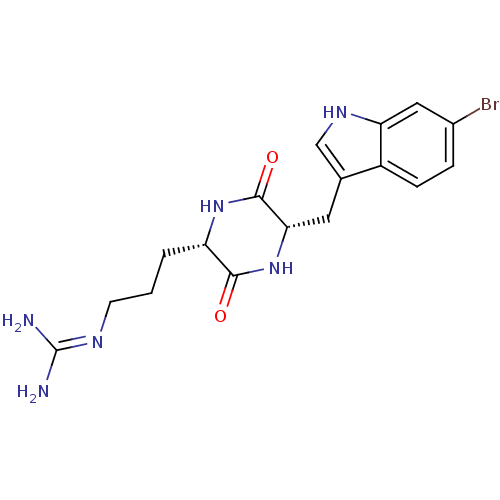 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT2C receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50241836 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50241836 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [1,2-3H]5-carboxamidotryptamine from human 5HT7A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [1,2-3H]5-carboxamidotryptamine from human 5HT1A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [1,2-3H]5-carboxamidotryptamine from human 5HT1D receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50241836 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [1,2-3H]5-carboxamidotryptamine from human 5HT5A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50241836 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50241836 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [9-methyl-3H]BRL43694 from human 5HT3A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50241836 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT2A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50241836 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [1,2-3H]5-carboxamidotryptamine from human 5HT1D receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50241836 (CHEMBL511609 | cyclo[(6-bromotryptophan)arginine]) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [1,2-3H]5-carboxamidotryptamine from human 5HT1A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [9-methyl-3H]BRL43694 from human 5HT3A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [1,2-3H]5-carboxamidotryptamine from human 5HT7A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [1,2-3H]5-carboxamidotryptamine from human 5HT5A receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50234920 ((cyclo[(6-bromo-8-entryptophan)arginine]) | Barett...