 Found 195 hits with Last Name = 'heap' and Initial = 'c'
Found 195 hits with Last Name = 'heap' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50065428
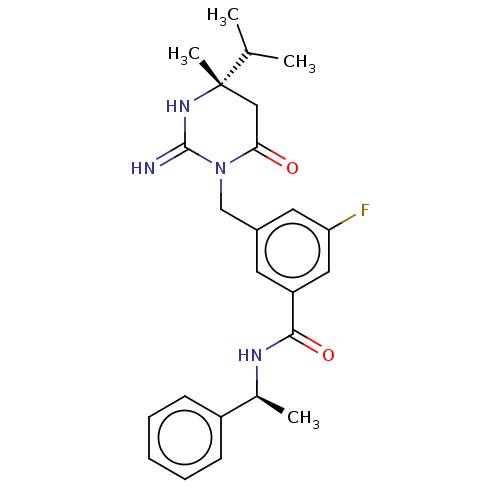
(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065395

(CHEMBL3401345)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2CC(CC2=O)c2cccc(Cl)c2)C(=N)N1 |r| Show InChI InChI=1S/C25H29ClN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-6-4-9-21(10-17)29-15-19(12-22(29)31)18-7-5-8-20(26)11-18/h4-11,16,19H,12-15H2,1-3H3,(H2,27,28)/t19?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065426
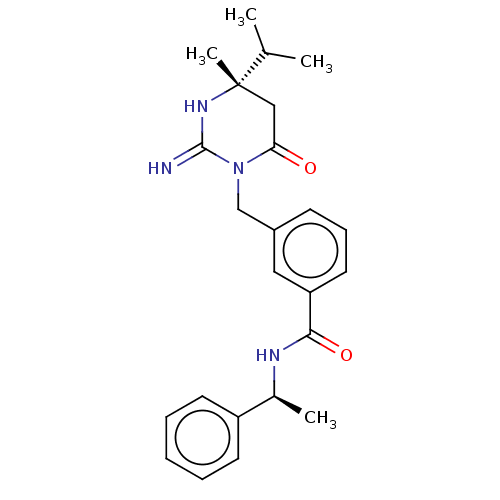
(CHEMBL3401348)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065424
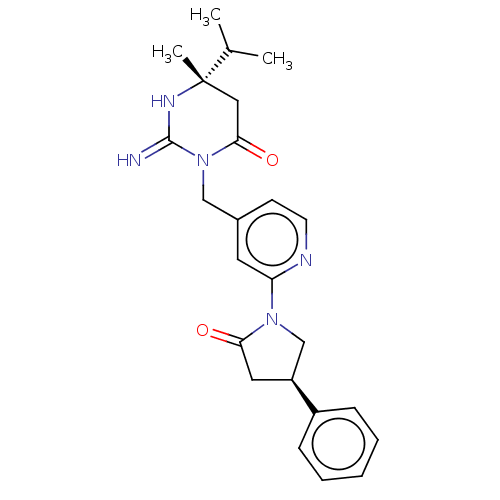
(CHEMBL3401346)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29N5O2/c1-16(2)24(3)13-22(31)29(23(25)27-24)14-17-9-10-26-20(11-17)28-15-19(12-21(28)30)18-7-5-4-6-8-18/h4-11,16,19H,12-15H2,1-3H3,(H2,25,27)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065425
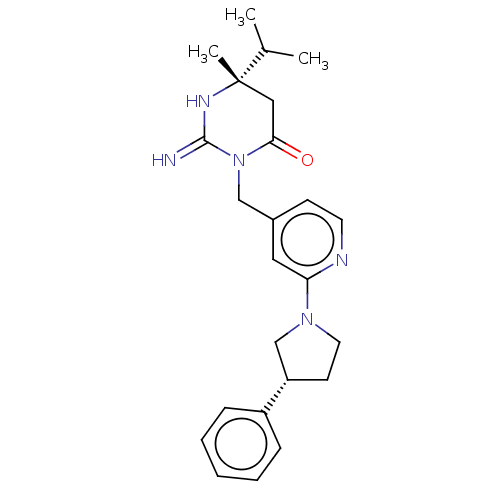
(CHEMBL3401347)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2CC[C@@H](C2)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H31N5O/c1-17(2)24(3)14-22(30)29(23(25)27-24)15-18-9-11-26-21(13-18)28-12-10-20(16-28)19-7-5-4-6-8-19/h4-9,11,13,17,20H,10,12,14-16H2,1-3H3,(H2,25,27)/t20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065393

(CHEMBL3401344)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H29FN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-9-20(26)12-21(10-17)29-15-19(11-22(29)31)18-7-5-4-6-8-18/h4-10,12,16,19H,11,13-15H2,1-3H3,(H2,27,28)/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065391
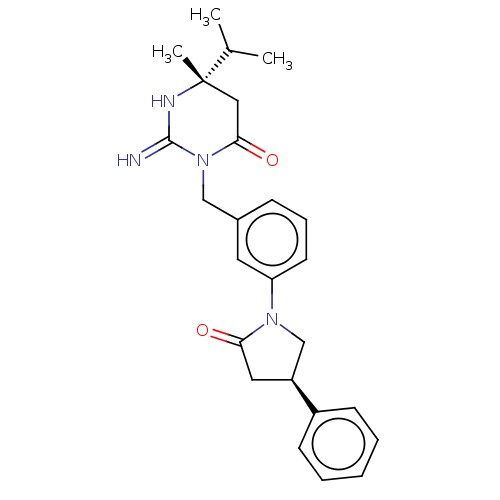
(CHEMBL3401342)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H30N4O2/c1-17(2)25(3)14-23(31)29(24(26)27-25)15-18-8-7-11-21(12-18)28-16-20(13-22(28)30)19-9-5-4-6-10-19/h4-12,17,20H,13-16H2,1-3H3,(H2,26,27)/t20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065427

(CHEMBL3401349)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065392
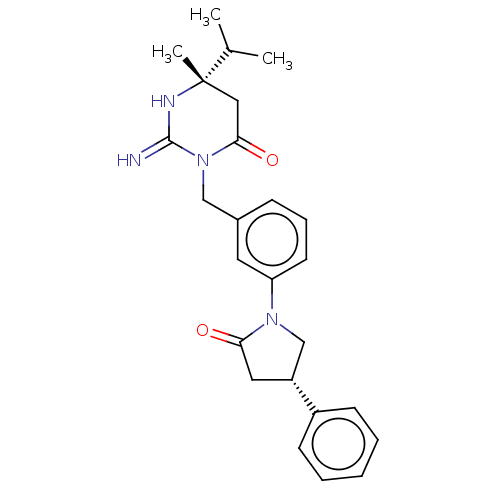
