

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-1 (Homo sapiens (Human)) | BDBM12197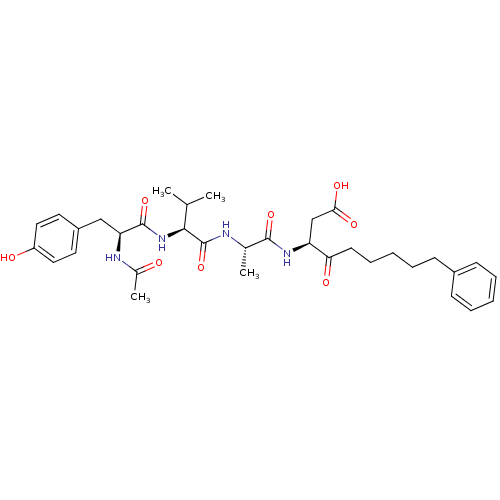 ((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12055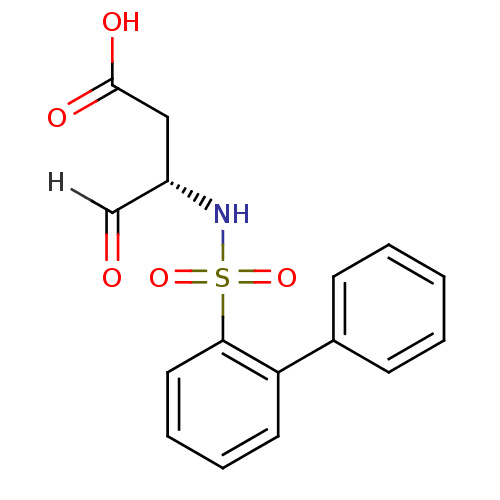 ((3S)-4-oxo-3-[(2-phenylbenzene)sulfonamido]butanoi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12049 ((3S)-3-[(3-methyl-2-phenylbenzene)sulfonamido]-4-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12054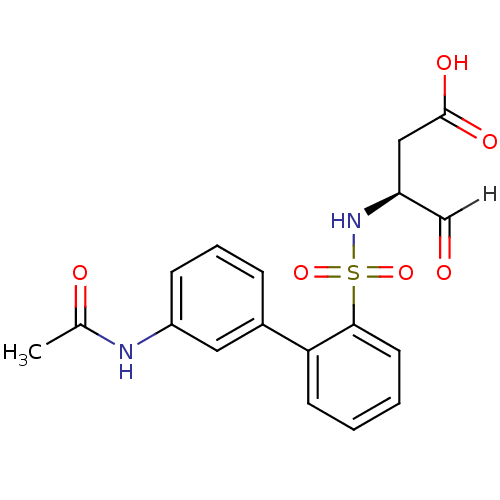 ((3S)-3-{[2-(3-acetamidophenyl)benzene]sulfonamido}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12051 ((3S)-3-{[2-(3-methylphenyl)benzene]sulfonamido}-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12050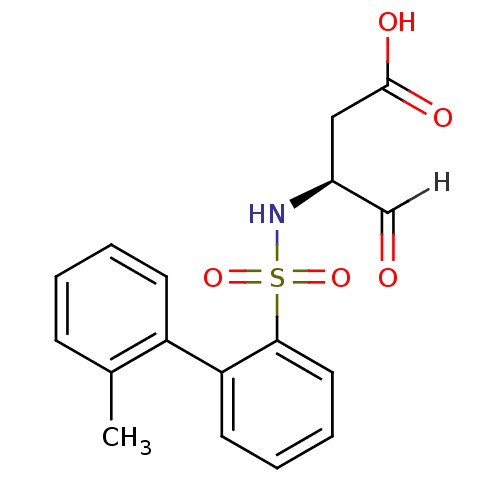 ((3S)-3-{[2-(2-methylphenyl)benzene]sulfonamido}-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12053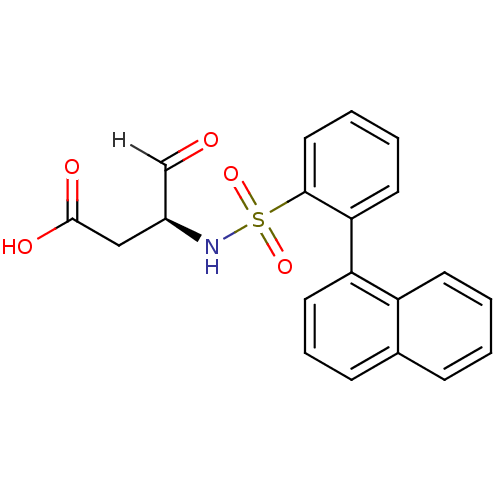 ((3S)-3-{[2-(naphthalen-1-yl)benzene]sulfonamido}-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.90E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12052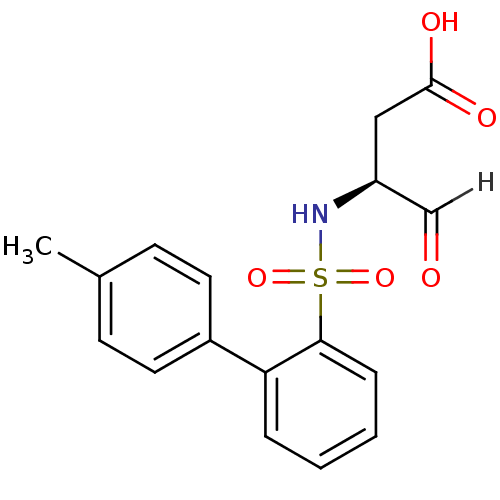 ((3S)-3-{[2-(4-methylphenyl)benzene]sulfonamido}-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.20E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12047 ((3S)-3-benzenesulfonamido-4-oxobutanoic acid | (S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.40E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12045 ((3S)-3-benzenesulfonamido-4-oxo-9-phenylnonanoic a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12046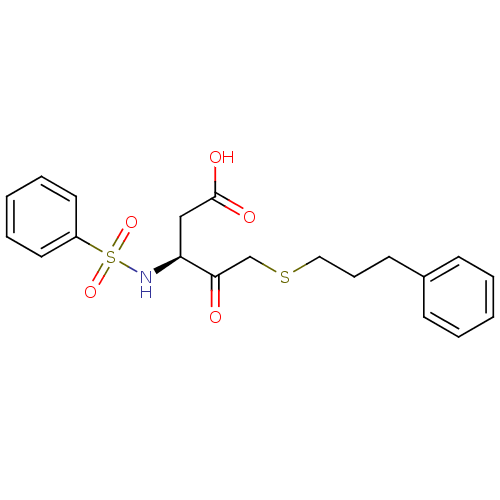 ((3S)-3-benzenesulfonamido-4-oxo-5-[(3-phenylpropyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12044 ((3S)-3-{[(benzyloxy)carbonyl]amino}-4-oxo-5-[(3-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | -27.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM12043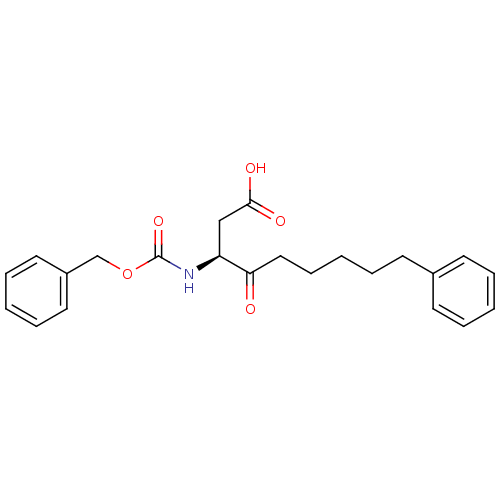 ((3S)-3-{[(benzyloxy)carbonyl]amino}-4-oxo-9-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.19E+5 | -23.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | Bioorg Med Chem 10: 31-40 (2002) Article DOI: 10.1016/s0968-0896(01)00250-4 BindingDB Entry DOI: 10.7270/Q2BP011G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50344821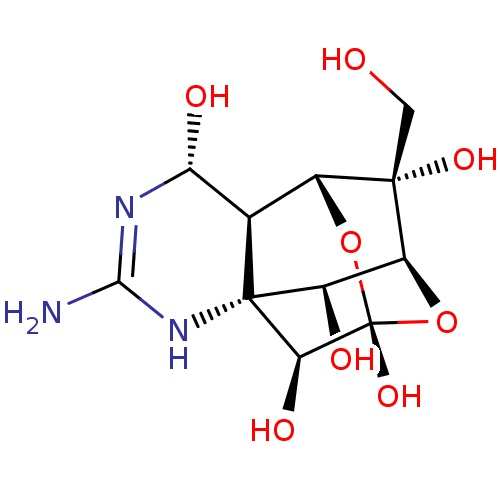 (10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Na+ influx in chinese hamster ovary cells expressing rat brain sodium channel type IIA | J Med Chem 37: 268-74 (1994) BindingDB Entry DOI: 10.7270/Q2CJ8F39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro for inhibition of purified bovine trypsin. | Bioorg Med Chem Lett 9: 815-20 (1999) BindingDB Entry DOI: 10.7270/Q29G5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was evaluated to inhibit trypsinand is expressed in IC50 (The concentration required to inhibit 50% of the enzyme). | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Trypsin. | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against C1s serine protease . | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063701 (2-(2-Iodo-phenylamino)-naphtho[2,3-d][1,3]oxazin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against plasmin. | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Thrombin. | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063745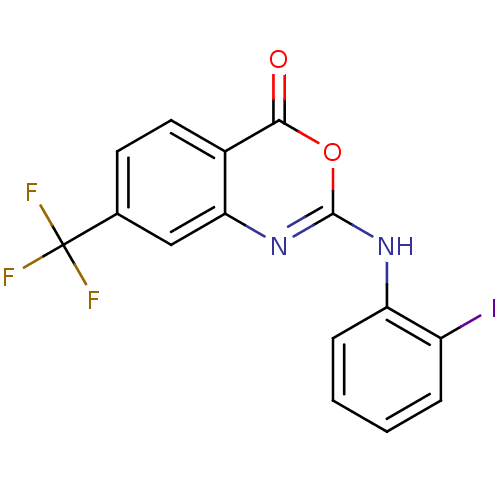 (2-(2-Iodo-phenylamino)-7-trifluoromethyl-benzo[d][...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for inhibitory activity against purified human C1r protease protease | Bioorg Med Chem Lett 9: 815-20 (1999) BindingDB Entry DOI: 10.7270/Q29G5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063745 (2-(2-Iodo-phenylamino)-7-trifluoromethyl-benzo[d][...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063726 (7-Chloro-2-(2,6-dichloro-phenylamino)-benzo[d][1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50017702 (1-(bis(4-fluorophenyl)methyl)-4-cinnamylpiperazine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of veratridine-induced Na+ influx in chinese hamster ovary cells expressing alpha subunit of rat brain type voltage-gated sodium channel t... | J Med Chem 37: 268-74 (1994) BindingDB Entry DOI: 10.7270/Q2CJ8F39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063722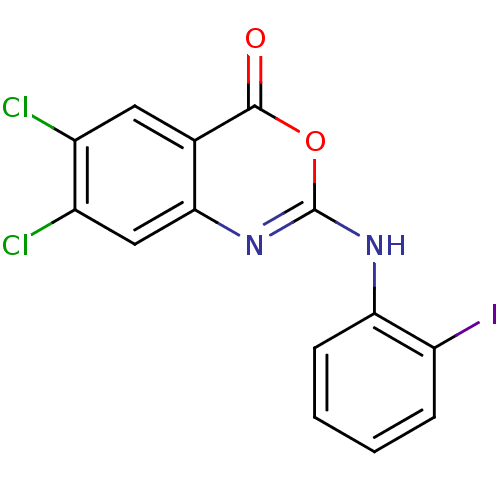 (6,7-Dichloro-2-(2-iodo-phenylamino)-benzo[d][1,3]o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063703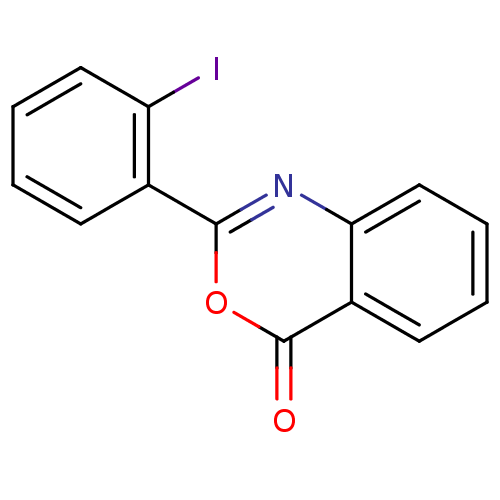 (2-(2-Iodo-phenyl)-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Thrombin. | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Kallikrein. | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Bos