

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139844 (CHEMBL3763503) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50139843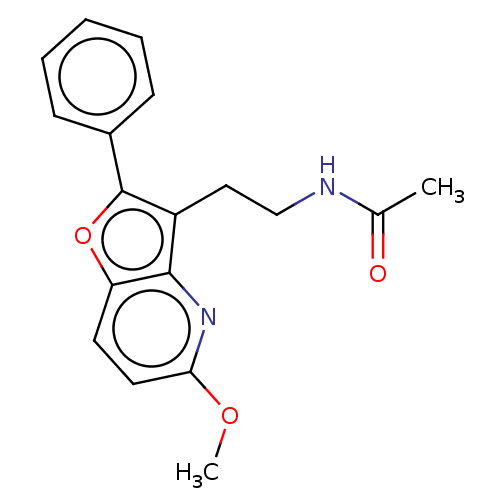 (CHEMBL3765540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139842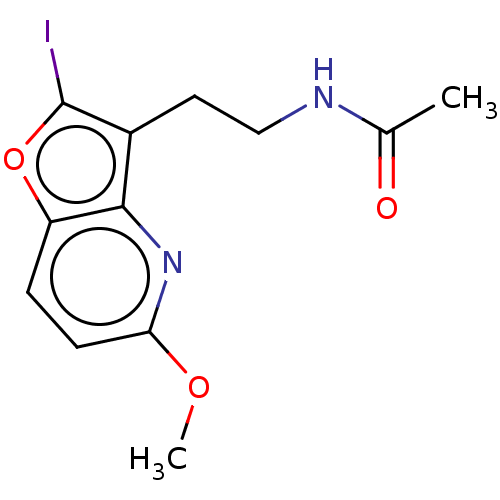 (CHEMBL3765401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139847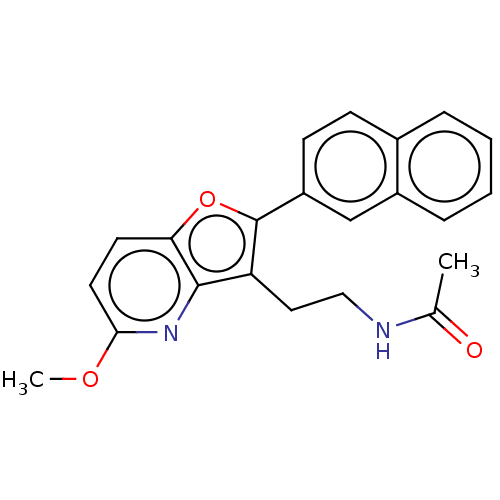 (CHEMBL3764765) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139843 (CHEMBL3765540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50037242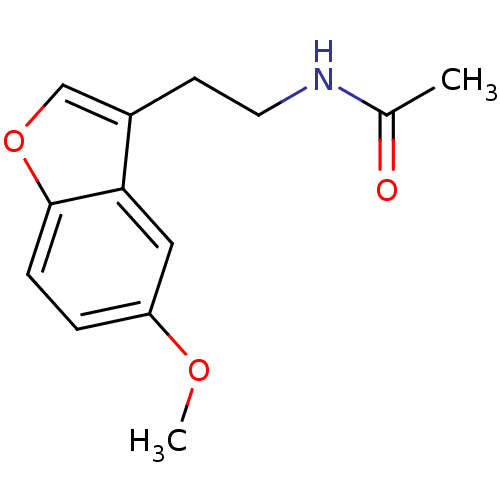 (CHEMBL323332 | N-(2-(5-methoxybenzofuran-3-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176019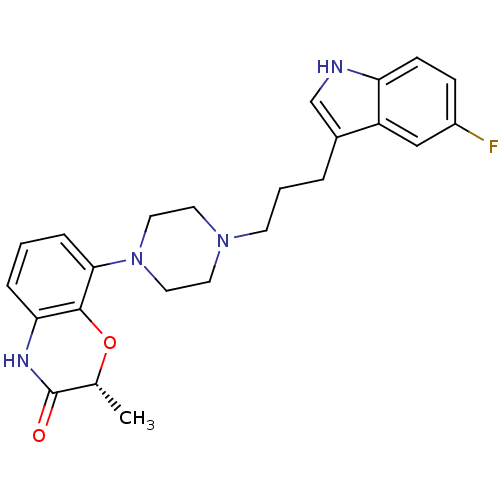 ((R)-8-(4-(3-(5-fluoro-1H-indol-3-yl)propyl)piperaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50139844 (CHEMBL3763503) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50139842 (CHEMBL3765401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513620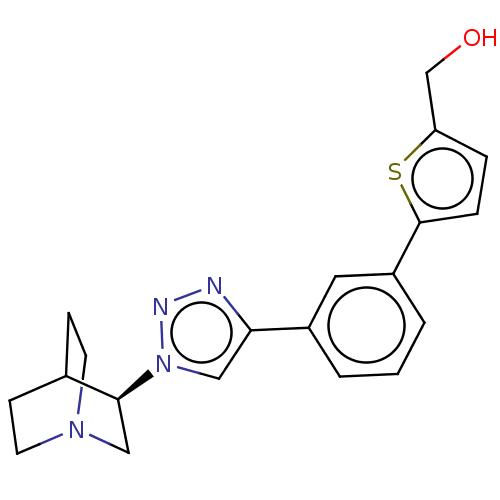 (CHEMBL4563574) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50340329 (4-azamelatonin | CHEMBL1760944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176024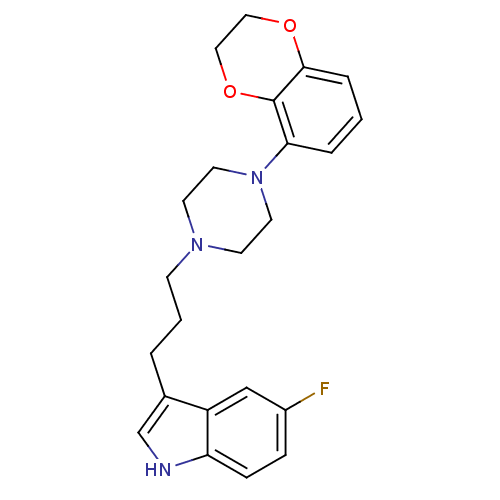 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513605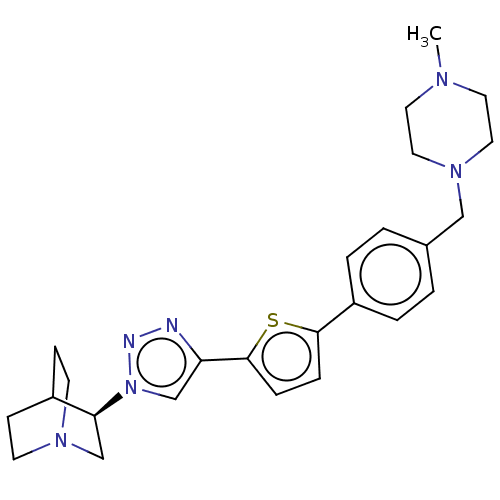 (CHEMBL4563839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513594 (CHEMBL4556722) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513583 (CHEMBL4533685) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513617 (CHEMBL4459802) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50037242 (CHEMBL323332 | N-(2-(5-methoxybenzofuran-3-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50340329 (4-azamelatonin | CHEMBL1760944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176049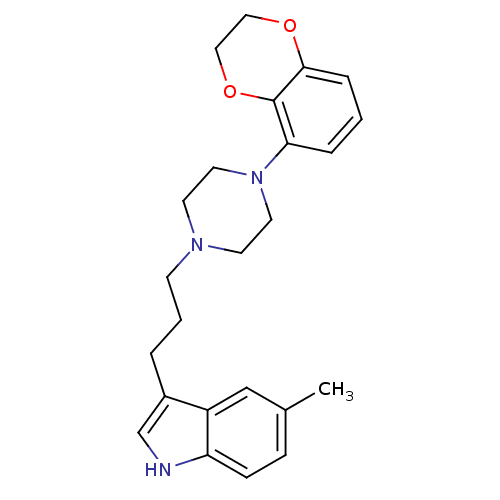 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176035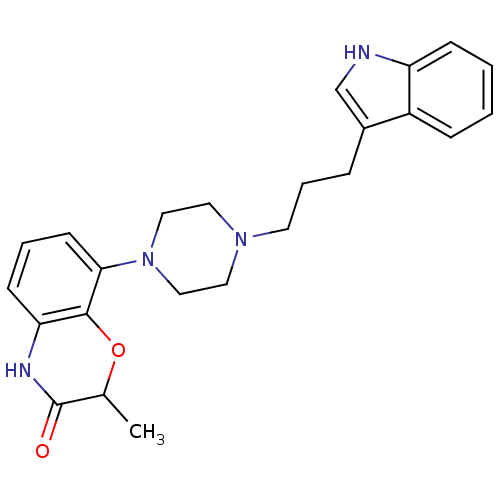 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176021 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176044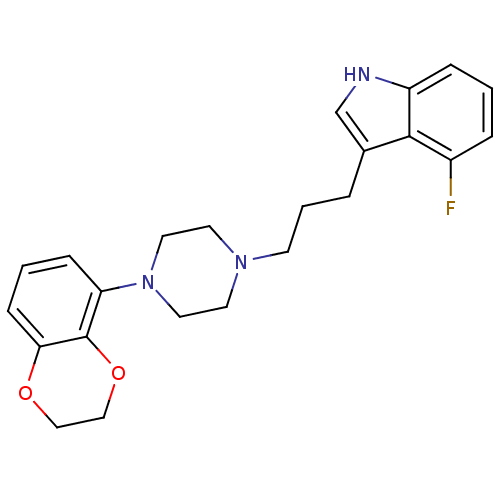 