

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139906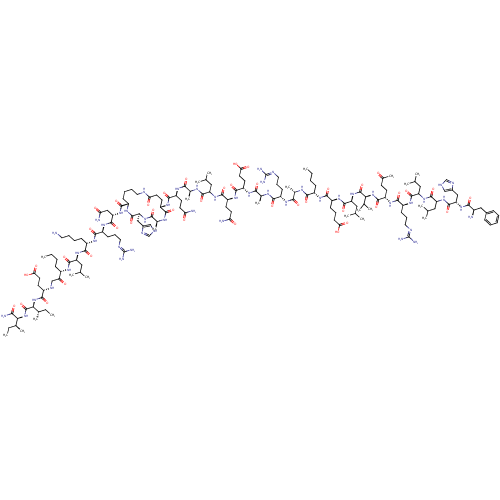 (CHEMBL414991 | DPhe-His-Leu-Leu-Arg-Glu-Val-Leu-Gl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139920 (Ac-(Glu-Aib-Glu-Lys)-Leu-Arg-Lys-Leu-Cha-Asp-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139907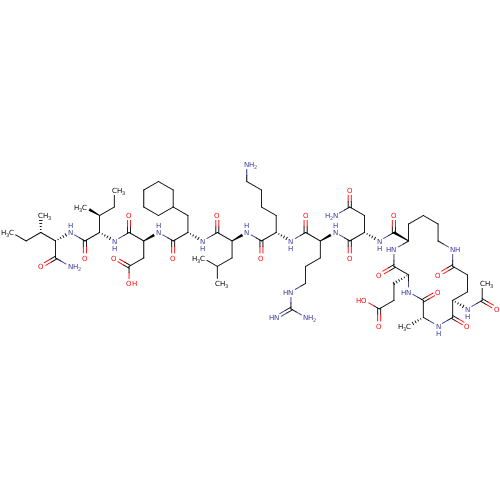 (Ac-(Glu-Ala-Glu-Lys)-Leu-Arg-Lys-Leu-Cha-Asp-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139905 (Ac-(Glu-Ala-Glu-Lys)-Leu-Arg-Lys-Leu-Phe-Asp-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139915 (Ac-(Glu-Ala-Glu-Lys)-Leu-Arg-Lys-Leu-Nle-Asp-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139909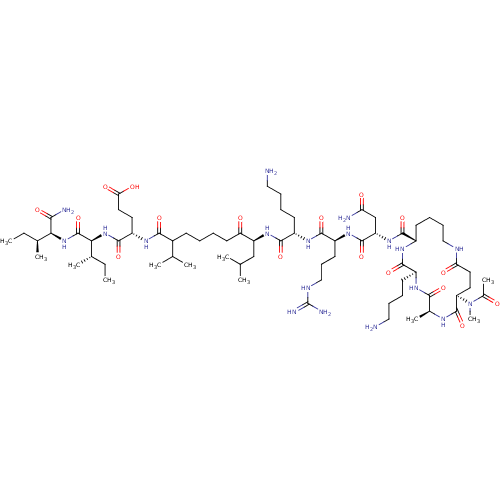 (Ac-(Glu-Ala-Lys-Lys)-Leu-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139901 (Ac-(Glu-Ala-His-Lys)-Leu-Lys-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139904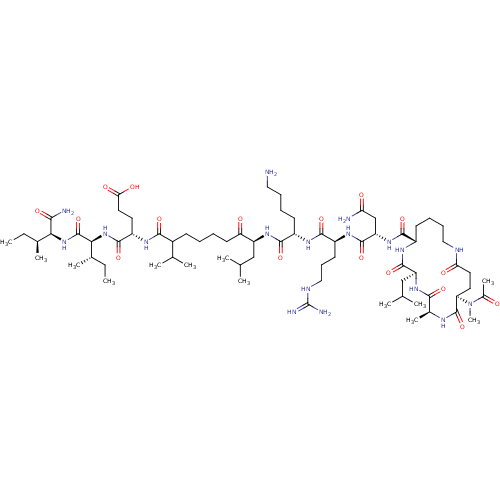 (Ac-(Glu-Ala-Leu-Lys)-Leu-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139908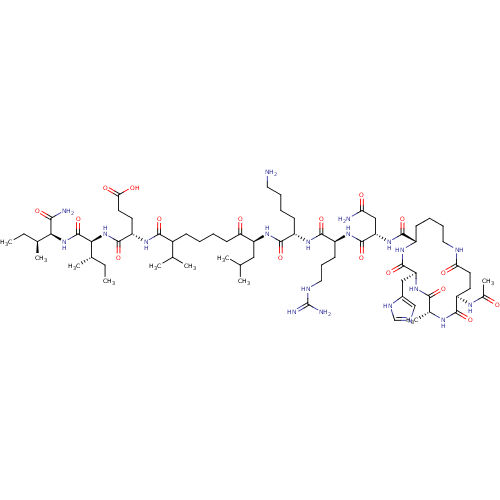 (Ac-(Glu-Ala-His-Lys)-Asn-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139910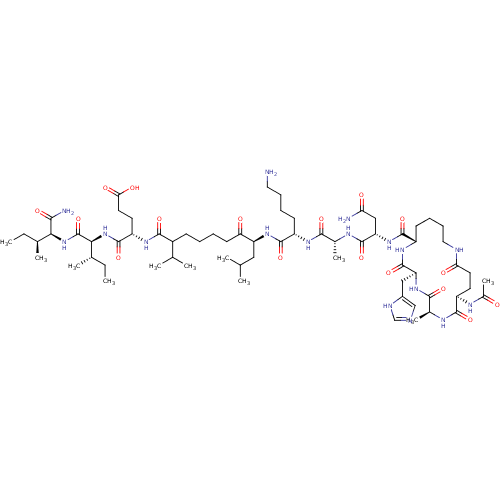 (Ac-(Glu-Ala-His-Lys)-Leu-Ala-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139913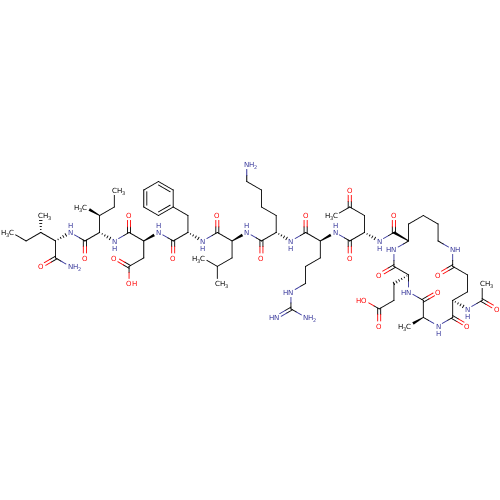 (Ac-(Glu-Ala-Glu-Lys)-Leu-Arg-Lys-Leu-Phe-Asp-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139914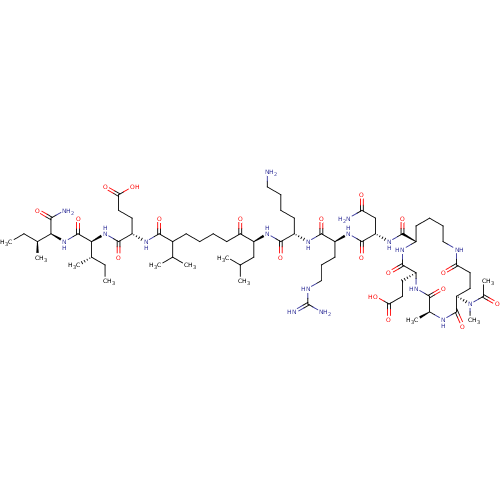 (Ac-(Glu-Ala-Glu-Lys)-Leu-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139912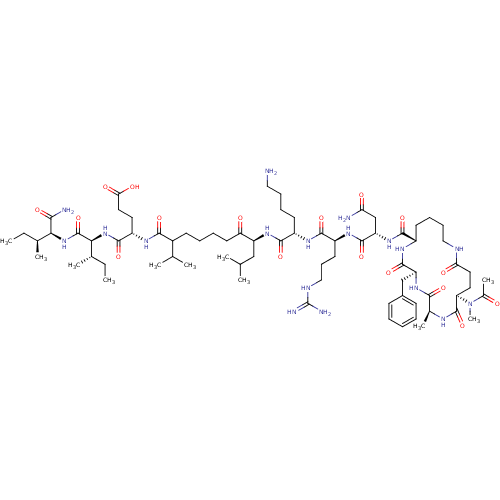 (Ac-(Glu-Ala-Phe-Lys)-Leu-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139911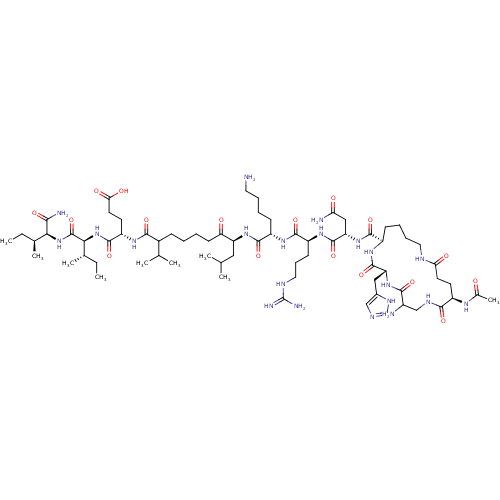 (Ac-(Glu-Dap-His-Lys)-Asn-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139903 (Ac-(Glu-Ala-His-Lys)-Dab-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139902 (Ac-(Glu-Ala-His-Lys)-Asp-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139900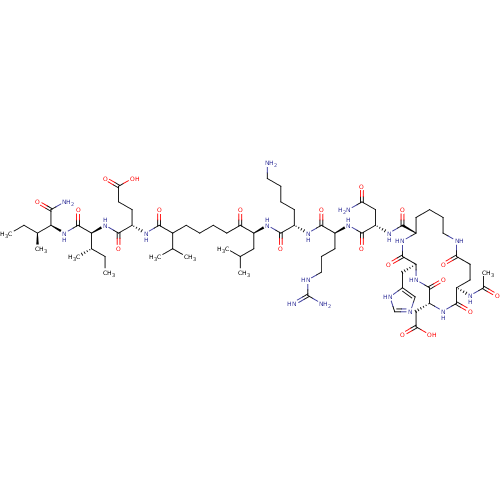 (Ac-(Glu-Asp-His-Lys)-Asn-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139917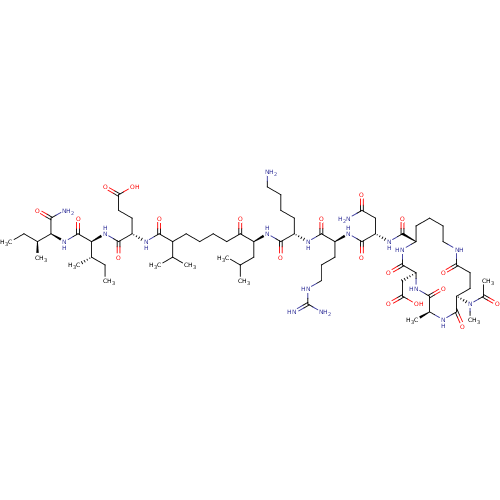 (Ac-(Glu-Ala-Asp-Lys)-Leu-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139919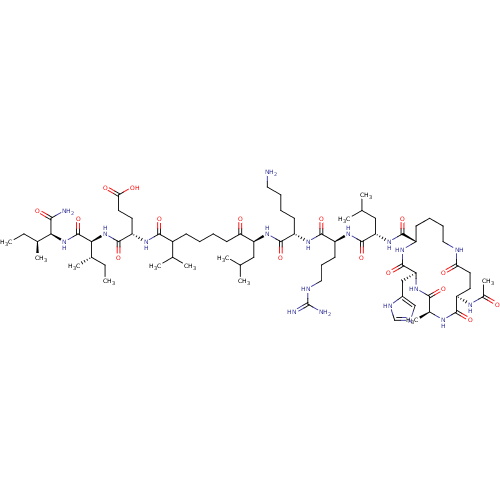 (Ac-(Glu-Ala-His-Lys)-Leu-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139918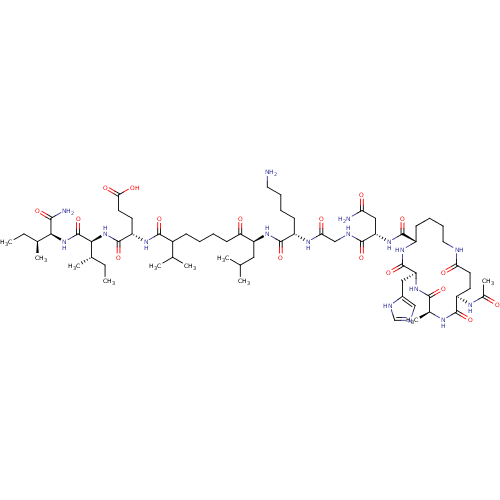 (Ac-(Glu-Ala-His-Lys)-Leu-Gly-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139916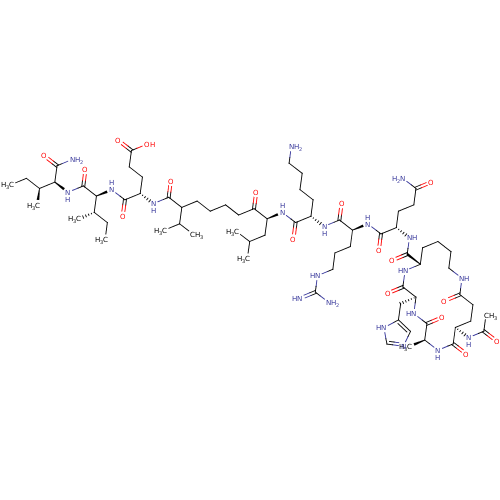 (Ac-(Glu-Ala-His-Lys)-Gln-Arg-Lys-Leu-Nle-Glu-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110994 (4-[2-Methyl-4-(5-methyl-thiophen-2-yl)-oxazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human purified Prostaglandin G/H synthase 2 | J Med Chem 45: 1511-7 (2002) BindingDB Entry DOI: 