

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050933 ((S)-2-((S)-2-{(S)-2-[3-(4-Chloro-phenyl)-ureido]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050929 (2-((S)-2-{(S)-2-[3-(4-Methoxy-phenyl)-ureido]-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050935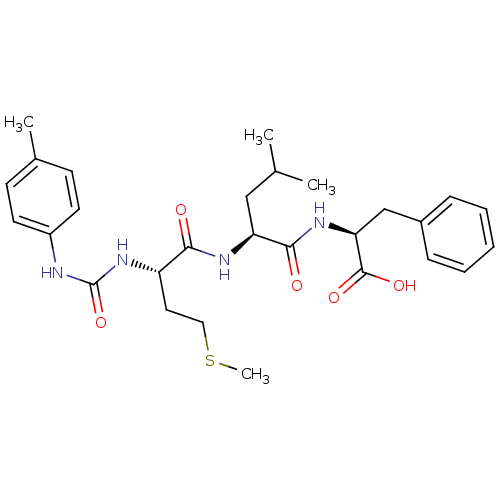 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050945 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Adamantan-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050928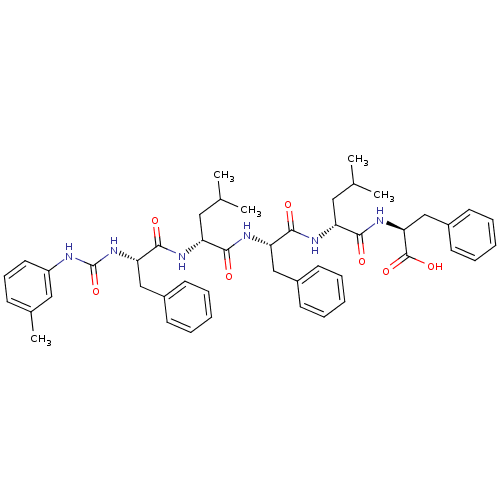 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937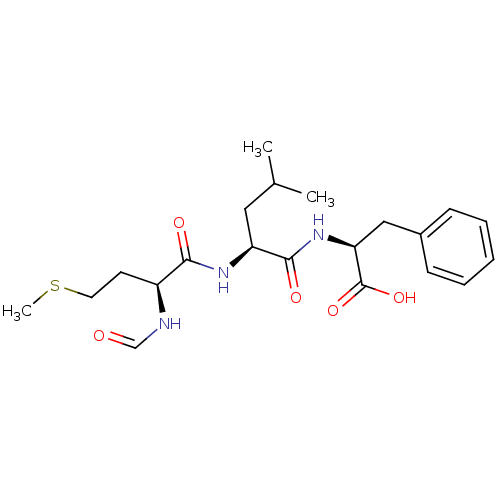 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050946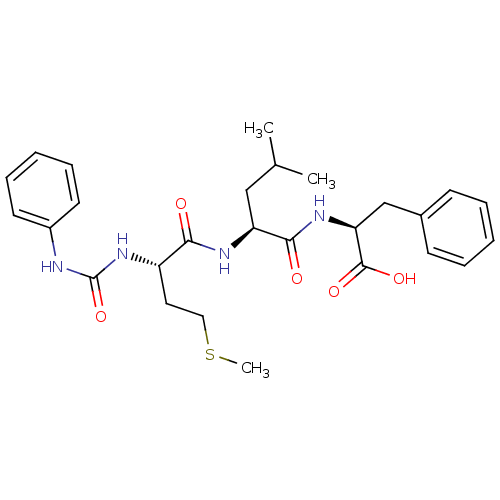 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050942 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050939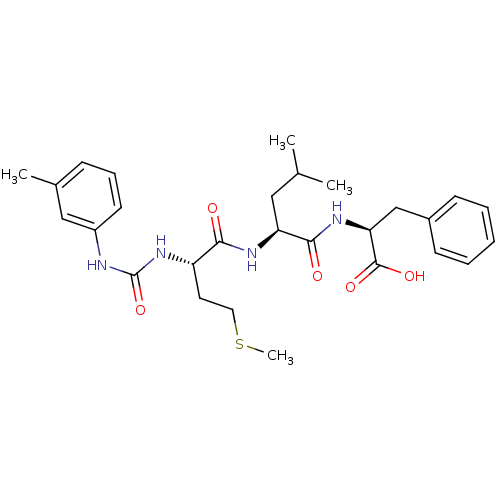 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050926 ((S)-2-((R)-4-Methyl-2-{(S)-2-[(R)-4-methyl-2-((S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050928 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050943 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Isopropyl-ure...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050931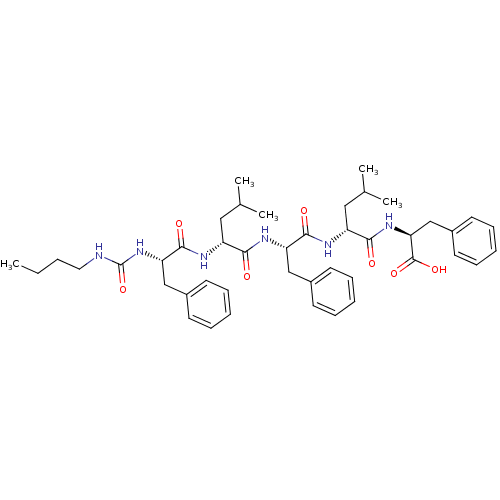 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Butyl-ureido)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050927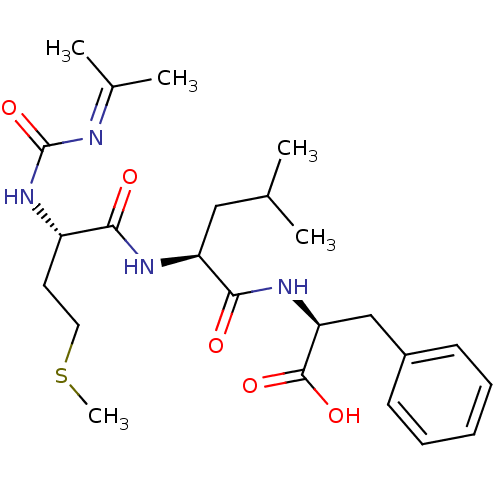 ((S)-2-{(S)-2-[(S)-2-(3-Isopropenyl-ureido)-4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050934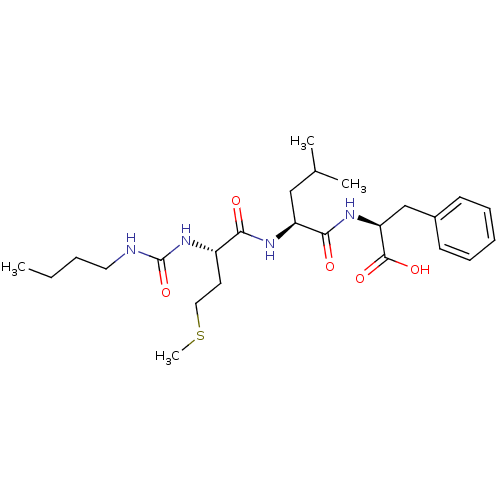 ((S)-2-{(S)-2-[(S)-2-(3-Butyl-ureido)-4-methylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050945 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Adamantan-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050942 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050943 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Isopropyl-ure...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050931 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Butyl-ureido)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050940 ((S)-2-{(S)-2-[(S)-2-(3-Benzyl-ureido)-4-methylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050940 ((S)-2-{(S)-2-[(S)-2-(3-Benzyl-ureido)-4-methylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050926 ((S)-2-((R)-4-Methyl-2-{(S)-2-[(R)-4-methyl-2-((S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050944 ((S)-2-{(S)-2-[(S)-2-(3-Adamantan-1-yl-ureido)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050948 ((S)-2-{(S)-2-[(S)-2-(3-Ethyl-ureido)-4-methylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050938 ((S)-2-{(S)-2-[(S)-2-(3-Isobutyl-ureido)-4-methylsu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050939 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050941 ((S)-2-[(S)-4-Methyl-2-((S)-4-methylsulfanyl-2-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050930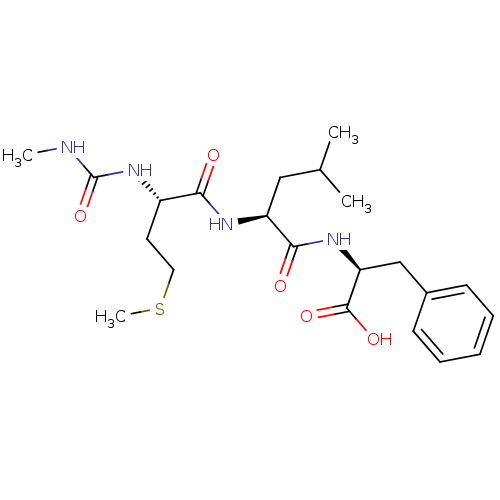 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050947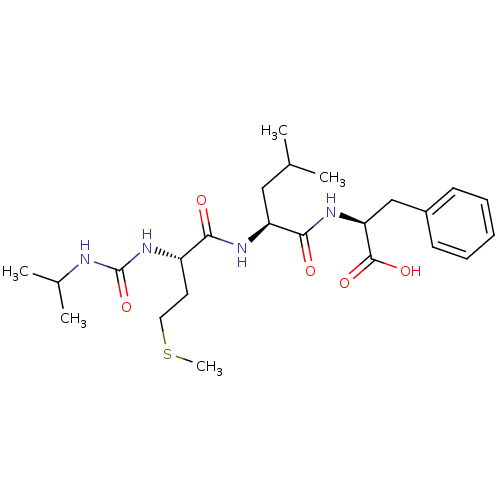 ((S)-2-{(S)-2-[(S)-2-(3-Isopropyl-ureido)-4-methyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050936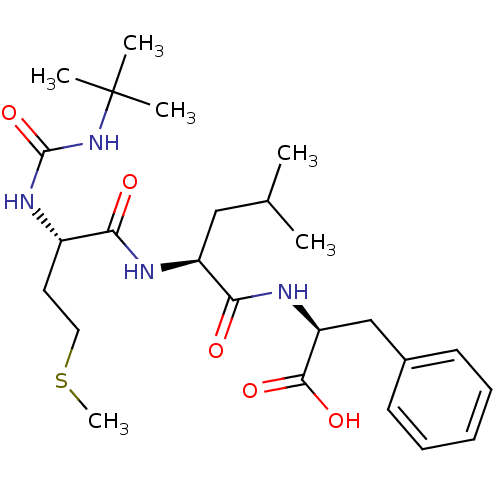 ((S)-2-{(S)-2-[(S)-2-(3-tert-Butyl-ureido)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050944 ((S)-2-{(S)-2-[(S)-2-(3-Adamantan-1-yl-ureido)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050932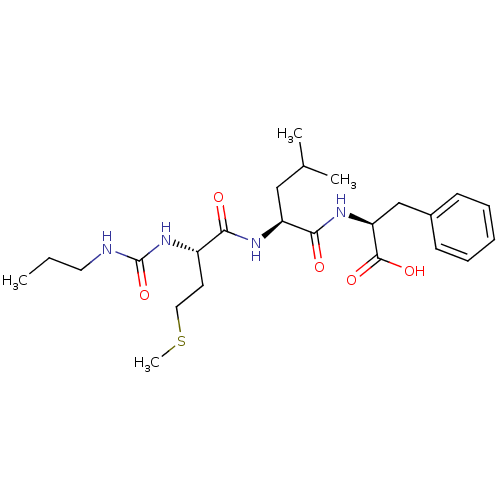 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050932 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050948 ((S)-2-{(S)-2-[(S)-2-(3-Ethyl-ureido)-4-methylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050936 ((S)-2-{(S)-2-[(S)-2-(3-tert-Butyl-ureido)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050930 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050938 ((S)-2-{(S)-2-[(S)-2-(3-Isobutyl-ureido)-4-methylsu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050947 ((S)-2-{(S)-2-[(S)-2-(3-Isopropyl-ureido)-4-methyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050946 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050935 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050939 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050934 ((S)-2-{(S)-2-[(S)-2-(3-Butyl-ureido)-4-methylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050933 ((S)-2-((S)-2-{(S)-2-[3-(4-Chloro-phenyl)-ureido]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050929 (2-((S)-2-{(S)-2-[3-(4-Methoxy-phenyl)-ureido]-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050941 ((S)-2-[(S)-4-Methyl-2-((S)-4-methylsulfanyl-2-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||