taurus) | BDBM50028169 (3-(1,1-Dimethyl-2-phenyl-ethyl)-pyridine | CHEMBL4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibition of bovine adrenal cortical mitochondrial 11 beta-hydroxylase | J Med Chem 27: 15-9 (1984) BindingDB Entry DOI: 10.7270/Q25Q4V3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50075982 (4-Methoxy-N-methyl-N-(4-oxo-4H-benzo[d][1,3]oxazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of purified human C1r protease. | Bioorg Med Chem Lett 9: 815-20 (1999) BindingDB Entry DOI: 10.7270/Q29G5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50289007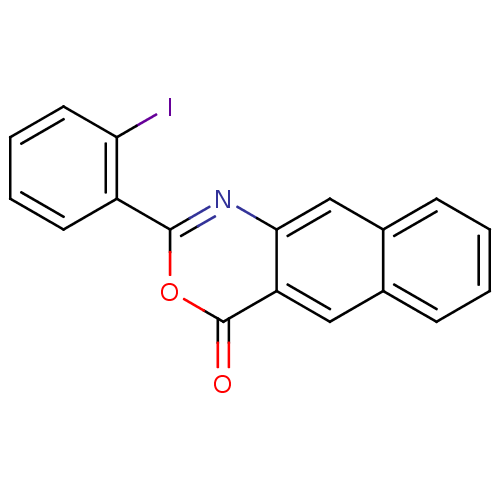 (2-(2-Iodo-phenyl)-naphtho[2,3-d][1,3]oxazin-4-one ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063711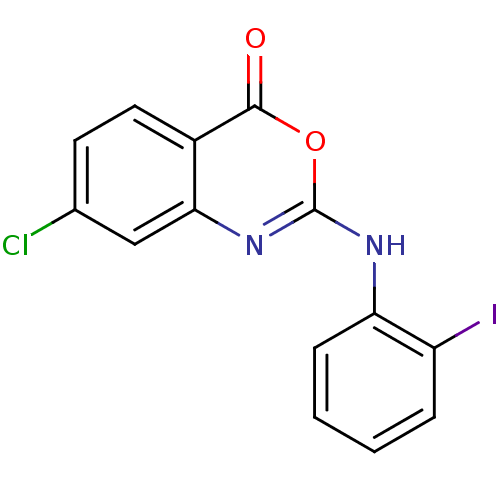 (7-Chloro-2-(2-iodo-phenylamino)-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063717 (7-Chloro-2-(2-chloro-phenylamino)-benzo[d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063721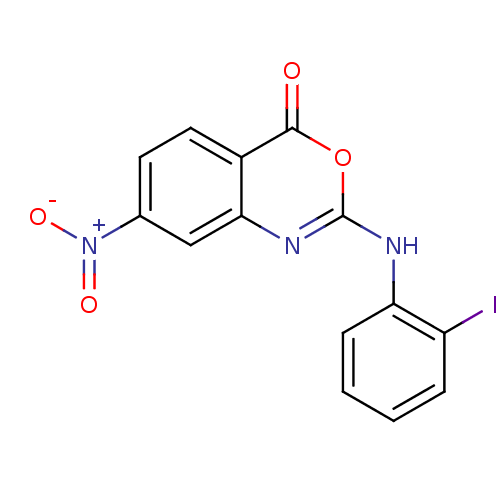 (2-(2-Iodo-phenylamino)-7-nitro-benzo[d][1,3]oxazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063717 (7-Chloro-2-(2-chloro-phenylamino)-benzo[d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063723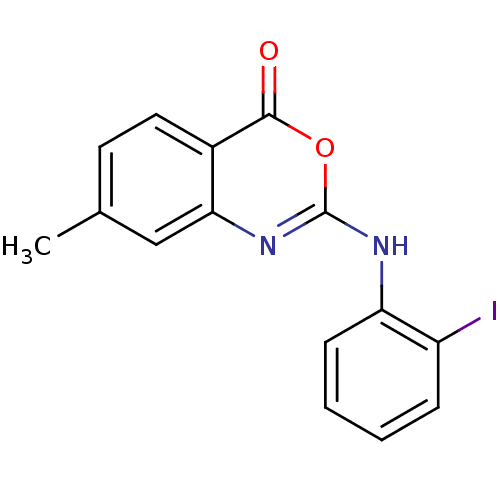 (2-(2-Iodo-phenylamino)-7-methyl-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063724 (2-(2,6-Dichloro-phenylamino)-benzo[d][1,3]oxazin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063711 (7-Chloro-2-(2-iodo-phenylamino)-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063726 (7-Chloro-2-(2,6-dichloro-phenylamino)-benzo[d][1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063724 (2-(2,6-Dichloro-phenylamino)-benzo[d][1,3]oxazin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063732 (2-(2-Chloro-phenylamino)-7-methyl-benzo[d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50075980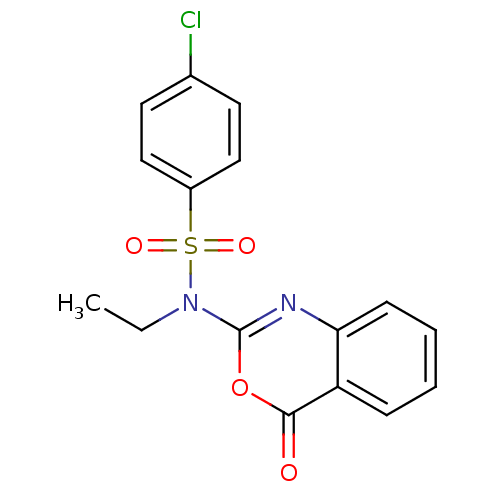 (4-Chloro-N-ethyl-N-(4-oxo-4H-benzo[d][1,3]oxazin-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro for inhibition of purified bovine trypsin. | Bioorg Med Chem Lett 9: 815-20 (1999) BindingDB Entry DOI: 10.7270/Q29G5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50063703 (2-(2-Iodo-phenyl)-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against C1s serine protease . | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50075979 (4-Chloro-N-methyl-N-(4-oxo-4H-benzo[d][1,3]oxazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro for inhibition of purified bovine trypsin. | Bioorg Med Chem Lett 9: 815-20 (1999) BindingDB Entry DOI: 10.7270/Q29G5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063703 (2-(2-Iodo-phenyl)-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063703 (2-(2-Iodo-phenyl)-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063704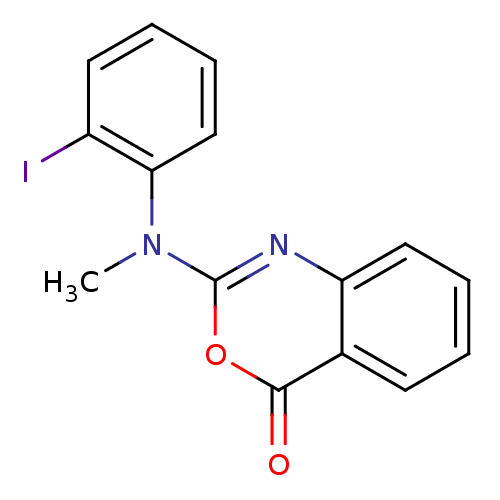 (2-[(2-Iodo-phenyl)-methyl-amino]-benzo[d][1,3]oxaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063732 (2-(2-Chloro-phenylamino)-7-methyl-benzo[d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 271 total ) | Next | Last >> |