 Found 118 hits with Last Name = 'henry' and Initial = 'e'
Found 118 hits with Last Name = 'henry' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013689
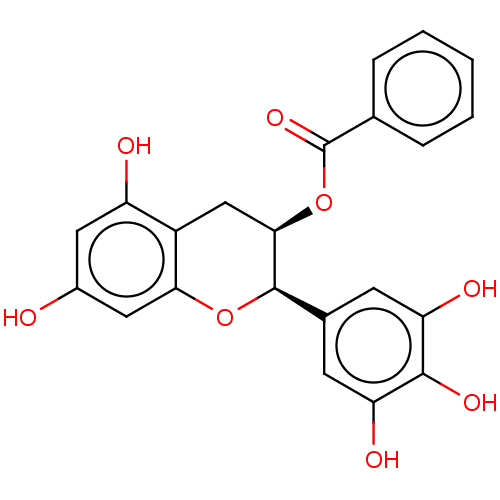
(CHEMBL3264525)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3ccccc3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O8/c23-13-8-15(24)14-10-19(30-22(28)11-4-2-1-3-5-11)21(29-18(14)9-13)12-6-16(25)20(27)17(26)7-12/h1-9,19,21,23-27H,10H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013693
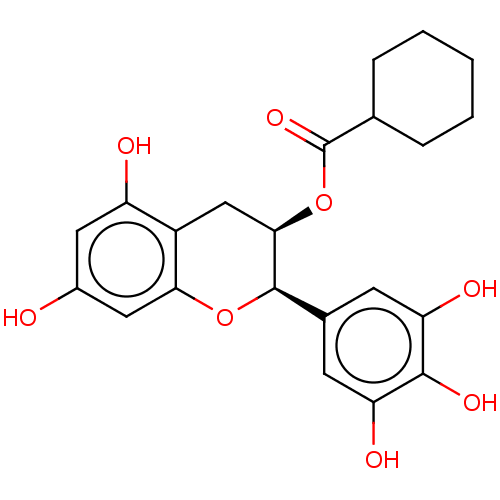
(CHEMBL3264529)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)C3CCCCC3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H24O8/c23-13-8-15(24)14-10-19(30-22(28)11-4-2-1-3-5-11)21(29-18(14)9-13)12-6-16(25)20(27)17(26)7-12/h6-9,11,19,21,23-27H,1-5,10H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50236531
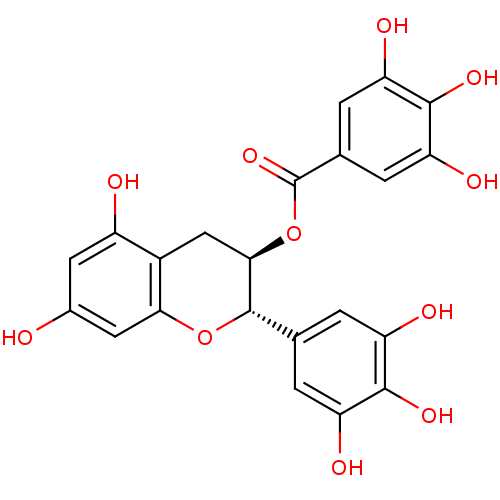
((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013710

(CHEMBL1923070)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1Cc2ccccc2O[C@@H]1c1ccccc1 |r| Show InChI InChI=1S/C22H18O6/c23-16-10-15(11-17(24)20(16)25)22(26)28-19-12-14-8-4-5-9-18(14)27-21(19)13-6-2-1-3-7-13/h1-11,19,21,23-25H,12H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013690
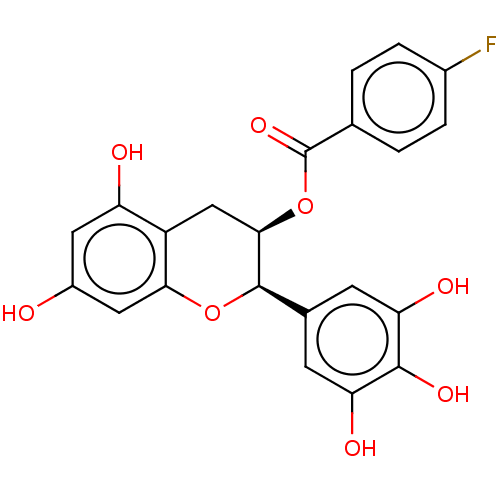
(CHEMBL3264526)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3ccc(F)cc3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H17FO8/c23-12-3-1-10(2-4-12)22(29)31-19-9-14-15(25)7-13(24)8-18(14)30-21(19)11-5-16(26)20(28)17(27)6-11/h1-8,19,21,24-28H,9H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013695
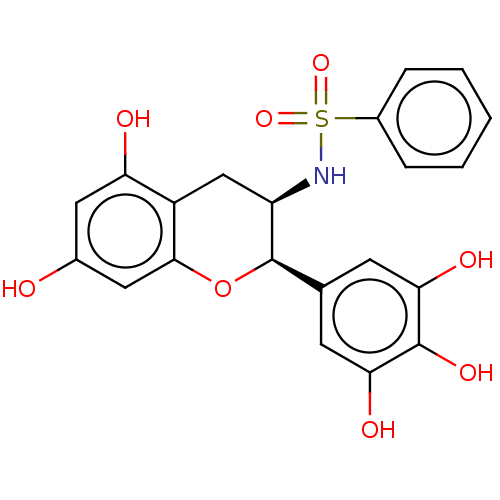
(CHEMBL3264531)Show SMILES Oc1cc(O)c2C[C@@H](NS(=O)(=O)c3ccccc3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C21H19NO8S/c23-12-8-16(24)14-10-15(22-31(28,29)13-4-2-1-3-5-13)21(30-19(14)9-12)11-6-17(25)20(27)18(26)7-11/h1-9,15,21-27H,10H2/t15-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013691
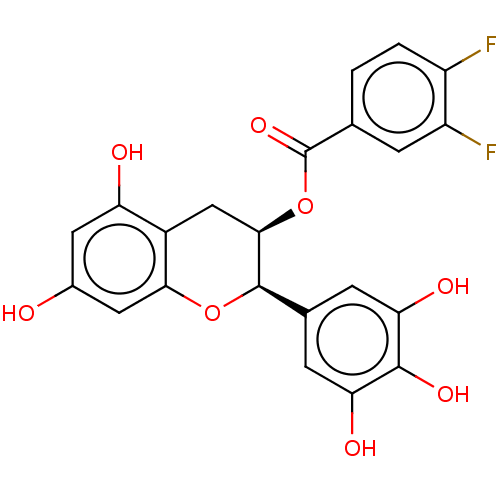