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Nat Prod 69: 1421-4 (2006) Article DOI: 10.1021/np0601760 BindingDB Entry DOI: 10.7270/Q2H9950V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50250114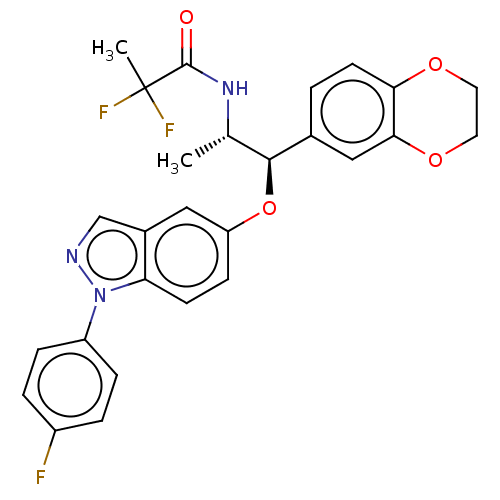 (CHEMBL4104286) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in PMA-stimulated human ChaGoK1 cells expressing TRE-LacZ construct assessed as inhibition of AP-1 mediate... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50250125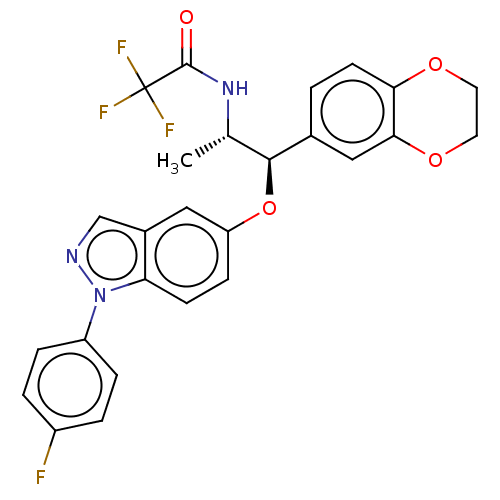 (CHEMBL4061359) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in PMA-stimulated human ChaGoK1 cells expressing TRE-LacZ construct assessed as inhibition of AP-1 mediate... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203432 (CHEMBL3911831) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human ChaGoK1 cells assessed as inhibition of AP1-mediated transcriptional activity by measuring reduc... | Bioorg Med Chem Lett 26: 5741-5748 (2016) Article DOI: 10.1016/j.bmcl.2016.10.052 BindingDB Entry DOI: 10.7270/Q2NP26D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50250115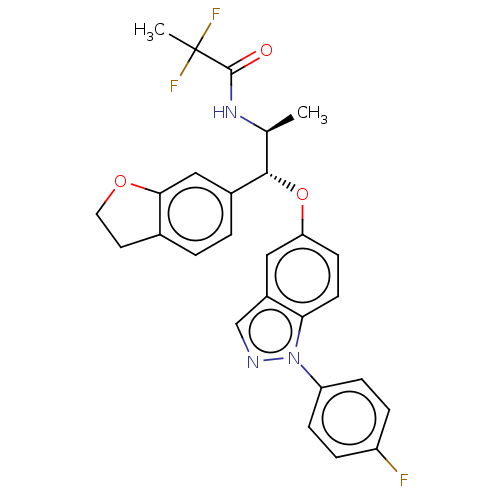 (CHEMBL4086388) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141378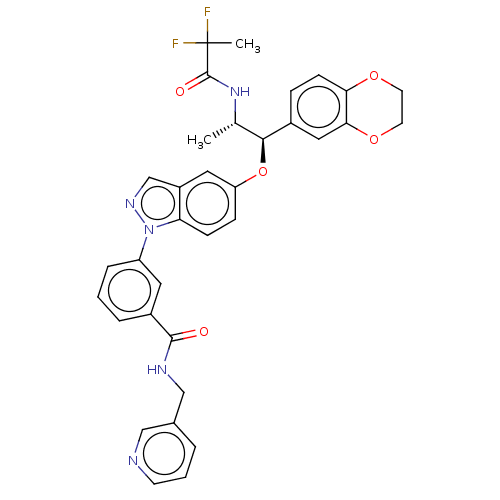 (US8916600, 12 | US9738632, Example 12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in PMA-stimulated human ChaGoK1 cells expressing TRE-LacZ construct assessed as inhibition of AP-1 mediate... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203418 (CHEMBL3902818) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human ChaGoK1 cells assessed as inhibition of AP1-mediated transcriptional activity by measuring reduc... | Bioorg Med Chem Lett 26: 5741-5748 (2016) Article DOI: 10.1016/j.bmcl.2016.10.052 BindingDB Entry DOI: 10.7270/Q2NP26D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203441 (CHEMBL3973775) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human ChaGoK1 cells assessed as inhibition of AP1-mediated transcriptional activity by measuring reduc... | Bioorg Med Chem Lett 26: 5741-5748 (2016) Article DOI: 10.1016/j.bmcl.2016.10.052 BindingDB Entry DOI: 10.7270/Q2NP26D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354849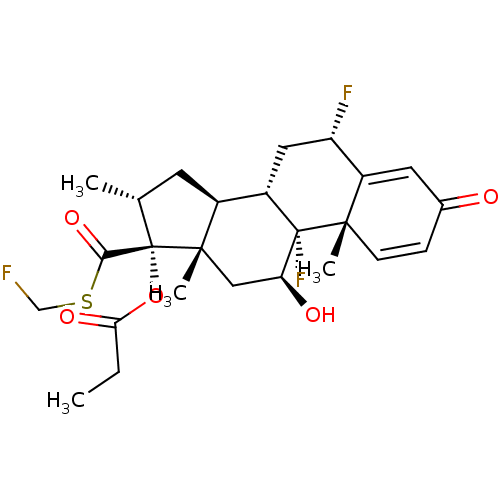 (CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354849 (CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50250112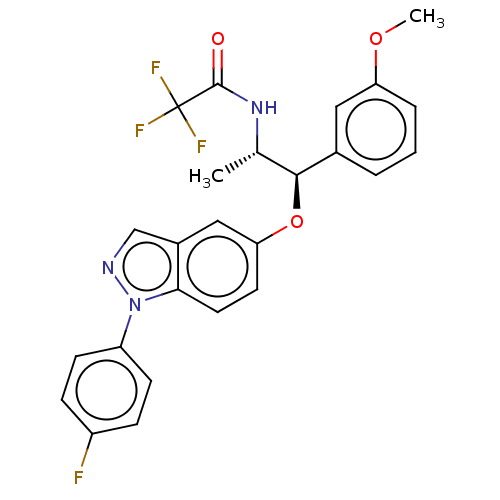 (CHEMBL4072756) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203413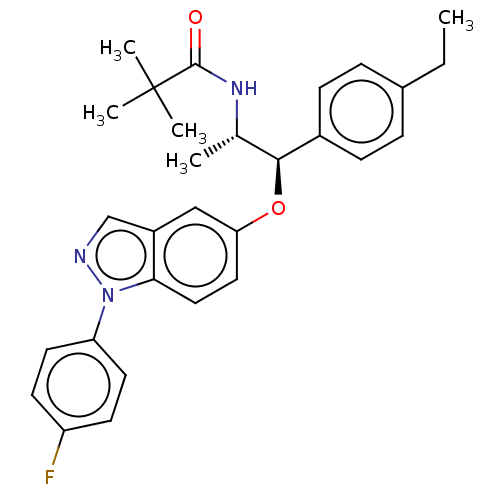 (CHEMBL3920760) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human ChaGoK1 cells assessed as inhibition of AP1-mediated transcriptional activity by measuring reduc... | Bioorg Med Chem Lett 26: 5741-5748 (2016) Article DOI: 10.1016/j.bmcl.2016.10.052 BindingDB Entry DOI: 10.7270/Q2NP26D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141371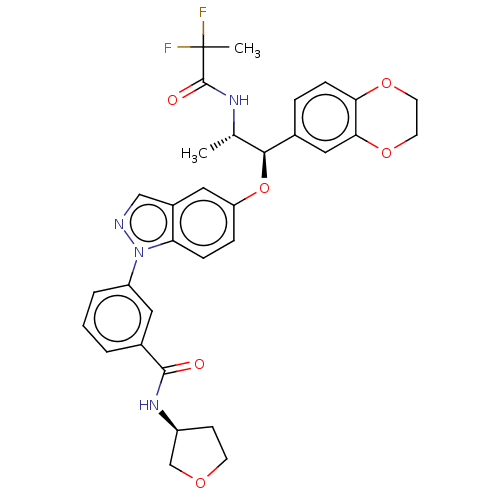 (US8916600, 5 | US9738632, Example 5) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in PMA-stimulated human ChaGoK1 cells expressing TRE-LacZ construct assessed as inhibition of AP-1 mediate... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141372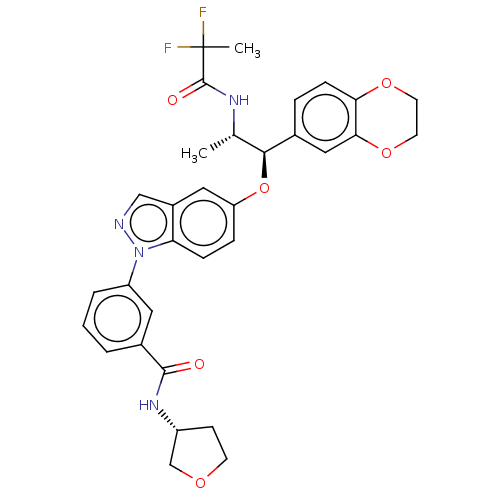 (US8916600, 6 | US9738632, Example 6) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in PMA-stimulated human ChaGoK1 cells expressing TRE-LacZ construct assessed as inhibition of AP-1 mediate... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141379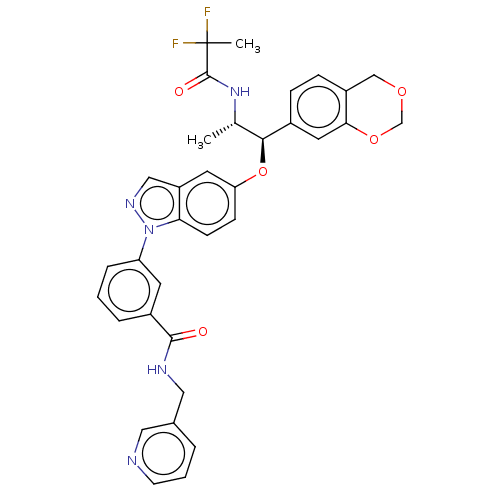 (US8916600, 13 | US9738632, Example 13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in PMA-stimulated human ChaGoK1 cells expressing TRE-LacZ construct assessed as inhibition of AP-1 mediate... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50250126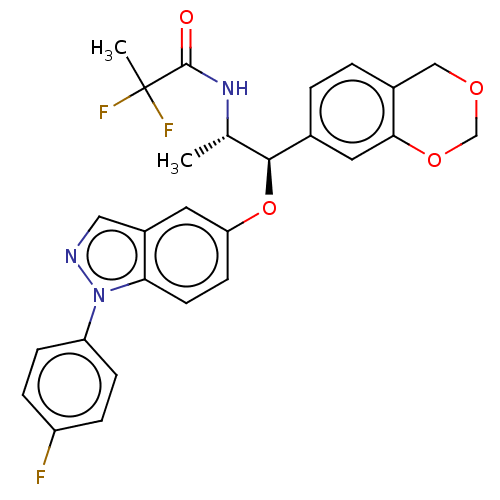 (CHEMBL4094154) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50250125 (CHEMBL4061359) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50250114 (CHEMBL4104286) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203430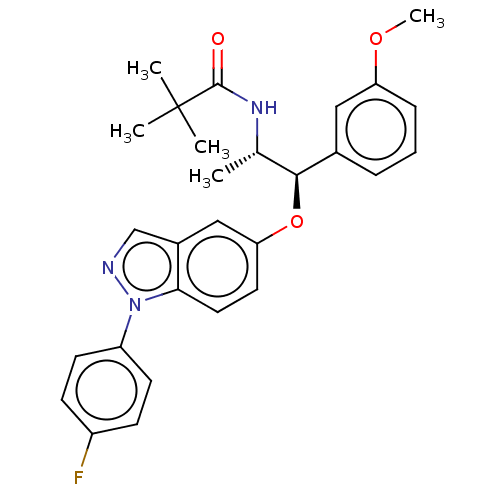 (CHEMBL3901719) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human ChaGoK1 cells assessed as inhibition of AP1-mediated transcriptional activity by measuring reduc... | Bioorg Med Chem Lett 26: 5741-5748 (2016) Article DOI: 10.1016/j.bmcl.2016.10.052 BindingDB Entry DOI: 10.7270/Q2NP26D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203423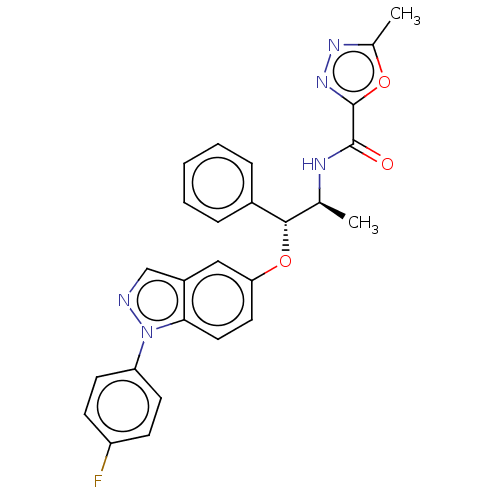 (CHEMBL3960682) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human ChaGoK1 cells assessed as inhibition of AP1-mediated transcriptional activity by measuring reduc... | Bioorg Med Chem Lett 26: 5741-5748 (2016) Article DOI: 10.1016/j.bmcl.2016.10.052 BindingDB Entry DOI: 10.7270/Q2NP26D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 745 total ) | Next | Last >> |