10.7270/Q2H995XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50107528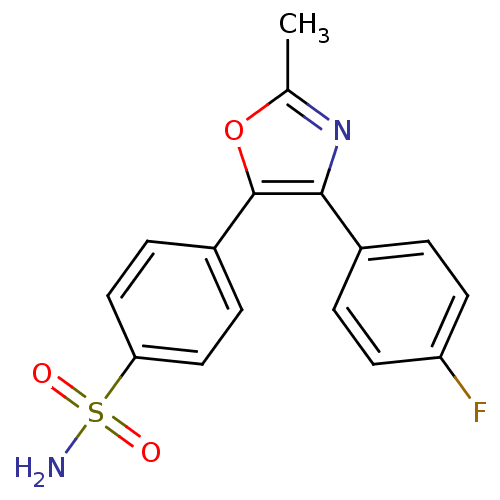 (4-[4-(4-Fluoro-phenyl)-2-methyl-oxazol-5-yl]-benze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human purified Prostaglandin G/H synthase 2 | J Med Chem 45: 1511-7 (2002) BindingDB Entry DOI: 10.7270/Q2H995XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50107530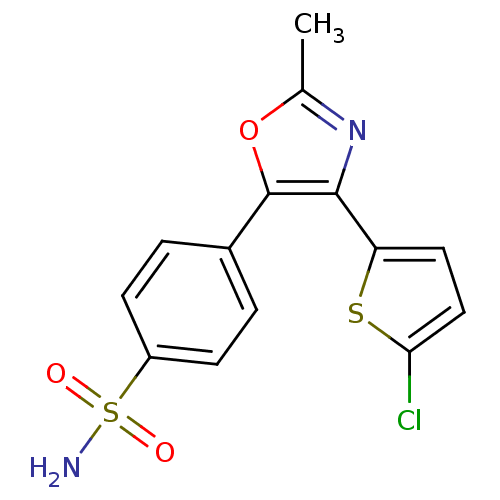 (4-[4-(5-Chloro-thiophen-2-yl)-2-methyl-oxazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound towards human recombinant Prostaglandin G/H synthase 2 enzyme | Bioorg Med Chem Lett 12: 65-8 (2001) BindingDB Entry DOI: 10.7270/Q24F1Q1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50107528 (4-[4-(4-Fluoro-phenyl)-2-methyl-oxazol-5-yl]-benze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound towards human recombinant Prostaglandin G/H synthase 2 enzyme | Bioorg Med Chem Lett 12: 65-8 (2001) BindingDB Entry DOI: 