(CHEMBL3264527)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3ccc(F)c(F)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H16F2O8/c23-13-2-1-9(3-14(13)24)22(30)32-19-8-12-15(26)6-11(25)7-18(12)31-21(19)10-4-16(27)20(29)17(28)5-10/h1-7,19,21,25-29H,8H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013709
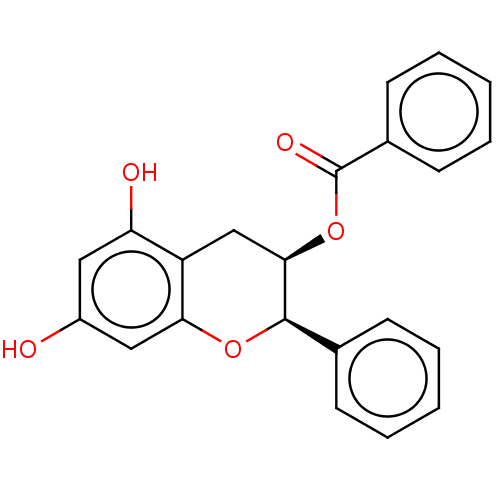
(CHEMBL3264541)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3ccccc3)[C@H](Oc2c1)c1ccccc1 |r| Show InChI InChI=1S/C22H18O5/c23-16-11-18(24)17-13-20(27-22(25)15-9-5-2-6-10-15)21(26-19(17)12-16)14-7-3-1-4-8-14/h1-12,20-21,23-24H,13H2/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013692
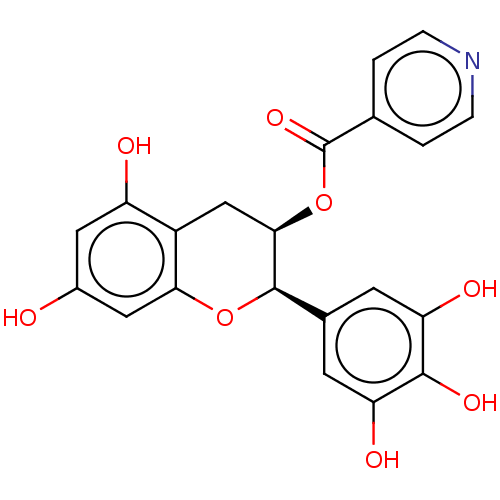
(CHEMBL3264528)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3ccncc3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C21H17NO8/c23-12-7-14(24)13-9-18(30-21(28)10-1-3-22-4-2-10)20(29-17(13)8-12)11-5-15(25)19(27)16(26)6-11/h1-8,18,20,23-27H,9H2/t18-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013700
(CHEMBL3264535)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)[C@@H]3CC[C@H](F)CC3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r,wU:11.10,14.14,wD:18.24,7.7,(-.5,-47.41,;.84,-48.18,;.84,-49.73,;2.17,-50.5,;2.17,-52.04,;3.5,-49.73,;4.84,-50.51,;6.19,-49.73,;7.52,-50.51,;7.51,-52.05,;6.18,-52.81,;8.84,-52.82,;8.83,-54.36,;10.16,-55.13,;11.5,-54.37,;12.83,-55.14,;11.5,-52.82,;10.17,-52.05,;6.19,-48.18,;4.84,-47.4,;3.5,-48.18,;2.17,-47.41,;7.52,-47.41,;8.85,-48.19,;10.19,-47.43,;10.19,-45.89,;11.53,-45.12,;8.85,-45.11,;8.85,-43.57,;7.52,-45.88,)| Show InChI InChI=1S/C22H23FO7/c23-13-4-1-11(2-5-13)22(28)30-20-10-15-17(26)8-14(24)9-19(15)29-21(20)12-3-6-16(25)18(27)7-12/h3,6-9,11,13,20-21,24-27H,1-2,4-5,10H2/t11-,13+,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013701