(CHEMBL3401343)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2C[C@@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H30N4O2/c1-17(2)25(3)14-23(31)29(24(26)27-25)15-18-8-7-11-21(12-18)28-16-20(13-22(28)30)19-9-5-4-6-10-19/h4-12,17,20H,13-16H2,1-3H3,(H2,26,27)/t20-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathE (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM98678
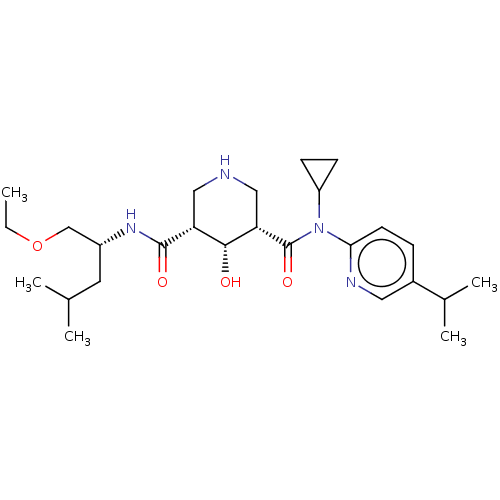
(US8497286, 154)Show SMILES CCOC[C@@H](CC(C)C)NC(=O)[C@@H]1CNC[C@@H]([C@@H]1O)C(=O)N(C1CC1)c1ccc(cn1)C(C)C |r| Show InChI InChI=1S/C26H42N4O4/c1-6-34-15-19(11-16(2)3)29-25(32)21-13-27-14-22(24(21)31)26(33)30(20-8-9-20)23-10-7-18(12-28-23)17(4)5/h7,10,12,16-17,19-22,24,27,31H,6,8-9,11,13-15H2,1-5H3,(H,29,32)/t19-,21-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50382334
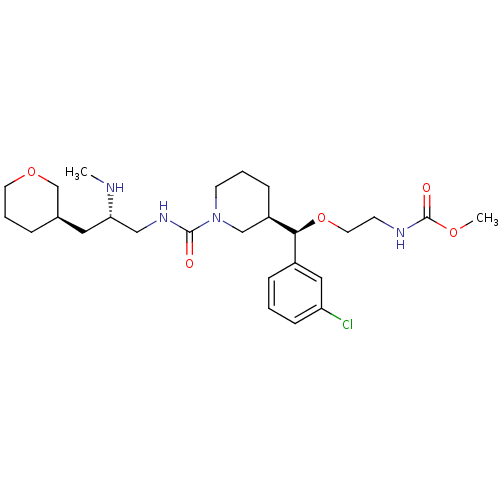
(CHEMBL1276678)Show SMILES CN[C@H](CNC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)C[C@H]1CCCOC1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-23(14-19-6-5-12-35-18-19)16-30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950
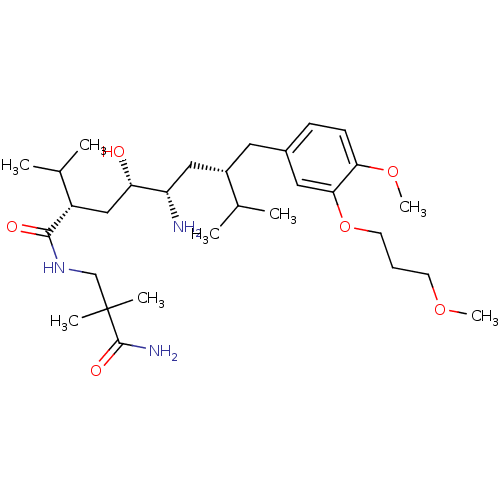
((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50392953
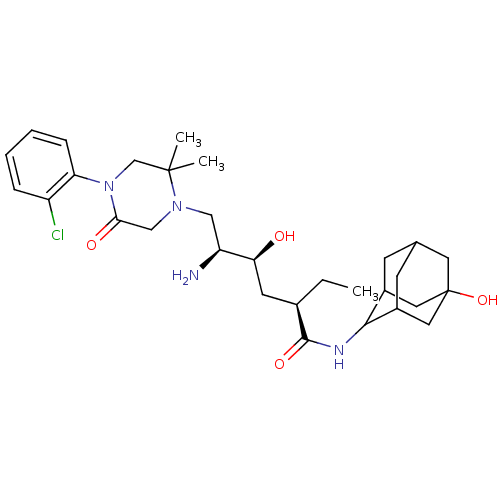
(CHEMBL2152353)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NC1C2CC3CC1CC(O)(C3)C2 |r,wU:4.4,2.2,wD:6.6,TLB:38:35:28.29.30:32,36:35:28:30.31.32,27:28:34.38.35:30.31.32,27:28:32:34.35.37,THB:38:29:32:34.35.37,37:35:28:30.31.32,37:31:28:34.38.35,(13.51,-6.54,;12.17,-7.31,;12.17,-8.85,;10.84,-9.62,;9.51,-8.85,;9.51,-7.31,;8.17,-9.62,;6.84,-8.85,;8.17,-11.16,;6.84,-11.93,;5.5,-11.17,;4.16,-11.94,;2.83,-11.17,;4.18,-13.47,;5.51,-14.24,;6.84,-13.48,;7.61,-14.81,;8.38,-13.48,;2.85,-14.25,;1.51,-13.49,;.18,-14.26,;.18,-15.8,;1.52,-16.57,;2.85,-15.8,;4.19,-16.57,;13.51,-9.62,;13.51,-11.16,;14.84,-8.86,;16.17,-9.63,;17.67,-9.21,;17.67,-7.62,;18.71,-6.39,;17.36,-6.87,;17.37,-8.35,;18.7,-8.84,;20.09,-8.5,;21.42,-9.26,;20.1,-6.97,;19.08,-9.77,)| Show InChI InChI=1S/C30H45ClN4O4/c1-4-19(28(38)33-27-20-9-18-10-21(27)14-30(39,12-18)13-20)11-25(36)23(32)15-34-16-26(37)35(17-29(34,2)3)24-8-6-5-7-22(24)31/h5-8,18-21,23,25,27,36,39H,4,9-17,32H2,1-3H3,(H,33,38)/t18?,19-,20?,21?,23+,25+,27?,30?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50347010
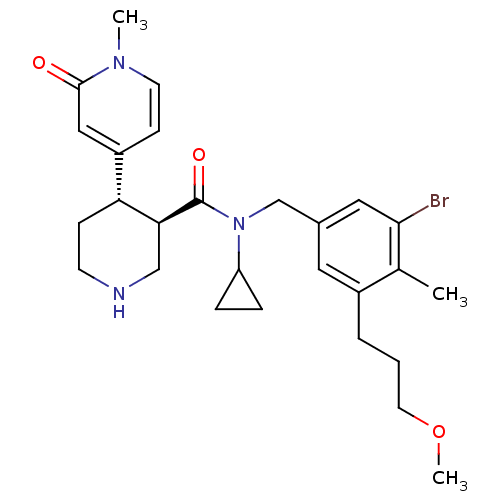