10.7270/Q24F1Q1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110998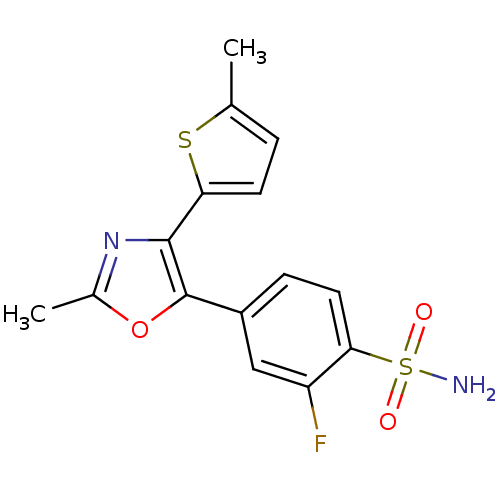 (2-Fluoro-4-[2-methyl-4-(5-methyl-thiophen-2-yl)-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human purified Prostaglandin G/H synthase 2 | J Med Chem 45: 1511-7 (2002) BindingDB Entry DOI: 10.7270/Q2H995XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50107522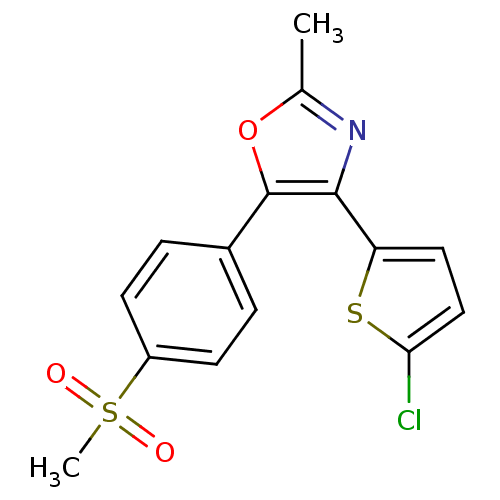 (4-(5-Chloro-thiophen-2-yl)-5-(4-methanesulfonyl-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound towards human recombinant Prostaglandin G/H synthase 2 enzyme | Bioorg Med Chem Lett 12: 65-8 (2001) BindingDB Entry DOI: 10.7270/Q24F1Q1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50111000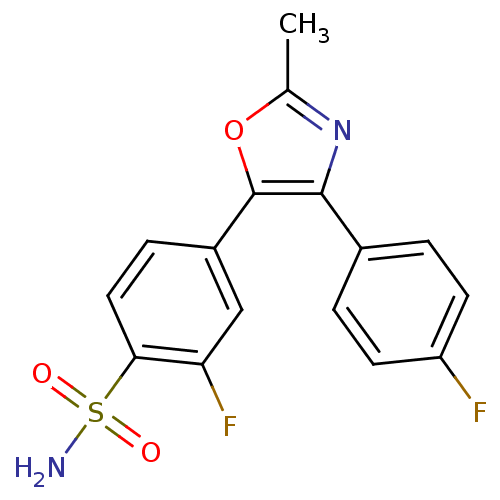 (2-Fluoro-4-[4-(4-fluoro-phenyl)-2-methyl-oxazol-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human purified Prostaglandin G/H synthase 2 | J Med Chem 45: 1511-7 (2002) BindingDB Entry DOI: 