(CHEMBL3264536)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)[C@H]3CC[C@H](F)CC3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r,wU:11.10,wD:18.24,7.7,14.14,(11.85,-47.98,;13.19,-48.75,;13.19,-50.29,;14.52,-51.07,;14.52,-52.61,;15.85,-50.3,;17.19,-51.07,;18.54,-50.3,;19.87,-51.08,;19.86,-52.62,;18.53,-53.38,;21.2,-53.39,;22.52,-52.62,;23.85,-53.39,;23.85,-54.94,;25.18,-55.71,;22.51,-55.7,;21.18,-54.93,;18.54,-48.75,;17.19,-47.96,;15.85,-48.74,;14.52,-47.98,;19.87,-47.98,;21.2,-48.76,;22.54,-48,;22.54,-46.45,;23.88,-45.69,;21.2,-45.68,;21.2,-44.14,;19.87,-46.45,)| Show InChI InChI=1S/C22H23FO7/c23-13-4-1-11(2-5-13)22(28)30-20-10-15-17(26)8-14(24)9-19(15)29-21(20)12-3-6-16(25)18(27)7-12/h3,6-9,11,13,20-21,24-27H,1-2,4-5,10H2/t11-,13-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013694
(CHEMBL3264530)Show SMILES Oc1cc(O)c2C[C@@H](NC(=O)c3ccccc3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H19NO7/c24-13-8-16(25)14-10-15(23-22(29)11-4-2-1-3-5-11)21(30-19(14)9-13)12-6-17(26)20(28)18(27)7-12/h1-9,15,21,24-28H,10H2,(H,23,29)/t15-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013704
(CHEMBL3264537)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)C3CCCCC3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C22H24O7/c23-14-9-17(25)15-11-20(29-22(27)12-4-2-1-3-5-12)21(28-19(15)10-14)13-6-7-16(24)18(26)8-13/h6-10,12,20-21,23-26H,1-5,11H2/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013696
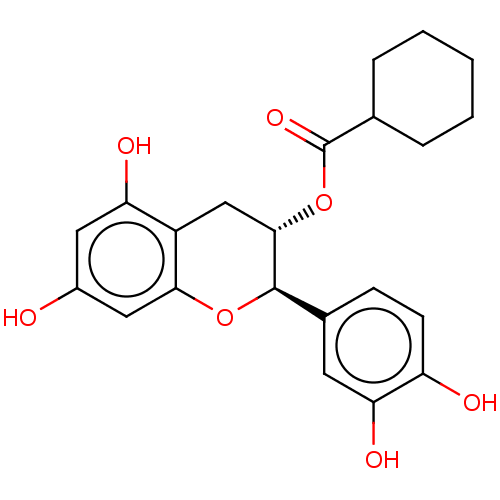
(CHEMBL3264532)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)C3CCCCC3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C22H24O7/c23-14-9-17(25)15-11-20(29-22(27)12-4-2-1-3-5-12)21(28-19(15)10-14)13-6-7-16(24)18(26)8-13/h6-10,12,20-21,23-26H,1-5,11H2/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503625

(CHEMBL4442228)Show InChI InChI=1S/C20H28O4/c1-6-8-11-24-20(22)10-9-18(21)17-13-16(14(3)4)19(23-7-2)12-15(17)5/h6,8,12-14H,7,9-11H2,1-5H3/b8-6+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013697
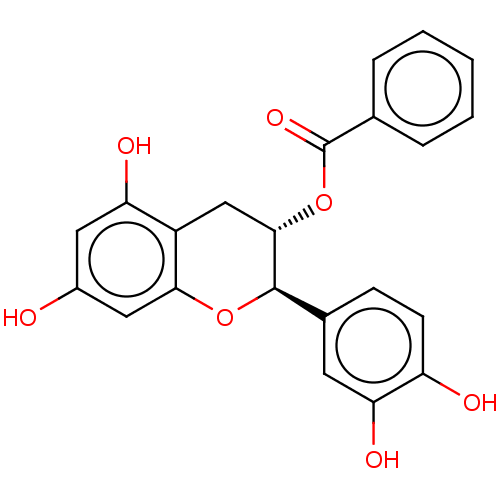
(CHEMBL3264533)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3ccccc3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O7/c23-14-9-17(25)15-11-20(29-22(27)12-4-2-1-3-5-12)21(28-19(15)10-14)13-6-7-16(24)18(26)8-13/h1-10,20-21,23-26H,11H2/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013713