(CHEMBL1796063)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccn(C)c(=O)c2)cc(Br)c1C |r| Show InChI InChI=1S/C27H36BrN3O3/c1-18-20(5-4-12-34-3)13-19(14-25(18)28)17-31(22-6-7-22)27(33)24-16-29-10-8-23(24)21-9-11-30(2)26(32)15-21/h9,11,13-15,22-24,29H,4-8,10,12,16-17H2,1-3H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184486
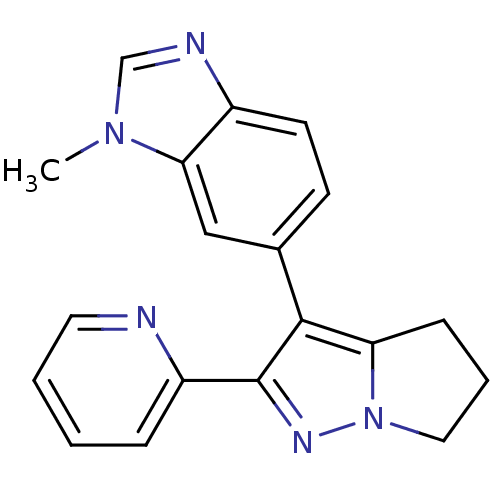
(1-methyl-6-(2-pyridin-2-yl-5,6-dihydro-4H-pyrrolo[...)Show InChI InChI=1S/C19H17N5/c1-23-12-21-14-8-7-13(11-17(14)23)18-16-6-4-10-24(16)22-19(18)15-5-2-3-9-20-15/h2-3,5,7-9,11-12H,4,6,10H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057402
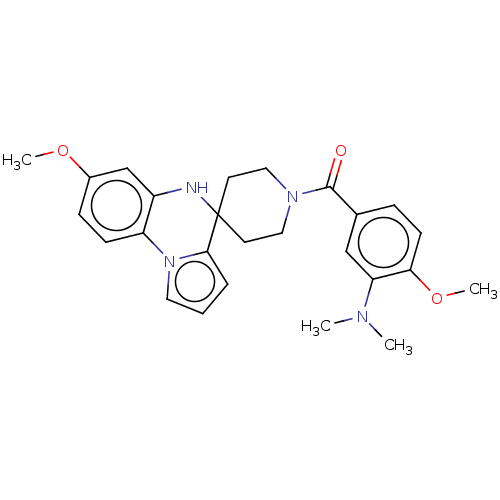
(CHEMBL3322777)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(OC)c(c3)N(C)C)c3cccn-23)c1 Show InChI InChI=1S/C26H30N4O3/c1-28(2)22-16-18(7-10-23(22)33-4)25(31)29-14-11-26(12-15-29)24-6-5-13-30(24)21-9-8-19(32-3)17-20(21)27-26/h5-10,13,16-17,27H,11-12,14-15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057398

(CHEMBL3322775)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(N(C)C)c(Cl)c3)c3cccn-23)c1 Show InChI InChI=1S/C25H27ClN4O2/c1-28(2)21-8-6-17(15-19(21)26)24(31)29-13-10-25(11-14-29)23-5-4-12-30(23)22-9-7-18(32-3)16-20(22)27-25/h4-9,12,15-16,27H,10-11,13-14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184488
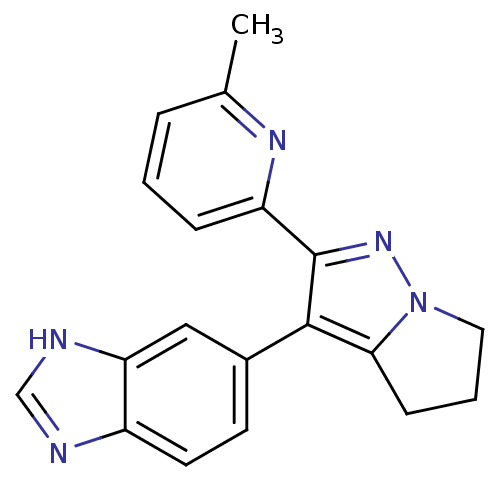
(6-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrol...)Show InChI InChI=1S/C19H17N5/c1-12-4-2-5-15(22-12)19-18(17-6-3-9-24(17)23-19)13-7-8-14-16(10-13)21-11-20-14/h2,4-5,7-8,10-11H,3,6,9H2,1H3,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057365

(CHEMBL3322762)Show SMILES COc1ccc-2c(NC3(CCN(Cc4ccccc4)CC3)c3cccn-23)c1 Show InChI InChI=1S/C23H25N3O/c1-27-19-9-10-21-20(16-19)24-23(22-8-5-13-26(21)22)11-14-25(15-12-23)17-18-6-3-2-4-7-18/h2-10,13,16,24H,11-12,14-15,17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184483
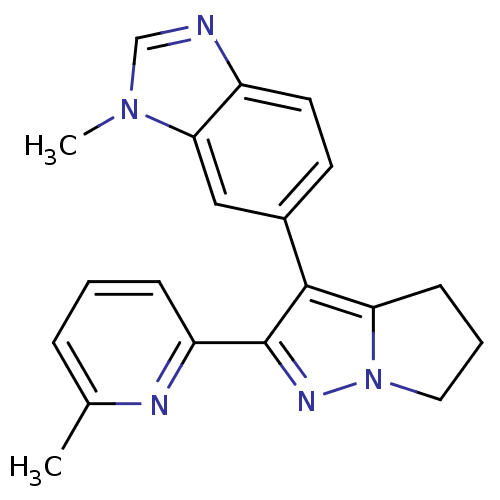
(1-methyl-6-[2-(6-methylpyridin-2-yl)-5,6-dihydro-4...)Show InChI InChI=1S/C20H19N5/c1-13-5-3-6-16(22-13)20-19(17-7-4-10-25(17)23-20)14-8-9-15-18(11-14)24(2)12-21-15/h3,5-6,8-9,11-12H,4,7,10H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1 [4-503,T204D]
(Homo sapiens (Human)) | BDBM21506
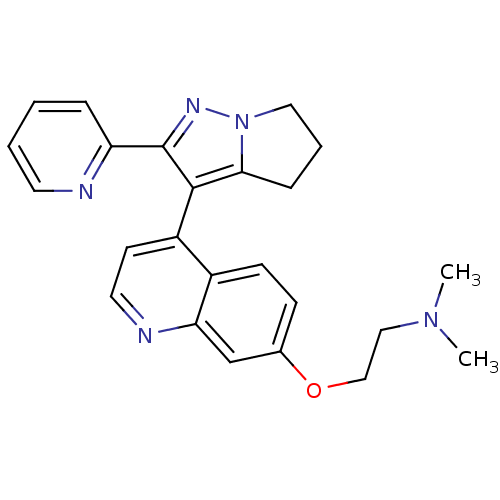