10.7270/Q2H995XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50107526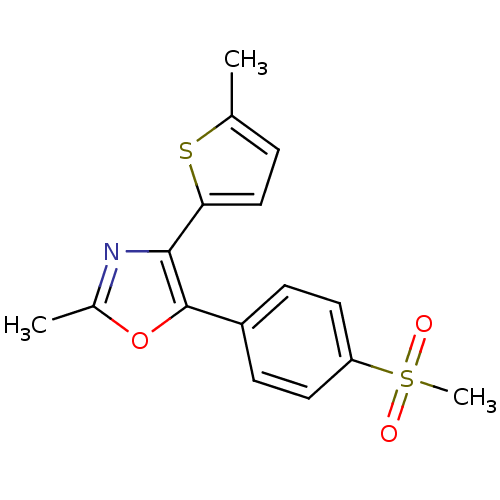 (5-(4-Methanesulfonyl-phenyl)-2-methyl-4-(5-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound towards human recombinant Prostaglandin G/H synthase 2 enzyme | Bioorg Med Chem Lett 12: 65-8 (2001) BindingDB Entry DOI: 10.7270/Q24F1Q1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389038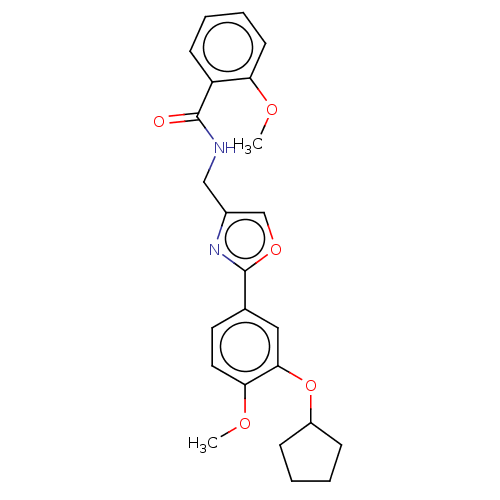 (USRE46792, 35) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389039 (USRE46792, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389040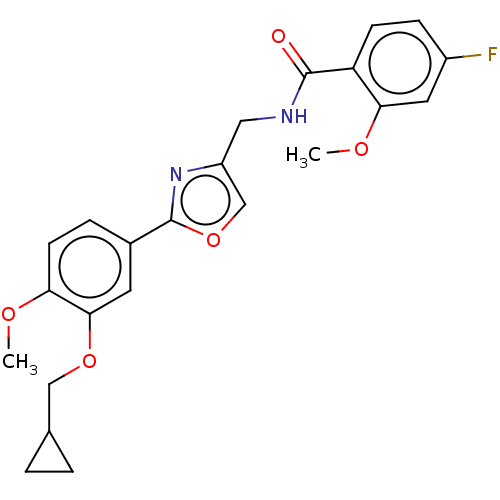 (USRE46792, 42) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389041 (USRE46792, 43) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389042 (USRE46792, 44) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389043 (USRE46792, 61) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389044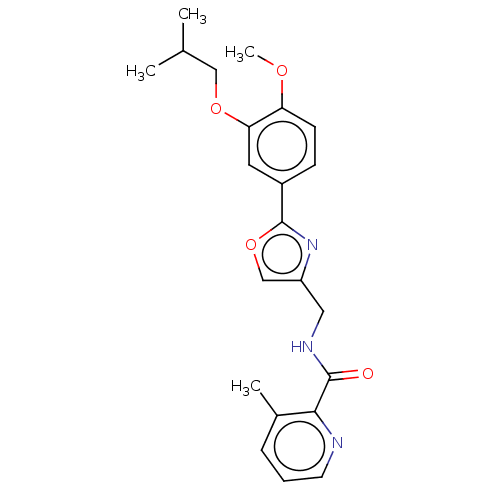 (USRE46792, 62) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389045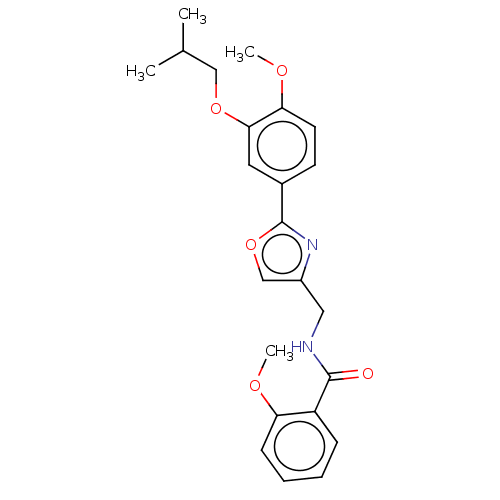 (USRE46792, 63) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389046 (USRE46792, 76) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389047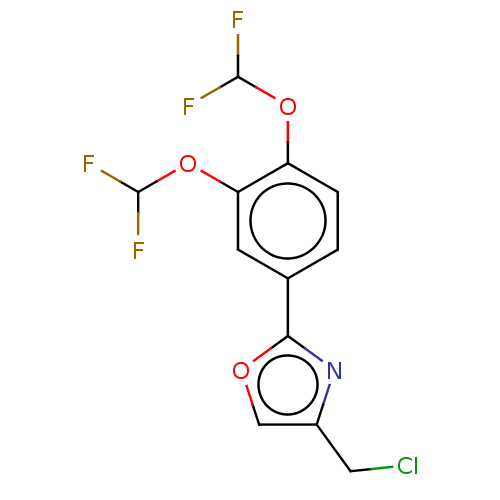 (USRE46792, 98) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389048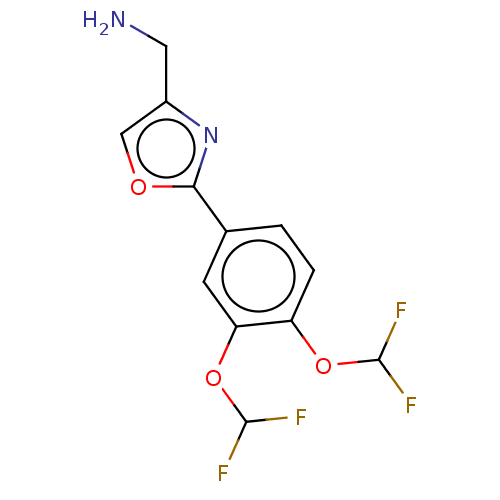 (USRE46792, 99) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389049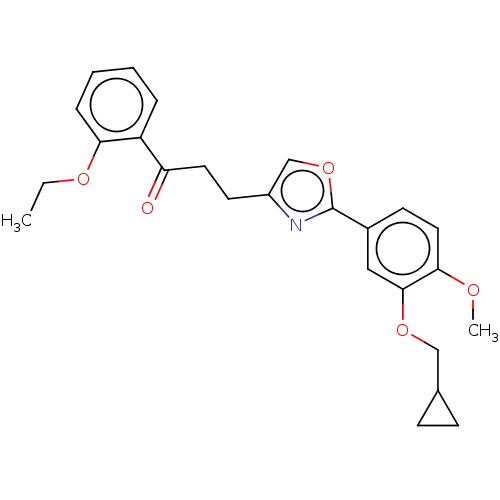 (USRE46792, 102) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389050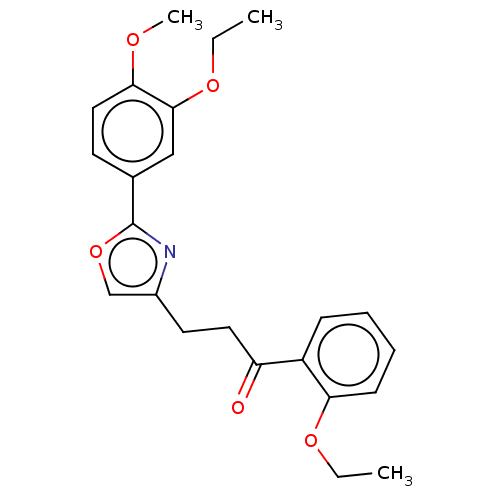 (USRE46792, 103) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389051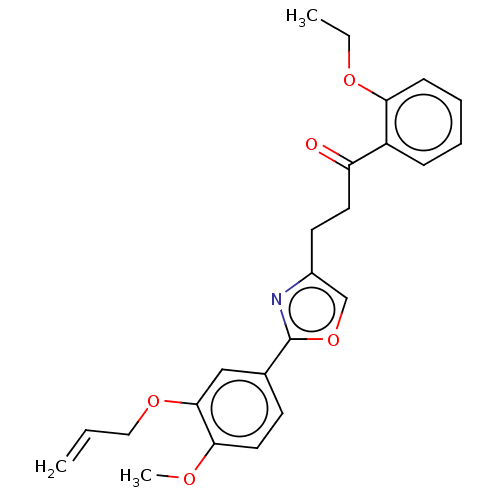 (USRE46792, 104) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389052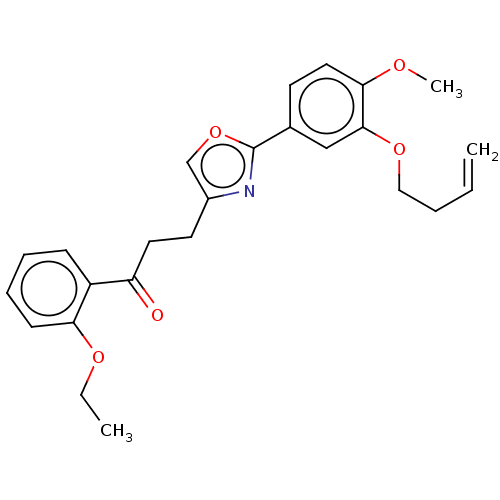 (USRE46792, 108) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389053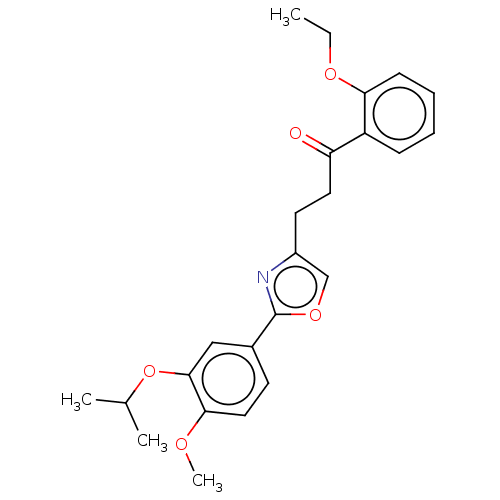 (USRE46792, 111) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389054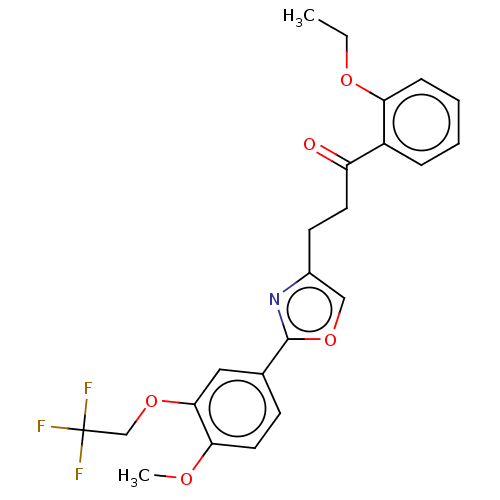 (USRE46792, 112) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389055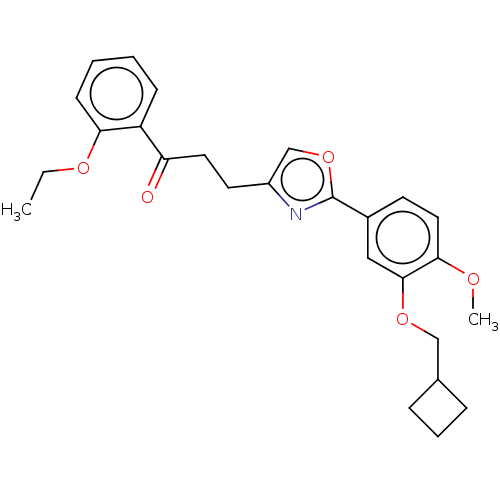 (USRE46792, 116) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389056 (USRE46792, 126) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389057 (USRE46792, 129) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM389058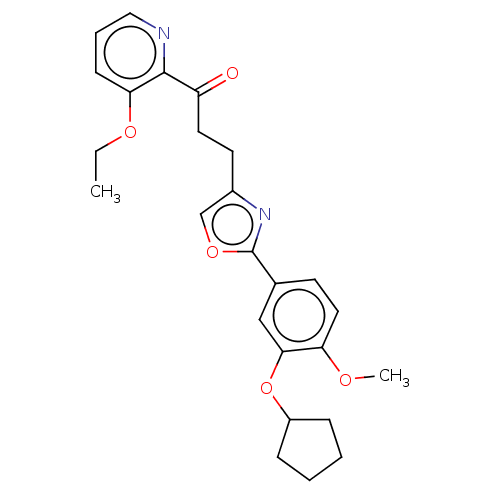 (USRE46792, 132) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere | Assay Description Necessary amounts of test compounds were weighed, and 100% dimethylsulfoxide (DMSO) was added thereto to adjust the concentration to 10 mM. The solut... | J Med Chem 52: 646-54 (2009) BindingDB Entry DOI: 10.7270/Q2DZ0BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 202 total ) | Next | Last >> |