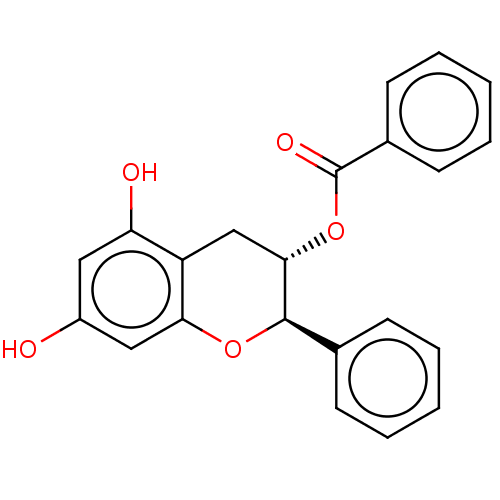
(CHEMBL3264542)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3ccccc3)[C@H](Oc2c1)c1ccccc1 |r| Show InChI InChI=1S/C22H18O5/c23-16-11-18(24)17-13-20(27-22(25)15-9-5-2-6-10-15)21(26-19(17)12-16)14-7-3-1-4-8-14/h1-12,20-21,23-24H,13H2/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503630

(CHEMBL4465955)Show InChI InChI=1S/C19H26O4/c1-6-10-23-19(21)9-8-17(20)16-12-15(13(3)4)18(22-7-2)11-14(16)5/h6,11-13H,1,7-10H2,2-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503622
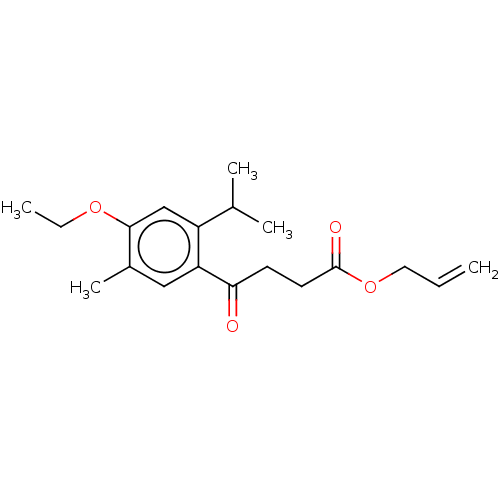
(CHEMBL4514018)Show InChI InChI=1S/C19H26O4/c1-6-10-23-19(21)9-8-17(20)16-11-14(5)18(22-7-2)12-15(16)13(3)4/h6,11-13H,1,7-10H2,2-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013714
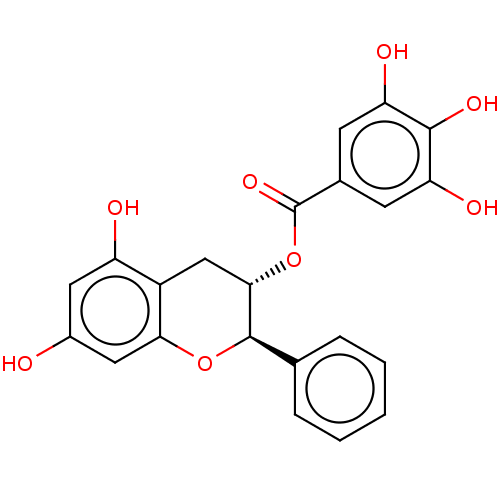
(CHEMBL3264543)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1ccccc1 |r| Show InChI InChI=1S/C22H18O8/c23-13-8-15(24)14-10-19(21(29-18(14)9-13)11-4-2-1-3-5-11)30-22(28)12-6-16(25)20(27)17(26)7-12/h1-9,19,21,23-27H,10H2/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503620
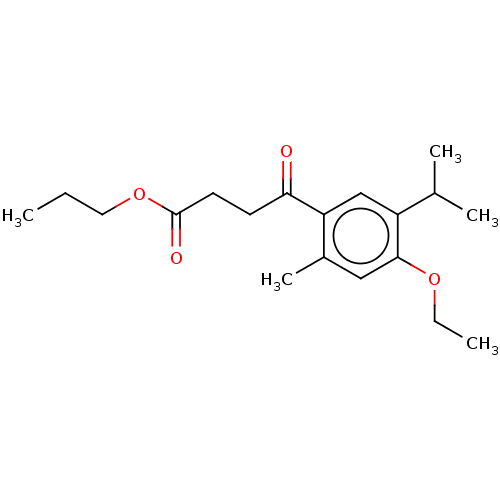
(CHEMBL4530299)Show InChI InChI=1S/C19H28O4/c1-6-10-23-19(21)9-8-17(20)16-12-15(13(3)4)18(22-7-2)11-14(16)5/h11-13H,6-10H2,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503617

(CHEMBL4482921)Show InChI InChI=1S/C20H30O4/c1-6-8-11-24-20(22)10-9-18(21)17-12-15(5)19(23-7-2)13-16(17)14(3)4/h12-14H,6-11H2,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503627
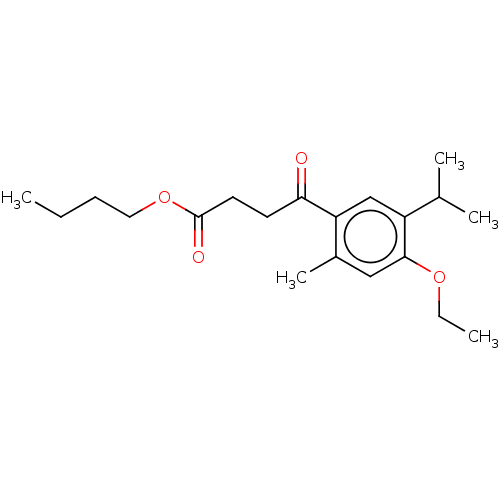
(CHEMBL4462546)Show InChI InChI=1S/C20H30O4/c1-6-8-11-24-20(22)10-9-18(21)17-13-16(14(3)4)19(23-7-2)12-15(17)5/h12-14H,6-11H2,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503629
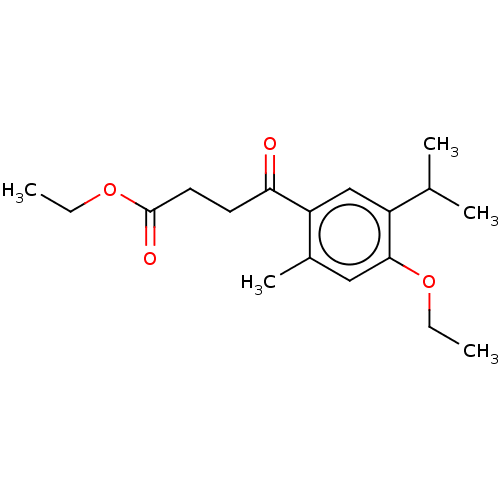
(CHEMBL4553753)Show InChI InChI=1S/C18H26O4/c1-6-21-17-10-13(5)15(11-14(17)12(3)4)16(19)8-9-18(20)22-7-2/h10-12H,6-9H2,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503628