(Dihydropyrrolopyrazole, 15a | dimethyl[2-({4-[2-(p...)Show SMILES CN(C)CCOc1ccc2c(ccnc2c1)-c1c2CCCn2nc1-c1ccccn1 |(-8.89,-1.84,;-7.56,-1.07,;-6.23,-1.84,;-7.56,.47,;-6.23,1.24,;-6.23,2.78,;-4.89,3.55,;-4.89,5.09,;-3.56,5.86,;-2.22,5.09,;-.89,5.86,;.44,5.09,;.44,3.55,;-.89,2.78,;-2.22,3.55,;-3.56,2.78,;-.89,7.4,;-1.89,8.57,;-3.39,8.93,;-3.51,10.46,;-2.09,11.05,;-1.09,9.88,;.41,9.52,;.53,7.99,;1.84,7.18,;1.86,5.64,;3.21,4.89,;4.53,5.67,;4.51,7.21,;3.17,7.97,)| Show InChI InChI=1S/C24H25N5O/c1-28(2)14-15-30-17-8-9-18-19(10-12-26-21(18)16-17)23-22-7-5-13-29(22)27-24(23)20-6-3-4-11-25-20/h3-4,6,8-12,16H,5,7,13-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | 29 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories
| Assay Description
The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... |
J Med Chem 51: 2302-2306 (2008)
Article DOI: 10.1021/jm701199p
BindingDB Entry DOI: 10.7270/Q2G15Z4K |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184483

(1-methyl-6-[2-(6-methylpyridin-2-yl)-5,6-dihydro-4...)Show InChI InChI=1S/C20H19N5/c1-13-5-3-6-16(22-13)20-19(17-7-4-10-25(17)23-20)14-8-9-15-18(11-14)24(2)12-21-15/h3,5-6,8-9,11-12H,4,7,10H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1 [4-503,T204D]
(Homo sapiens (Human)) | BDBM21505
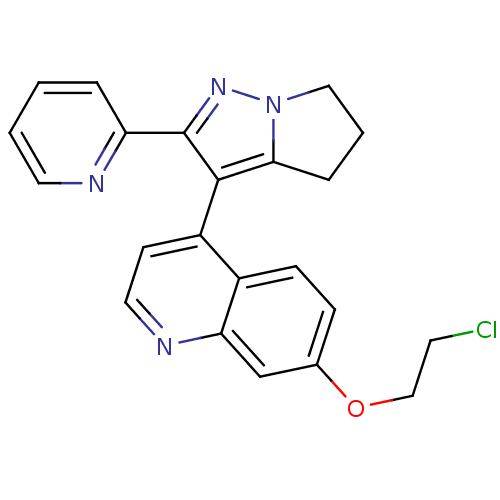
(7-(2-chloroethoxy)-4-[2-(pyridin-2-yl)-4H,5H,6H-py...)Show SMILES ClCCOc1ccc2c(ccnc2c1)-c1c2CCCn2nc1-c1ccccn1 |(-7.56,-1.07,;-7.56,.47,;-6.23,1.24,;-6.23,2.78,;-4.89,3.55,;-4.89,5.09,;-3.56,5.86,;-2.22,5.09,;-.89,5.86,;.44,5.09,;.44,3.55,;-.89,2.78,;-2.22,3.55,;-3.56,2.78,;-.89,7.4,;-1.89,8.57,;-3.39,8.93,;-3.51,10.46,;-2.09,11.05,;-1.09,9.88,;.41,9.52,;.53,7.99,;1.84,7.18,;1.86,5.64,;3.21,4.89,;4.53,5.67,;4.51,7.21,;3.17,7.97,)| Show InChI InChI=1S/C22H19ClN4O/c23-9-13-28-15-6-7-16-17(8-11-25-19(16)14-15)21-20-5-3-12-27(20)26-22(21)18-4-1-2-10-24-18/h1-2,4,6-8,10-11,14H,3,5,9,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | 66 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories
| Assay Description
The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... |
J Med Chem 51: 2302-2306 (2008)
Article DOI: 10.1021/jm701199p
BindingDB Entry DOI: 10.7270/Q2G15Z4K |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057401