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176020 ((R)-8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176042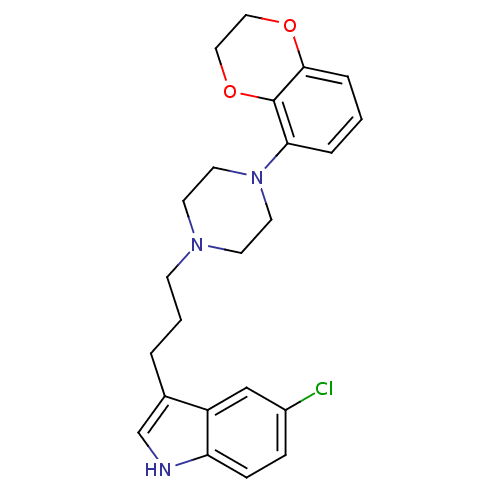 (5-Chloro-3-{3-[4-(2,3-dihydro-benzo[1,4]dioxin-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513613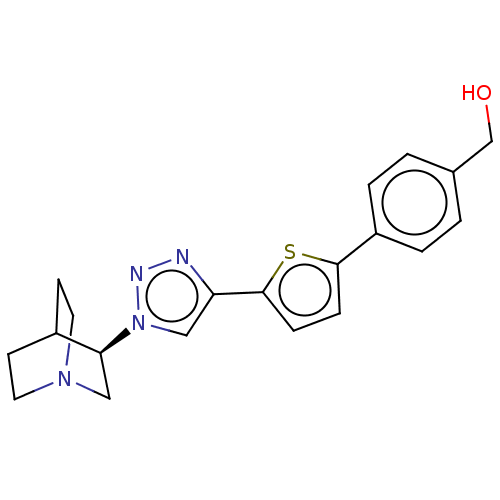 (CHEMBL4567300) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176045 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176034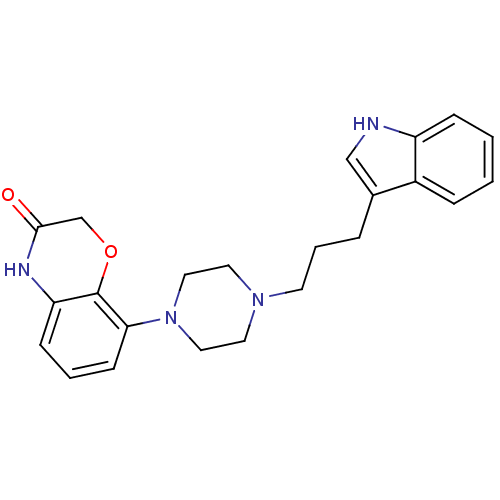 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-4H...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176041 ((S)-8-{4-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513577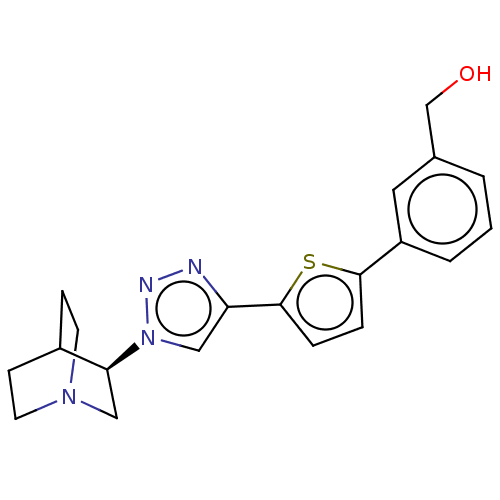 (CHEMBL4445961) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513611 (CHEMBL4452867) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50172158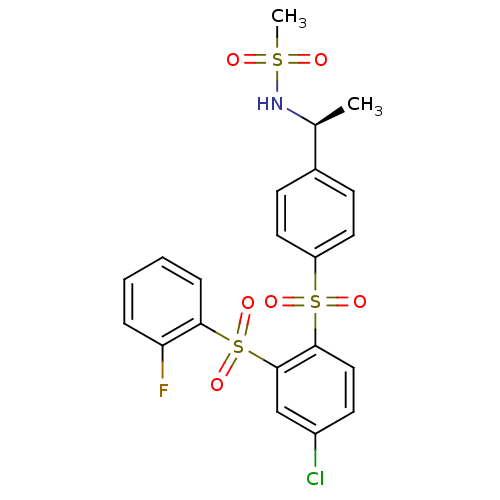 ((S)-N-(1-(4-(4-chloro-2-(2-fluorophenylsulfonyl)ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1084-9 (2010) Article DOI: 10.1016/j.bmcl.2009.12.032 BindingDB