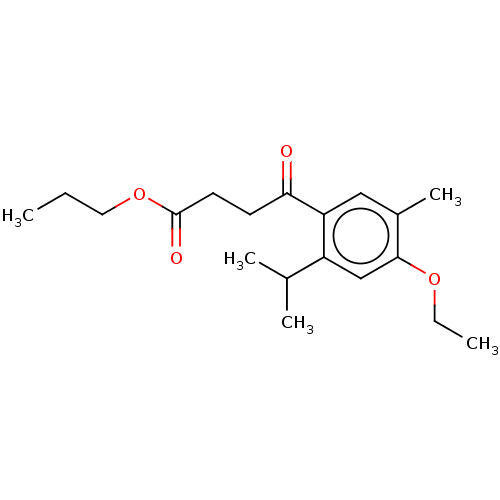
(CHEMBL4460162)Show InChI InChI=1S/C19H28O4/c1-6-10-23-19(21)9-8-17(20)16-11-14(5)18(22-7-2)12-15(16)13(3)4/h11-13H,6-10H2,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503624
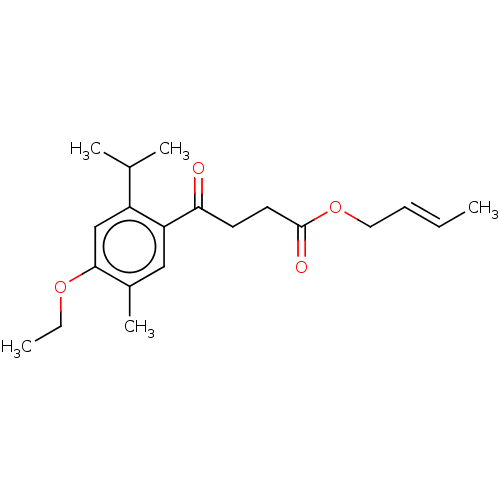
(CHEMBL4561682)Show InChI InChI=1S/C20H28O4/c1-6-8-11-24-20(22)10-9-18(21)17-12-15(5)19(23-7-2)13-16(17)14(3)4/h6,8,12-14H,7,9-11H2,1-5H3/b8-6+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013699
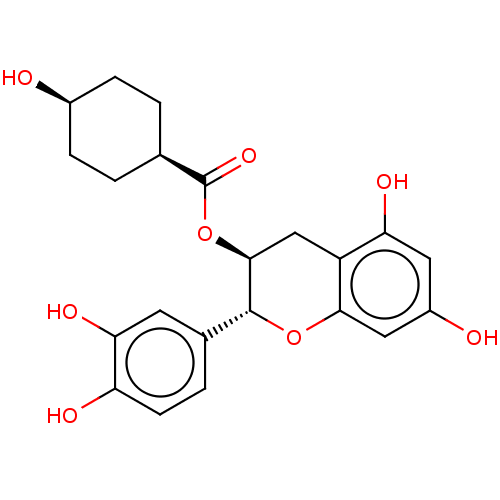
(CHEMBL3264534)Show SMILES O[C@H]1CC[C@H](CC1)C(=O)O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)c(O)c1 |r,wU:10.10,4.7,1.0,wD:21.24,(60.47,-41.94,;59.14,-41.17,;57.8,-41.94,;56.47,-41.16,;56.48,-39.62,;57.81,-38.85,;59.14,-39.62,;55.15,-38.85,;53.82,-39.62,;55.16,-37.31,;53.82,-36.54,;52.48,-37.31,;51.14,-36.53,;49.81,-37.3,;49.81,-38.84,;48.47,-36.53,;48.48,-34.98,;47.14,-34.21,;49.8,-34.21,;51.14,-34.98,;52.48,-34.2,;53.83,-34.98,;55.16,-34.21,;56.49,-35,;57.83,-34.23,;57.83,-32.69,;59.17,-31.92,;56.49,-31.92,;56.49,-30.38,;55.16,-32.69,)| Show InChI InChI=1S/C22H24O8/c23-13-4-1-11(2-5-13)22(28)30-20-10-15-17(26)8-14(24)9-19(15)29-21(20)12-3-6-16(25)18(27)7-12/h3,6-9,11,13,20-21,23-27H,1-2,4-5,10H2/t11-,13+,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013705
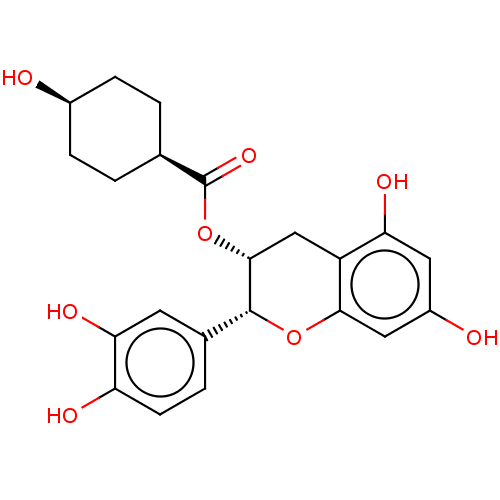
(CHEMBL3264538)Show SMILES O[C@H]1CC[C@H](CC1)C(=O)O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)c(O)c1 |r,wU:4.7,1.0,wD:21.24,10.10,(52.18,-55.64,;50.85,-54.87,;49.51,-55.64,;48.18,-54.86,;48.19,-53.33,;49.52,-52.56,;50.85,-53.32,;46.86,-52.55,;45.52,-53.32,;46.86,-51.01,;45.53,-50.24,;44.18,-51.01,;42.85,-50.23,;41.52,-51,;41.52,-52.54,;40.18,-50.23,;40.18,-48.69,;38.85,-47.92,;41.51,-47.92,;42.85,-48.68,;44.19,-47.9,;45.53,-48.68,;46.87,-47.92,;48.2,-48.7,;49.53,-47.93,;49.54,-46.39,;50.87,-45.62,;48.2,-45.62,;48.2,-44.08,;46.87,-46.39,)| Show InChI InChI=1S/C22H24O8/c23-13-4-1-11(2-5-13)22(28)30-20-10-15-17(26)8-14(24)9-19(15)29-21(20)12-3-6-16(25)18(27)7-12/h3,6-9,11,13,20-21,23-27H,1-2,4-5,10H2/t11-,13+,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013708