(CHEMBL3322776)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(OC)c(Cl)c3)c3cccn-23)c1 Show InChI InChI=1S/C24H24ClN3O3/c1-30-17-6-7-20-19(15-17)26-24(22-4-3-11-28(20)22)9-12-27(13-10-24)23(29)16-5-8-21(31-2)18(25)14-16/h3-8,11,14-15,26H,9-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM21492
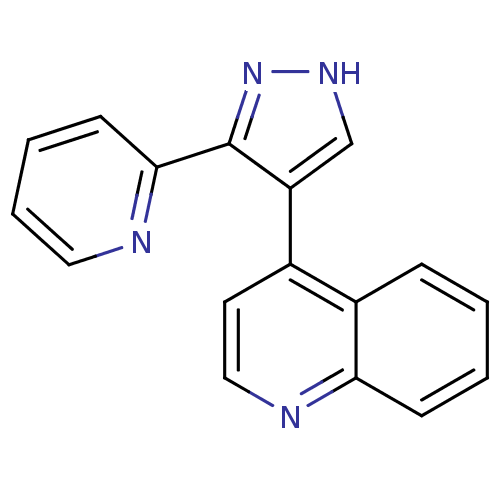
(4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...)Show InChI InChI=1S/C17H12N4/c1-2-6-15-13(5-1)12(8-10-19-15)14-11-20-21-17(14)16-7-3-4-9-18-16/h1-11H,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184489
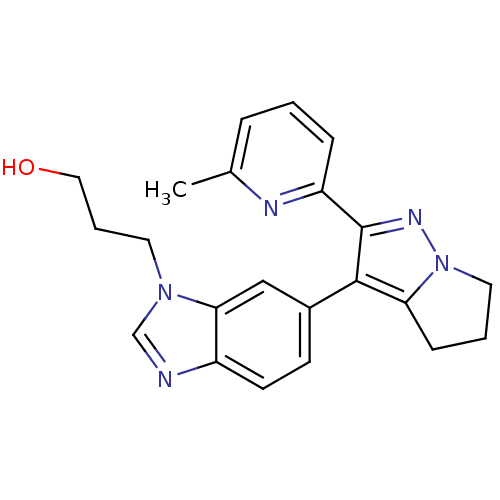
(3-(6-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrr...)Show SMILES Cc1cccc(n1)-c1nn2CCCc2c1-c1ccc2ncn(CCCO)c2c1 Show InChI InChI=1S/C22H23N5O/c1-15-5-2-6-18(24-15)22-21(19-7-3-11-27(19)25-22)16-8-9-17-20(13-16)26(14-23-17)10-4-12-28/h2,5-6,8-9,13-14,28H,3-4,7,10-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184488

(6-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrol...)Show InChI InChI=1S/C19H17N5/c1-12-4-2-5-15(22-12)19-18(17-6-3-9-24(17)23-19)13-7-8-14-16(10-13)21-11-20-14/h2,4-5,7-8,10-11H,3,6,9H2,1H3,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1 [4-503,T204D]
(Homo sapiens (Human)) | BDBM21510

(Dihydropyrrolopyrazole, 16a | dimethyl[5-({4-[2-(p...)Show SMILES CN(C)CCCCCOc1ccc2c(ccnc2c1)-c1c2CCCn2nc1-c1ccccn1 |(-4.89,-5.69,;-4.89,-4.15,;-3.56,-3.38,;-6.23,-3.38,;-6.23,-1.84,;-7.56,-1.07,;-7.56,.47,;-6.23,1.24,;-6.23,2.78,;-4.89,3.55,;-4.89,5.09,;-3.56,5.86,;-2.22,5.09,;-.89,5.86,;.44,5.09,;.44,3.55,;-.89,2.78,;-2.22,3.55,;-3.56,2.78,;-.89,7.4,;-1.89,8.57,;-3.39,8.93,;-3.51,10.46,;-2.09,11.05,;-1.09,9.88,;.41,9.52,;.53,7.99,;1.84,7.18,;1.86,5.64,;3.21,4.89,;4.53,5.67,;4.51,7.21,;3.17,7.97,)| Show InChI InChI=1S/C27H31N5O/c1-31(2)16-6-3-7-18-33-20-11-12-21-22(13-15-29-24(21)19-20)26-25-10-8-17-32(25)30-27(26)23-9-4-5-14-28-23/h4-5,9,11-15,19H,3,6-8,10,16-18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | 24 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories
| Assay Description
The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... |
J Med Chem 51: 2302-2306 (2008)
Article DOI: 10.1021/jm701199p
BindingDB Entry DOI: 10.7270/Q2G15Z4K |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057356
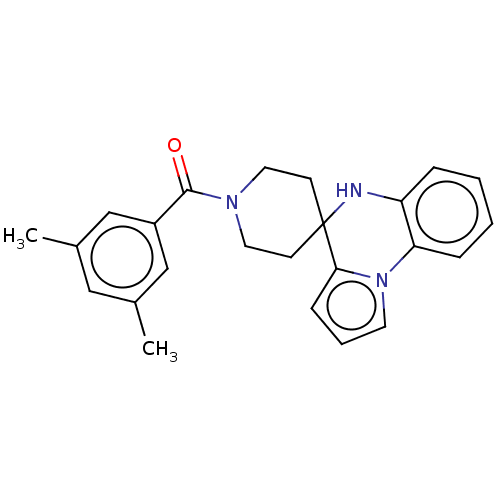