Entry DOI: 10.7270/Q22J6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513606 (CHEMBL4547917) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11644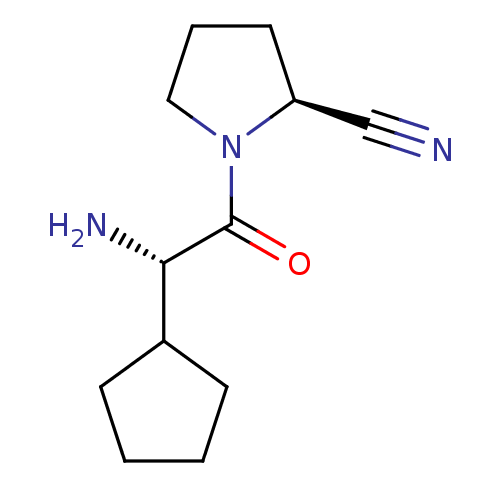 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176043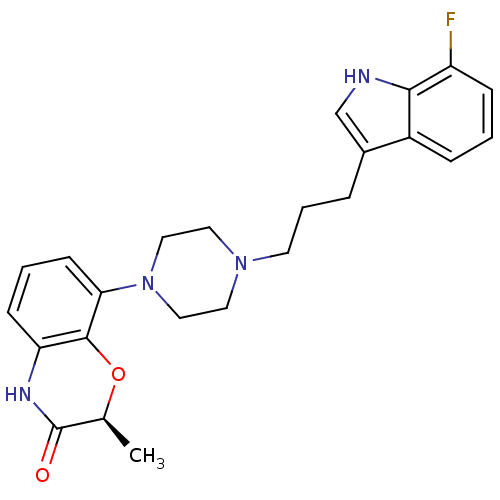 ((S)-8-{4-[3-(7-Fluoro-1H-indol-3-yl)-propyl]-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176023 ((S)-8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176023 ((S)-8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12648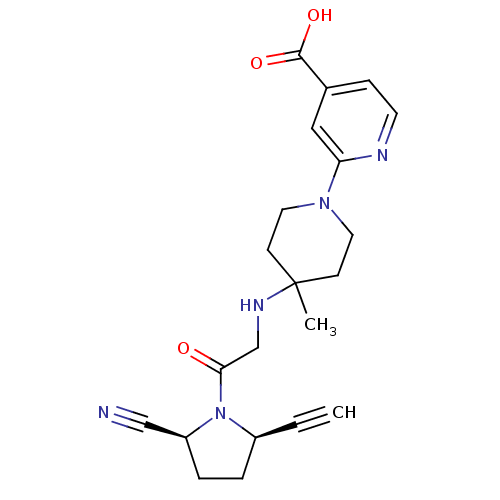 ((2S,5R)-5-Ethynyl-1-{N-(4-methyl-1-(4-carboxy-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50309538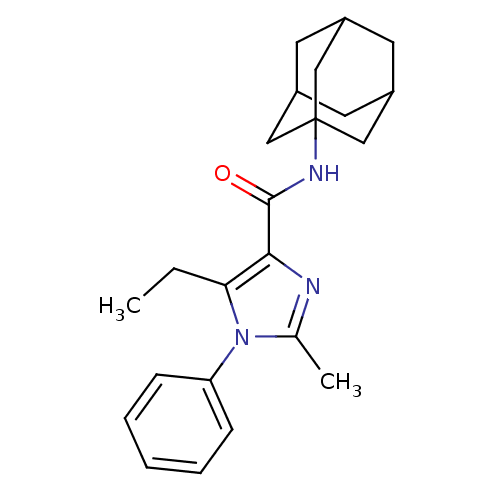 (5-Ethyl-2-methyl-1-phenyl-1H-imidazole-4-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1084-9 (2010) Article DOI: 10.1016/j.bmcl.2009.12.032 BindingDB Entry DOI: 10.7270/Q22J6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176032 (3-[3-(4-Chroman-5-yl-piperazin-1-yl)-propyl]-1H-in...