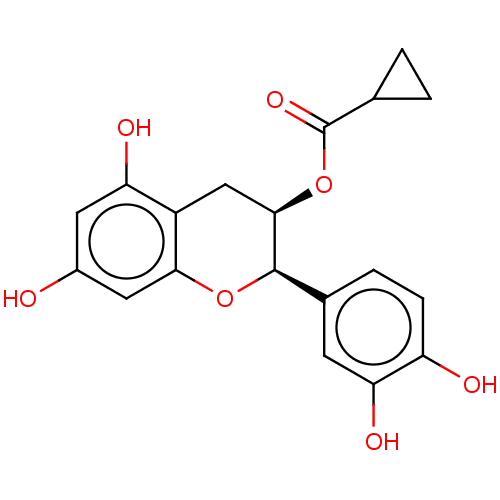
(CHEMBL3264540)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)C3CC3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C19H18O7/c20-11-6-14(22)12-8-17(26-19(24)9-1-2-9)18(25-16(12)7-11)10-3-4-13(21)15(23)5-10/h3-7,9,17-18,20-23H,1-2,8H2/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50013707
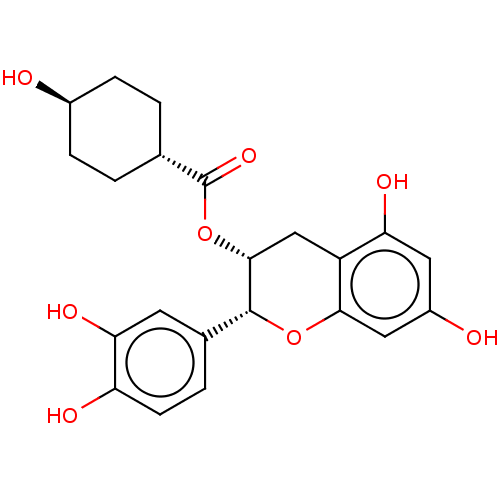
(CHEMBL3264539)Show SMILES O[C@H]1CC[C@@H](CC1)C(=O)O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)c(O)c1 |r,wU:4.7,wD:21.24,10.10,1.0,(67.59,-55.41,;66.26,-54.64,;66.25,-53.09,;64.92,-52.32,;63.6,-53.09,;63.59,-54.63,;64.92,-55.41,;62.27,-52.32,;60.93,-53.09,;62.27,-50.78,;60.94,-50.01,;59.59,-50.78,;58.25,-50,;56.92,-50.77,;56.92,-52.31,;55.59,-50,;55.59,-48.45,;54.26,-47.68,;56.92,-47.68,;58.26,-48.45,;59.6,-47.67,;60.94,-48.45,;62.28,-47.68,;63.61,-48.47,;64.94,-47.7,;64.95,-46.16,;66.28,-45.39,;63.61,-45.39,;63.6,-43.85,;62.28,-46.16,)| Show InChI InChI=1S/C22H24O8/c23-13-4-1-11(2-5-13)22(28)30-20-10-15-17(26)8-14(24)9-19(15)29-21(20)12-3-6-16(25)18(27)7-12/h3,6-9,11,13,20-21,23-27H,1-2,4-5,10H2/t11-,13-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503619
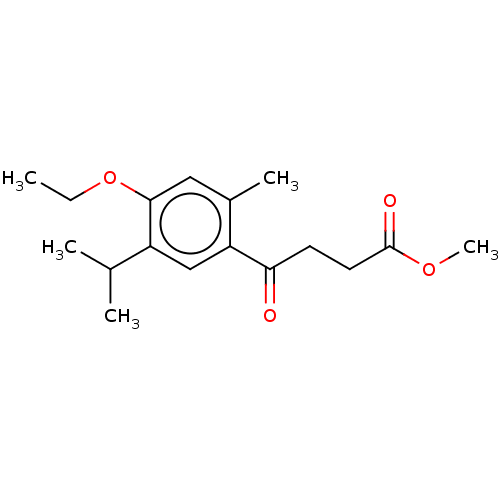
(CHEMBL4476244)Show InChI InChI=1S/C17H24O4/c1-6-21-16-9-12(4)14(10-13(16)11(2)3)15(18)7-8-17(19)20-5/h9-11H,6-8H2,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503621

(CHEMBL4467127)Show SMILES CCOc1cc(C)c(cc1C(C)C)C(=O)CCC(=O)OCc1ccccc1 Show InChI InChI=1S/C23H28O4/c1-5-26-22-13-17(4)20(14-19(22)16(2)3)21(24)11-12-23(25)27-15-18-9-7-6-8-10-18/h6-10,13-14,16H,5,11-12,15H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503623
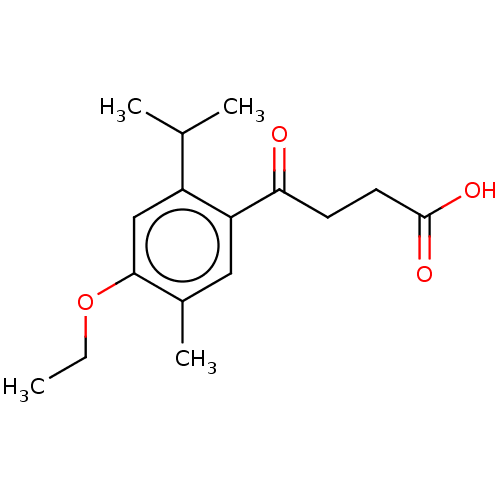
(CHEMBL4578501)Show InChI InChI=1S/C16H22O4/c1-5-20-15-9-12(10(2)3)13(8-11(15)4)14(17)6-7-16(18)19/h8-10H,5-7H2,1-4H3,(H,18,19) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503626