(CHEMBL3322666)Show SMILES Cc1cc(C)cc(c1)C(=O)N1CCC2(CC1)Nc1ccccc1-n1cccc21 Show InChI InChI=1S/C24H25N3O/c1-17-14-18(2)16-19(15-17)23(28)26-12-9-24(10-13-26)22-8-5-11-27(22)21-7-4-3-6-20(21)25-24/h3-8,11,14-16,25H,9-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057374

(CHEMBL3322759)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3cccc(Cl)c3)c3cccn-23)c1 Show InChI InChI=1S/C23H22ClN3O2/c1-29-18-7-8-20-19(15-18)25-23(21-6-3-11-27(20)21)9-12-26(13-10-23)22(28)16-4-2-5-17(24)14-16/h2-8,11,14-15,25H,9-10,12-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1 [4-503,T204D]
(Homo sapiens (Human)) | BDBM21511
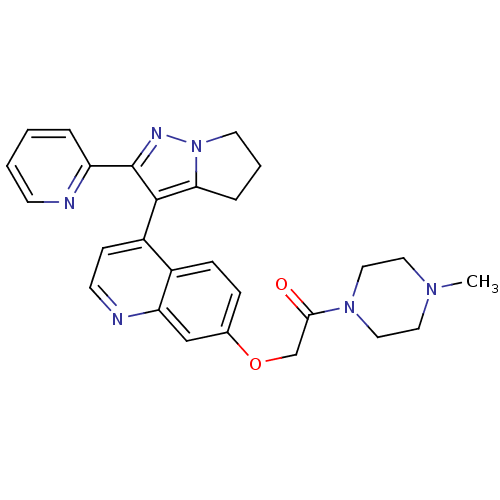
(1-(4-methylpiperazin-1-yl)-2-({4-[2-(pyridin-2-yl)...)Show SMILES CN1CCN(CC1)C(=O)COc1ccc2c(ccnc2c1)-c1c2CCCn2nc1-c1ccccn1 |(-7.56,-5.69,;-7.56,-4.15,;-8.89,-3.38,;-8.89,-1.84,;-7.56,-1.07,;-6.23,-1.84,;-6.23,-3.38,;-7.56,.47,;-8.89,1.24,;-6.23,1.24,;-6.23,2.78,;-4.89,3.55,;-4.89,5.09,;-3.56,5.86,;-2.22,5.09,;-.89,5.86,;.44,5.09,;.44,3.55,;-.89,2.78,;-2.22,3.55,;-3.56,2.78,;-.89,7.4,;-1.89,8.57,;-3.39,8.93,;-3.51,10.46,;-2.09,11.05,;-1.09,9.88,;.41,9.52,;.53,7.99,;1.84,7.18,;1.86,5.64,;3.21,4.89,;4.53,5.67,;4.51,7.21,;3.17,7.97,)| Show InChI InChI=1S/C27H28N6O2/c1-31-13-15-32(16-14-31)25(34)18-35-19-7-8-20-21(9-11-29-23(20)17-19)26-24-6-4-12-33(24)30-27(26)22-5-2-3-10-28-22/h2-3,5,7-11,17H,4,6,12-16,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | 260 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories
| Assay Description
The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... |
J Med Chem 51: 2302-2306 (2008)
Article DOI: 10.1021/jm701199p
BindingDB Entry DOI: 10.7270/Q2G15Z4K |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184494
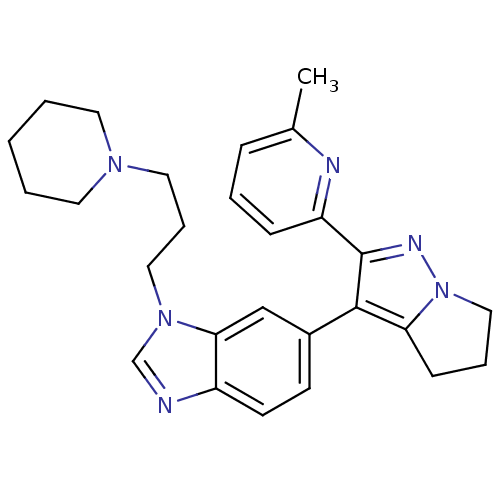
(6-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrol...)Show SMILES Cc1cccc(n1)-c1nn2CCCc2c1-c1ccc2ncn(CCCN3CCCCC3)c2c1 Show InChI InChI=1S/C27H32N6/c1-20-8-5-9-23(29-20)27-26(24-10-6-17-33(24)30-27)21-11-12-22-25(18-21)32(19-28-22)16-7-15-31-13-3-2-4-14-31/h5,8-9,11-12,18-19H,2-4,6-7,10,13-17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184496

(CHEMBL441176 | dimethyl-(3-{6-[2-(6-methyl-pyridin...)Show SMILES CN(C)CCCn1cnc2ccc(cc12)-c1c2CCCn2nc1-c1cccc(C)n1 Show InChI InChI=1S/C24H28N6/c1-17-7-4-8-20(26-17)24-23(21-9-5-14-30(21)27-24)18-10-11-19-22(15-18)29(16-25-19)13-6-12-28(2)3/h4,7-8,10-11,15-16H,5-6,9,12-14H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057394