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176028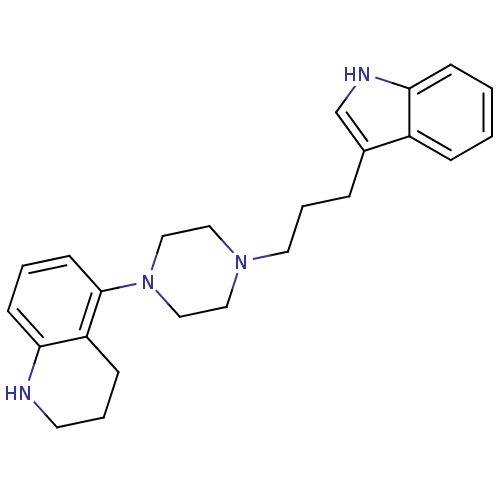 (5-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139841 (CHEMBL3763281) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139846 (CHEMBL3764879) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12656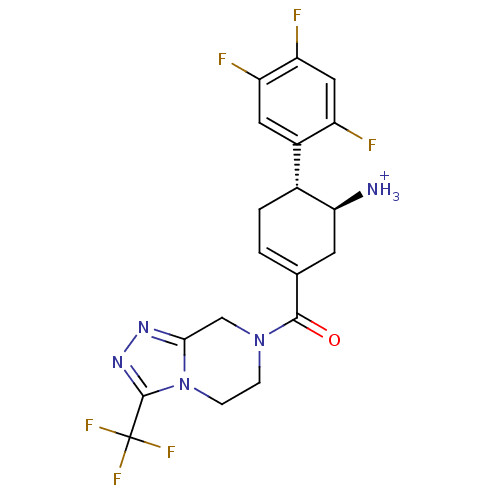 (((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6439-42 (2006) Article DOI: 10.1021/jm060955d BindingDB Entry DOI: 10.7270/Q2G73BXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12647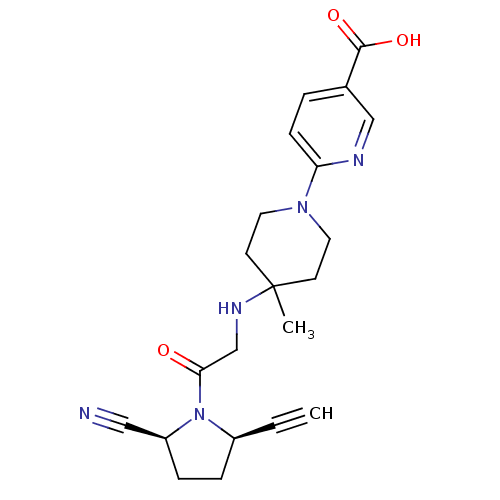 ((2S,5R)-5-ethynyl-1-(N-(4-methyl-1-(5-carboxy-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176025 (4-Chloro-3-{3-[4-(2,3-dihydro-benzo[1,4]dioxin-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513579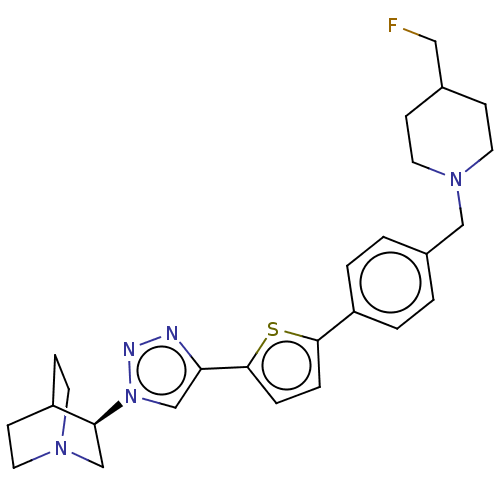 (CHEMBL4534225) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513616 (CHEMBL4532801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176039 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-2,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5565 total ) | Next | Last >> |