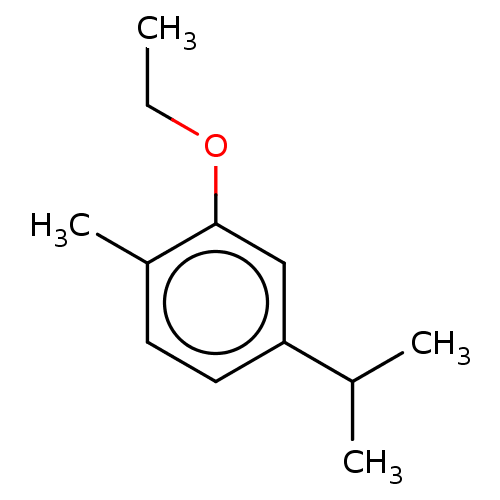
(CHEMBL4439425)Show InChI InChI=1S/C12H18O/c1-5-13-12-8-11(9(2)3)7-6-10(12)4/h6-9H,5H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50240433
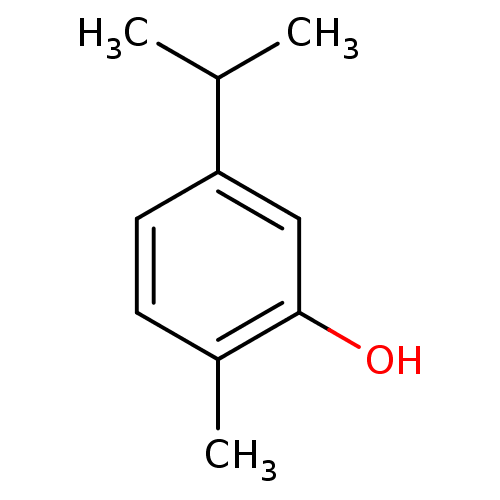
(5-Isopropyl-2-methyl-phenol | CARVACROL | CHEMBL28...)Show InChI InChI=1S/C10H14O/c1-7(2)9-5-4-8(3)10(11)6-9/h4-7,11H,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503618

(CHEMBL4438192)Show SMILES CCOc1cc(C(C)C)c(cc1C)C(=O)CCC(=O)OCc1ccccc1 Show InChI InChI=1S/C23H28O4/c1-5-26-22-14-19(16(2)3)20(13-17(22)4)21(24)11-12-23(25)27-15-18-9-7-6-8-10-18/h6-10,13-14,16H,5,11-12,15H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503613
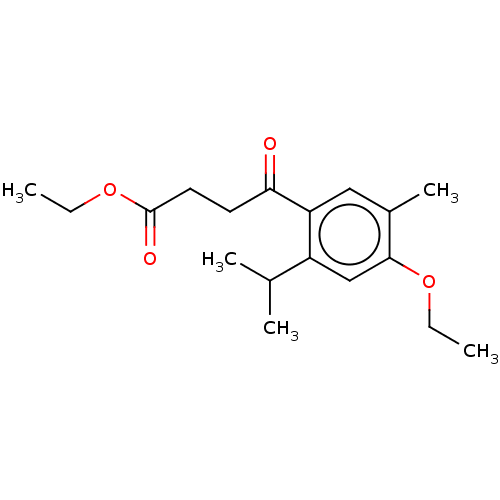
(CHEMBL4562120)Show InChI InChI=1S/C18H26O4/c1-6-21-17-11-14(12(3)4)15(10-13(17)5)16(19)8-9-18(20)22-7-2/h10-12H,6-9H2,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503616

(CHEMBL4450547)Show InChI InChI=1S/C17H24O4/c1-6-21-16-10-13(11(2)3)14(9-12(16)4)15(18)7-8-17(19)20-5/h9-11H,6-8H2,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50240432

(1-hydroxy-5-methyl-2-isopropylbenzene | 2-isopropy...)Show InChI InChI=1S/C10H14O/c1-7(2)9-5-4-8(3)6-10(9)11/h4-7,11H,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503614

(CHEMBL4452910)Show InChI InChI=1S/C12H18O/c1-5-13-12-8-10(4)6-7-11(12)9(2)3/h6-9H,5H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50503615
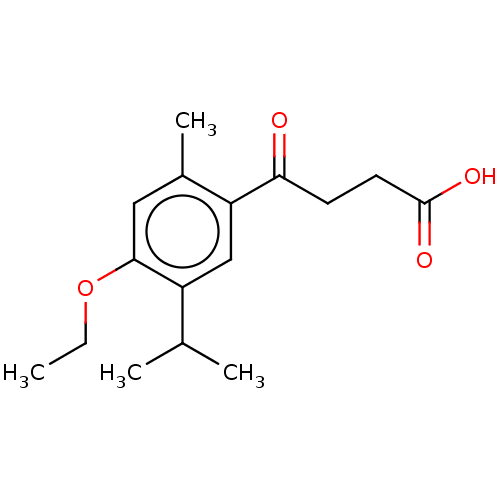
(CHEMBL4573816)Show InChI InChI=1S/C16H22O4/c1-5-20-15-8-11(4)13(9-12(15)10(2)3)14(17)6-7-16(18)19/h8-10H,5-7H2,1-4H3,(H,18,19) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Susquehanna University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate measured after 20 mins by spectrophotometric method |
Bioorg Med Chem Lett 29: 56-58 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.013
BindingDB Entry DOI: 10.7270/Q2CC13Z0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50044099