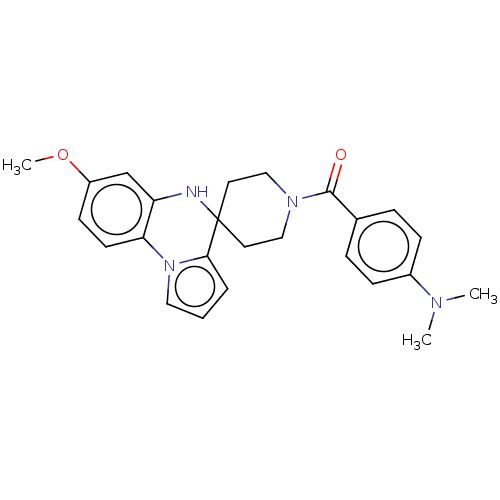
(CHEMBL3322773)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(cc3)N(C)C)c3cccn-23)c1 Show InChI InChI=1S/C25H28N4O2/c1-27(2)19-8-6-18(7-9-19)24(30)28-15-12-25(13-16-28)23-5-4-14-29(23)22-11-10-20(31-3)17-21(22)26-25/h4-11,14,17,26H,12-13,15-16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM21492

(4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...)Show InChI InChI=1S/C17H12N4/c1-2-6-15-13(5-1)12(8-10-19-15)14-11-20-21-17(14)16-7-3-4-9-18-16/h1-11H,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1 [4-503,T204D]
(Homo sapiens (Human)) | BDBM21492

(4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...)Show InChI InChI=1S/C17H12N4/c1-2-6-15-13(5-1)12(8-10-19-15)14-11-20-21-17(14)16-7-3-4-9-18-16/h1-11H,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 59 | n/a | 40 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories
| Assay Description
The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... |
J Med Chem 51: 2302-2306 (2008)
Article DOI: 10.1021/jm701199p
BindingDB Entry DOI: 10.7270/Q2G15Z4K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50184487
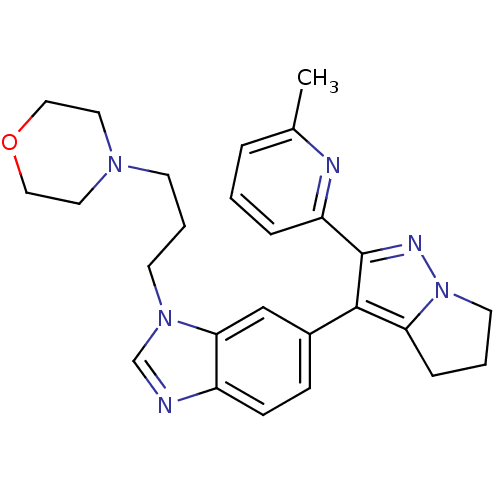
(4-(3-(6-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-p...)Show SMILES Cc1cccc(n1)-c1nn2CCCc2c1-c1ccc2ncn(CCCN3CCOCC3)c2c1 Show InChI InChI=1S/C26H30N6O/c1-19-5-2-6-22(28-19)26-25(23-7-3-12-32(23)29-26)20-8-9-21-24(17-20)31(18-27-21)11-4-10-30-13-15-33-16-14-30/h2,5-6,8-9,17-18H,3-4,7,10-16H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1 [4-503,T204D]
(Homo sapiens (Human)) | BDBM21509
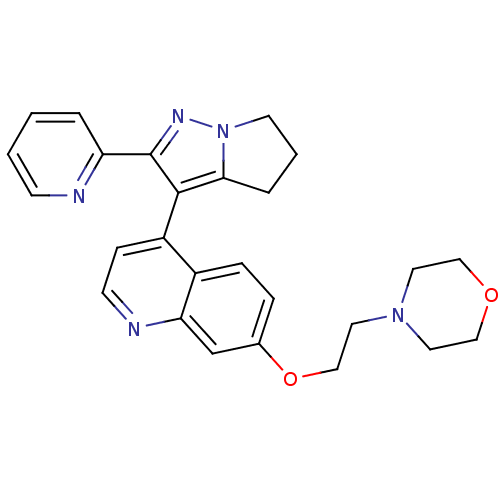
(4-[2-({4-[2-(pyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-a]...)Show SMILES C(CN1CCOCC1)Oc1ccc2c(ccnc2c1)-c1c2CCCn2nc1-c1ccccn1 |(-6.23,1.24,;-7.56,.47,;-7.56,-1.07,;-8.89,-1.84,;-8.89,-3.38,;-7.56,-4.15,;-6.23,-3.38,;-6.23,-1.84,;-6.23,2.78,;-4.89,3.55,;-4.89,5.09,;-3.56,5.86,;-2.22,5.09,;-.89,5.86,;.44,5.09,;.44,3.55,;-.89,2.78,;-2.22,3.55,;-3.56,2.78,;-.89,7.4,;-1.89,8.57,;-3.39,8.93,;-3.51,10.46,;-2.09,11.05,;-1.09,9.88,;.41,9.52,;.53,7.99,;1.84,7.18,;1.86,5.64,;3.21,4.89,;4.53,5.67,;4.51,7.21,;3.17,7.97,)| Show InChI InChI=1S/C26H27N5O2/c1-2-9-27-22(4-1)26-25(24-5-3-11-31(24)29-26)21-8-10-28-23-18-19(6-7-20(21)23)33-17-14-30-12-15-32-16-13-30/h1-2,4,6-10,18H,3,5,11-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | 180 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories
| Assay Description
The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... |
J Med Chem 51: 2302-2306 (2008)
Article DOI: 10.1021/jm701199p
BindingDB Entry DOI: 10.7270/Q2G15Z4K |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1 [4-503,T204D]
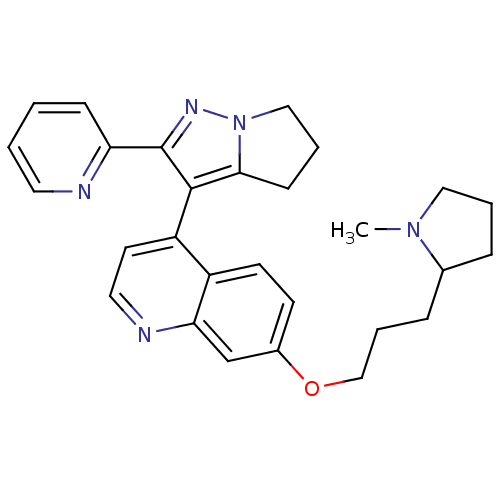
(Homo sapiens (Human)) | BDBM21513
(7-[3-(1-methylpyrrolidin-2-yl)propoxy]-4-[2-(pyrid...)Show SMILES CN1CCCC1CCCOc1ccc2c(ccnc2c1)-c1c2CCCn2nc1-c1ccccn1 |(-11.3,-.52,;-10.43,-1.79,;-10.96,-3.24,;-9.74,-4.19,;-8.46,-3.32,;-8.89,-1.84,;-7.56,-1.07,;-7.56,.47,;-6.23,1.24,;-6.23,2.78,;-4.89,3.55,;-4.89,5.09,;-3.56,5.86,;-2.22,5.09,;-.89,5.86,;.44,5.09,;.44,3.55,;-.89,2.78,;-2.22,3.55,;-3.56,2.78,;-.89,7.4,;-1.89,8.57,;-3.39,8.93,;-3.51,10.46,;-2.09,11.05,;-1.09,9.88,;.41,9.52,;.53,7.99,;1.84,7.18,;1.86,5.64,;3.21,4.89,;4.53,5.67,;4.51,7.21,;3.17,7.97,)| Show InChI InChI=1S/C28H31N5O/c1-32-16-4-7-20(32)8-6-18-34-21-11-12-22-23(13-15-30-25(22)19-21)27-26-10-5-17-33(26)31-28(27)24-9-2-3-14-29-24/h2-3,9,11-15,19-20H,4-8,10,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | 17 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories
| Assay Description
The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... |
J Med Chem 51: 2302-2306 (2008)
Article DOI: 10.1021/jm701199p
BindingDB Entry DOI: 10.7270/Q2G15Z4K |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
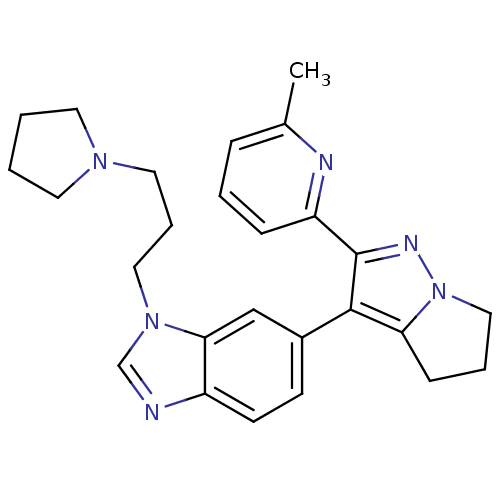
(Homo sapiens (Human)) | BDBM50184497
(6-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrol...)Show SMILES Cc1cccc(n1)-c1nn2CCCc2c1-c1ccc2ncn(CCCN3CCCC3)c2c1 Show InChI InChI=1S/C26H30N6/c1-19-7-4-8-22(28-19)26-25(23-9-5-16-32(23)29-26)20-10-11-21-24(17-20)31(18-27-21)15-6-14-30-12-2-3-13-30/h4,7-8,10-11,17-18H,2-3,5-6,9,12-16H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells |
J Med Chem 49: 2138-42 (2006)
Article DOI: 10.1021/jm058209g
BindingDB Entry DOI: 10.7270/Q2V40TS0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data