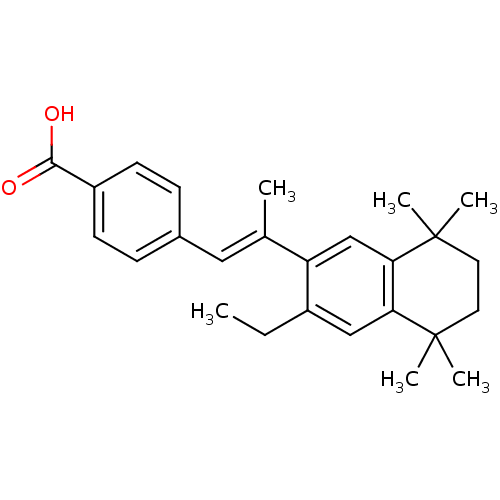
(4-[(E)-2-(3-Ethyl-5,5,8,8-tetramethyl-5,6,7,8-tetr...)Show SMILES CCc1cc2c(cc1\C(C)=C\c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H32O2/c1-7-19-15-22-23(26(5,6)13-12-25(22,3)4)16-21(19)17(2)14-18-8-10-20(11-9-18)24(27)28/h8-11,14-16H,7,12-13H2,1-6H3,(H,27,28)/b17-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RXR alpha receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RXR alpha receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50044099

(4-[(E)-2-(3-Ethyl-5,5,8,8-tetramethyl-5,6,7,8-tetr...)Show SMILES CCc1cc2c(cc1\C(C)=C\c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H32O2/c1-7-19-15-22-23(26(5,6)13-12-25(22,3)4)16-21(19)17(2)14-18-8-10-20(11-9-18)24(27)28/h8-11,14-16H,7,12-13H2,1-6H3,(H,27,28)/b17-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 961 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR beta receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50045276
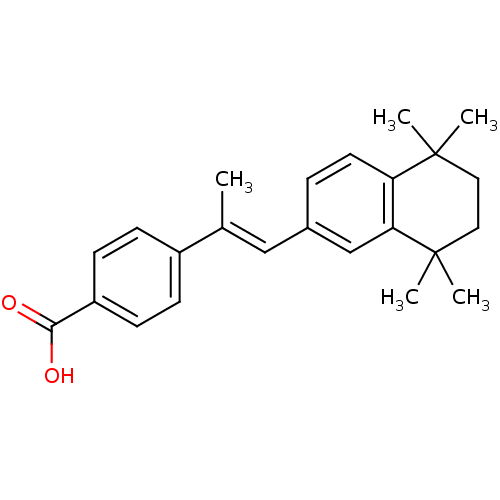
(4-[(E)-1-Methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tet...)Show SMILES C\C(=C/c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H28O2/c1-16(18-7-9-19(10-8-18)22(25)26)14-17-6-11-20-21(15-17)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR gamma receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
| n/a | n/a | n/a | n/a | 102 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR alpha receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50032219
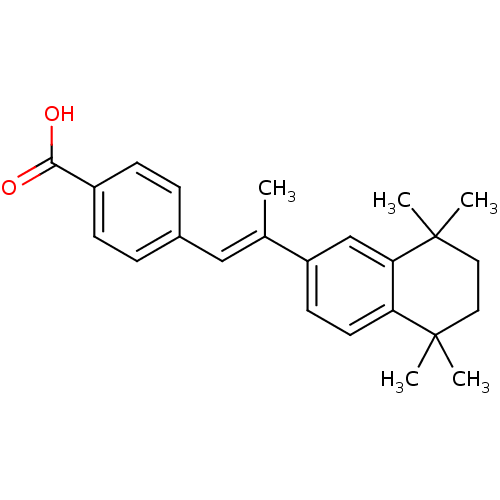
((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR beta receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50282692

(4-[(E)-2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(\C=C\c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C23H26O2/c1-22(2)13-14-23(3,4)20-15-17(9-12-19(20)22)6-5-16-7-10-18(11-8-16)21(24)25/h5-12,15H,13-14H2,1-4H3,(H,24,25)/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR beta receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data